Abstract
The skeletal muscle satellite cell was first described and named based on its anatomic location between the myofiber plasma and basement membranes. In 1961, two independent studies by Alexander Mauro and Bernard Katz provided the first electron microscopic descriptions of satellite cells in frog and rat muscles. These cells were soon detected in other vertebrates and acquired candidacy as the source of myogenic cells needed for myofiber growth and repair throughout life. Cultures of isolated myofibers and, subsequently, transplantation of single myofibers demonstrated that satellite cells were myogenic progenitors. More recently, satellite cells were redefined as myogenic stem cells given their ability to self-renew in addition to producing differentiated progeny. Identification of distinctively expressed molecular markers, in particular Pax7, has facilitated detection of satellite cells using light microscopy. Notwithstanding the remarkable progress made since the discovery of satellite cells, researchers have looked for alternative cells with myogenic capacity that can potentially be used for whole body cell-based therapy of skeletal muscle. Yet, new studies show that inducible ablation of satellite cells in adult muscle impairs myofiber regeneration. Thus, on the 50th anniversary since its discovery, the satellite cell’s indispensable role in muscle repair has been reaffirmed.
Keywords: satellite cell, stem cell, self-renewal, Pax7, nestin-GFP, extrafusal, intrafusal, spindle, myofiber, pericyte, regeneration, aging
The functional units responsible for skeletal muscle contraction are cylindrical, multinucleated muscle fibers (myofibers). These contractile structures are established during embryogenesis, when mononuclear cells known as myoblasts fuse into immature myofibers (myotubes). The myofiber nuclei (myonuclei) are postmitotic and under normal conditions cannot re-enter a proliferative state to contribute additional nuclei. During postnatal life, myofiber growth, homeostasis, and repair rely on satellite cells, myogenic stem cells residing between the myofiber plasmalemma and basal lamina (Fig. 1) (Katz 1961; Mauro 1961; Hawke and Garry 2001; Yablonka-Reuveni and Day 2011). For many years following its discovery in 1961, electron microscopy provided the only definitive method of identification of the skeletal muscle satellite cell. More recently, several molecular markers have been described that can be used to detect satellite cells, making them more accessible for study at the light microscope level. With the extensive use of histological and cytological approaches in the studies of satellite cells, the Journal of Histochemistry and Cytochemistry has provided a visible platform for original publications and reviews on these fascinating cells. Here, we join the celebrations for the satellite cell at 50, discussing selective topics related to satellite cell biology.
Figure 1.
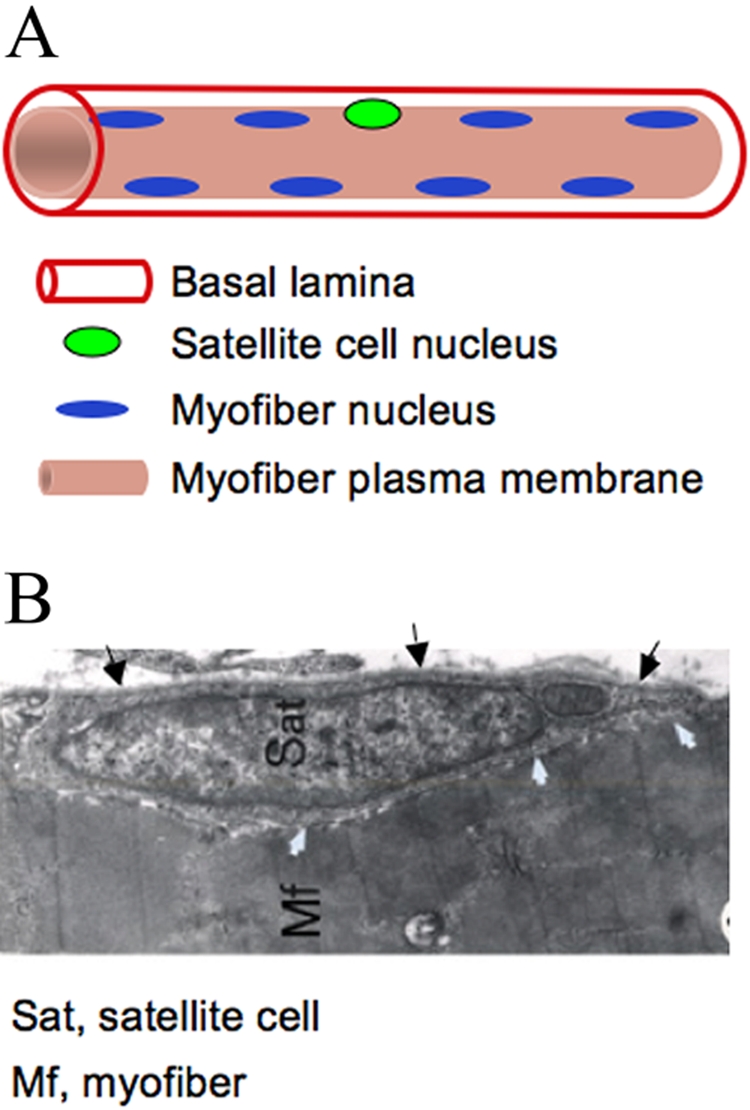
A schematic (A) and electron microscopy image (B) of the satellite cell location. In panel A, nuclei depicted at the myofiber periphery represent the state of healthy adult myofibers; immature myofibers present in regenerating muscles and in muscular dystrophy display centralized myofiber nuclei (not shown) (Yablonka-Reuveni and Day 2011, with kind permission of Springer Science+Business Media). In panel B, black arrows depict the basal lamina, and white arrows depict apposing satellite cell and myofiber membranes; note the sarcomeric organization within the myofiber (Yablonka-Reuveni 1995). The myofiber basement and plasma membranes have been routinely detected by immunostaining with antibodies against laminin and dystrophin, respectively.
Historical Perspective
The capacity of skeletal muscle to regenerate was documented in the 19th century, but it took another century before researchers unveiled the cellular basis of myofiber formation and regeneration (reviewed in Scharner and Zammit 2011). Seminal studies that set the stage for current cell biology of muscle regeneration were published in early 1960s. It was demonstrated that the multi-nucleated myofiber, the contractile unit of skeletal muscle, is formed by the fusion of mononucleated myoblasts and that single cells, but not myofiber nuclei, are involved in cell proliferation (Bintliff and Walker 1960; Capers 1960; Cooper and Konigsberg 1961; Stockdale and Holtzer 1961). This effectively resolved the “enigmatic” finding reported 44 years earlier (Lewis and Lewis 1917) that myofibers appeared to increase in size and in content of nuclei in the absence of any observable nuclear division within the myofiber. A complementary 1961 discovery consisted of electron microscopic descriptions of an apparently quiescent cell lying on the surface of the myofiber, but beneath its basement membrane, where its peripheral position earned it the name satellite cell (Katz 1961; Mauro 1961). Although first detected in frog muscle, the satellite cell presence was immediately confirmed in rat muscle (Mauro 1961), and it was soon shown to occupy a common anatomic position in the majority of vertebrates (reviewed in Grounds and Yablonka-Reuveni 1993).
Upon its discovery, the satellite cell acquired immediate candidacy as the source of myogenic cells for growth and repair of postnatal skeletal muscle. However, the debate about the actual source of myoblasts for muscle regeneration continued as there was no direct evidence that satellite cells were indeed myogenic progenitors (Carlson 1973; Scharner and Zammit 2011). In general, stem/progenitor cells have been identified and characterized in terms of molecular markers, which have then been used to trace them to their anatomic niche within a tissue. In the case of the satellite cell, attribution of a stem cell–like status to an anatomically defined entity made it difficult to devise stringent tests, because its activity during regeneration usually displaces the cell from its position beneath the basal lamina. Thus, the principal defining characteristics of a satellite cell are removed, destroying any formal connection between it and the myoblasts that appear upon injury and eventually form new myofibers. Evidence that satellite cells function as myogenic precursors was initially based on studies of the distribution of labeled thymidine in growing or regenerating muscles (Grounds and Yablonka-Reuveni 1993). Studies using this approach collectively led to the commonly accepted view that satellite cells divide to provide myonuclei to growing myofibers (Moss and Leblond 1971) before becoming mitotically quiescent in normal mature muscle (Schultz et al. 1978).
Conclusive proof that myofibers harbor cells that give rise to myoblasts and multinucleated myotubes was eventually shown with isolated myofibers (Bischoff 1975; Konigsberg et al. 1975). The isolation of viable myofibers was subsequently optimized using collagenase digestion for studies of the myofiber itself (Bekoff and Betz 1977a, 1977b) and for satellite cell studies (Bischoff 1986). This procedure has facilitated effective isolation of intact myofibers with their complete cohort of satellite cells still resident beneath the basal lamina (Bischoff 1986; Yablonka-Reuveni and Rivera 1994; Rosenblatt et al. 1995). Upon myofiber culturing, the satellite cells proliferate, giving rise to satellite cell–derived myoblasts that can differentiate and form multinucleated myotubes. Transplantation of single myofibers into host muscle has provided important evidence that the satellite cells contributed by the donor myofiber indeed act as myogenic stem cells in vivo, able to give rise to both new myofibers and, importantly, many new satellite cells (Collins et al. 2005). The satellite cell therefore fulfills the basic definition of a stem cell in that it can give rise to a differentiated cell type and maintain itself by self-renewal.
With the development of contemporary means for genetic labeling of satellite cells, it has become possible to trace their progeny even after muscle tissue has been injured. Lineage-tracing studies relying on inducible activation of Pax7-driven reporter expression in adult muscle have confirmed that satellite cells can repair damaged muscle tissue (Lepper et al. 2009). Furthermore, recent studies using novel genetic models have enabled specific ablation of satellite cells in adult mice and have provided what might be considered not only strong, but likely the ultimate, evidence for the essential role of satellite cells in muscle regeneration (Lepper et al. 2011; McCarthy et al. 2011; Murphy et al. 2011; Sambasivan et al. 2011).
Functional Satellite Cells Are Required Throughout Life
The distinct anatomic position of the satellite cell on the surface of the myofiber beneath the basal lamina provides immediacy and sensitivity to a postmitotic tissue like skeletal muscle that is critically dependent on mechanical, structural, and functional integrity (Anderson 2006; Ciciliot and Schiaffino 2010). In the juvenile growth phase, when muscles enlarge, satellite cells are proliferative and add nuclei to growing myofibers (Moss and Leblond 1971; Campion 1984; Schultz 1996; Halevy et al. 2004). In most adult muscles, satellite cells are typically quiescent until their activation is invoked by muscle injury (Snow 1978; Grounds and Yablonka-Reuveni 1993; Schultz et al. 1978; Hawke and Garry 2001). Subtle injuries may lead to minimal proliferation of activated satellite cells, whereas major trauma can recruit greater numbers of satellite cells and promote prolonged proliferation prior to differentiation. As small myofiber injuries can occur routinely during daily activity, a mechanism for repair is essential for muscle maintenance throughout life.
Activation of satellite cells is controlled by proximal signals from the muscle niche and microvasculature and through an inflammatory response (Bischoff 1989; Yablonka-Reuveni, Seger et al. 1999; Wozniak et al. 2005; Christov et al. 2007; Carlson et al. 2008; Gopinath and Rando 2008; Shavlakadze et al. 2010). Systemic factors may also regulate satellite cell activation (Conboy et al. 2005; Carlson et al. 2009; Shavlakadze et al. 2010). Among the factors considered to be regulators of satellite cell activation, hepatocyte growth factor (HGF) and neuronal nitric oxide synthase (NOS) have been studied extensively (Tatsumi et al. 1998; Tatsumi et al. 2001; Wozniak et al. 2005; Anderson 2006; Leiter et al. 2011), and fibroblast growth factors (FGFs) appear to play a role in the process as well (Yablonka-Reuveni and Rivera 1994, 1997; Yablonka-Reuveni, Seger et al. 1999; Kastner et al. 2000; Jones et al. 2005; Shefer et al. 2006). We have also detected high-level transcript expression of FGF receptor 1 (FGFR1) and to a lesser extent FGFR4 in freshly isolated satellite cells prepared by FACS sorting from nestin-GFP mice (K. Day and Z. Yablonka-Reuveni, unpublished results). Clearly, additional in situ studies within the muscle tissue are needed for further confirmation of the FGF-FGFR role in satellite cell activation.
Following their activation, satellite cells may contribute to repair of damaged myofibers and also generate new myofibers following cell division and fusion of myoblast progeny (Grounds and Yablonka-Reuveni 1993; Collins et al. 2005). Satellite cell behavior is under stringent regulatory control in order to balance various actively maintained states, including quiescence, entry into proliferation and continuity of the cell cycle, and terminal differentiation (Shefer and Yablonka-Reuveni 2008; Day et al. 2009). Furthermore, apart from their ability to fortify myofibers and contribute to muscle regeneration, satellite cells have the capacity to replenish a reserve pool and self-renew, qualifying them as tissue-specific stem cells (Collins et al. 2005; Sacco et al. 2008). It is possible, however, that individual satellite cells differ with regard to their amplification and renewal potential (Kuang et al. 2007; Sacco et al. 2008; Day et al. 2010).
During early postnatal growth, muscle satellite cells can represent about 30% of the nuclei, whereas in the healthy adult satellite cells represent approximately 2%–7% of nuclei within skeletal muscle (Hawke and Garry 2001; Halevy et al. 2004). The number of satellite cells per myofiber or per cross-sectional area may vary immensely between muscles. For example, the fast twitch extensor digitorum longus (EDL) contains fewer satellite cells compared with the slow twitch soleus (Hawke and Garry 2001; Zammit et al. 2002; Shefer et al. 2006). Additionally, as shown in chicken muscle, myofiber ends may have a higher concentration of satellite cells than the rest of the myofiber (Allouh et al. 2008).
There are also reports of an age-associated decline in the number of satellite cells, where the presence and extent of decline may vary by muscle (Shefer et al. 2006; Collins et al. 2007; Day et al. 2010; Shefer et al. 2010). Satellite cell performance may also decline in the aging environment, a possible contributory factor to age-associated muscle deterioration known as sarcopenia (Thompson 2009; Shefer et al. 2010). However, additional studies suggest that initial performance of skeletal muscle progenitors is delayed but not necessarily impaired in aged muscle and that factors beyond satellite cell activity alone may play a role in reducing muscle repair in old age (Carlson and Faulkner 1996; Grounds 1998; Shavlakadze et al. 2010). Indeed, satellite cell activity can be rejuvenated upon exposure of old muscle to a juvenile environment by cross-transplantation or by parabiosis of young and old mice (Carlson and Faulkner 1989; Conboy et al. 2005). Muscle wasting associated with muscular dystrophy is also thought to lead to exhaustion of satellite cells because of the continuous demand for reparative myogenic cells (Blau et al. 1983; Webster and Blau 1990; Aguennouz et al. 2011). However, exercise supports an increase in satellite cells, even in old age when there is a drastic decline in satellite cell numbers at least in some limb muscles (Shefer et al. 2010; Leiter et al. 2011; Smith and Merry 2011). It would be advantageous to gain further insight into the mechanisms involved in this process and whether such an increase in satellite cells is merely an outcome of muscle activity or an underlying factor in the beneficial effect of exercise on muscle performance. Last, although it is commonly accepted that satellite cells are involved in myofiber growth during the early phase of postnatal life, the role, if any, of satellite cells in myofiber hypertrophy during adult life has been a subject of debate (McCarthy and Esser 2007; O’Connor and Pavlath 2007; O’Connor et al. 2007). A recent study using a genetic mouse model for specific ablation of satellite cells in adult mice concluded that effective myofiber hypertrophy can take place in satellite cell–depleted skeletal muscle, although the typical increase in myofiber nuclei associated with hypertrophy was absent (McCarthy et al. 2011). Also, a more “minor” component of the hypertrophic response, which involves de novo formation of myofibers, was significantly blunted following satellite cell depletion, indicating a distinct requirement for satellite cells in the hypertrophy-associated regenerative process (McCarthy et al. 2011).
Overall, satellite cells are vital to skeletal muscle homeostasis and regeneration throughout life, and understanding the regulation of myogenic stem cells will likely provide valuable insights into muscle wasting in disease and aging.
Detection, Molecular Markers, and Lineage Origin of Satellite Cells
Satellite cells were initially described by their anatomic location on the surface of muscle fibers, between the myofiber plasmalemma and the basal lamina, using electron microscopy (Katz 1961; Mauro 1961; Muir et al. 1965). More recent methods facilitate monitoring these cells by light microscopy based on expression of a range of specific markers that can be detected by immunostaining (reviewed in Biressi and Rando 2010; Boldrin et al. 2010). In particular, specific expression of the paired box transcription factor Pax7 (Seale et al. 2000) and availability of an excellent antibody for immunodetection of this protein provide a consistent means to identify satellite cells in their native position in a range of species including mouse (Seale et al. 2000; Zammit, Golding et al., 2004; Shefer et al. 2006; Day et al. 2007; Day et al. 2010), rat (Shefer et al. 2010), chicken (Halevy et al. 2004; Allouh et al. 2008), and human (Lindstrom and Thornell 2009; Lindstrom et al. 2010).
Genetically manipulated reporter mice that permit direct detection of satellite cells based on specific expression of a fluorophore (e.g., GFP) or β-galactosidase (β-gal) have also become available (Beauchamp et al. 2000; Montarras et al. 2005; Biressi et al. 2007; Day et al. 2007). We have demonstrated that transgenic expression of GFP under the control of nestin regulatory elements (Mignone et al. 2004) allows detection of satellite cells in freshly isolated myofibers (Day et al. 2007). These nestin-GFP mice also facilitate isolation of satellite cells using fluorescent-activated cell sorting (FACS) and subsequent studies of purified populations (Day et al. 2007). The Myf5nLacZ/+ mouse has also provided a means to identify satellite cells in intact muscle and isolated myofibers (Beauchamp et al. 2000; Collins et al. 2005; Zammit et al. 2006; Day et al. 2010; Ono et al. 2010). In this mouse, one of the Myf5 alleles was modified to direct lacZ expression (Tajbakhsh et al. 1996; Tajbakhsh et al. 1997), resulting in β-gal protein in the nuclei of satellite cells, as originally reported by Beauchamp and colleagues (Beauchamp et al. 2000). We frequently use crosses of nestin-GFP with Myf5nLacZ/+ mice, allowing the detection of satellite cells by means of direct fluorescence and X-gal staining (Day et al. 2010). The 3F-nlacZ-E transgenic mice (Kelly et al. 1995) (referred to as MLC3F-nLacZ mice in some studies) are also useful for distinguishing between satellite cells and myofiber nuclei. In these mice, regulatory elements of muscle-specific myosin light chain 3F (MLC3F) drive LacZ expression in myofiber nuclei but not in satellite cells (Beauchamp et al. 2000). A cross of nestin-GFP mice with MLC3F-nLacZ transgenic mice results in a clear distinction between satellite cells (which are GFP+ but negative for X-gal) and myofiber nuclei (which are X-gal+ but negative for GFP) (Day et al. 2010).
Although studies on the functional role of Pax7 during myogenesis have been published (Olguin et al. 2007; Collins et al. 2009), the role of Pax7 in the development and maintenance of satellite cells remains an enigma. The initial study reporting that satellite cells express Pax7 concluded that this transcription factor is required for specification of satellite cells; the survival of mice in which the Pax7 gene was inactivated was compromised (Seale et al. 2000). Notably, such early lethality in Pax7-null mice could have also been due to failure of Pax7-dependent systems other than muscle, such as the brain. Subsequent studies on Pax7 inactivation in mice showed that it may control survival of satellite cells beyond the neonatal period, because detectable satellite cells exhibited signs of apoptosis and declined rapidly postnatally when Pax7 was absent (Oustanina et al. 2004; Relaix et al. 2006). A more recent study using inducible inactivation of Pax7 indicated that it is only required postnatally up to the juvenile stage to support satellite cells, but not for satellite cell function and maintenance in adult muscle (Lepper et al. 2009). The study suggests a cell-intrinsic difference in Pax7 dependency between the myogenic progenitors supporting neonatal muscle development and adult satellite cells.
The conclusion of the lineage tracing study by Lepper and colleagues seems to reaffirm early studies proposing that satellite cells represent a distinct population of myogenic progenitors that replaces the fetal myogenic population (reviewed in Yablonka-Reuveni 1995). These early studies demonstrated that progeny of myogenic cells isolated from adult muscles displayed various morphological and biochemical features that were distinct from cells derived from myogenic progenitors present in the fetal phase of embryogenesis. These studies further identified the emergence of “adult type” myogenic progenitors in late fetal phase, corresponding with the development of mature basal lamina around myofibers (Cossu et al. 1987; Cossu and Molinaro 1987; Feldman and Stockdale 1992; Hartley et al. 1991, 1992; Yablonka-Reuveni 1995). In the absence of a direct means to trace the satellite cells themselves, the latter studies established the concept that myogenic progenitors in the adult could be distinct from those present in fetal muscle based on the features of cells cultured from adult muscle. Notably, there is also heterogeneity in the myogenic cells that contribute to muscle development during earlier and later phases of embryogenesis (White et al. 1975; Seed and Hauschka 1988; Stockdale 1992).
Satellite cells share Pax7 expression (as well as Myf5nLacZ and nestin-GFP expression) across all muscles regardless of the embryonic origin of the parent muscle (Day et al. 2007; Harel et al. 2009; Ono et al. 2010). Skeletal muscles of body and limb are derived from somites, but most head muscles originate from cranial mesoderm (Noden and Francis-West 2006; Schienda et al. 2006; Zammit et al. 2006; Harel et al. 2009; Sambasivan et al. 2009; Ono et al. 2010). Lineage tracing studies indicate a common origin for the satellite cells and their parent muscle (Armand et al. 1983; Schienda et al. 2006; Harel et al. 2009; also our unpublished studies with limb, diaphragm, extraocular, and jaw muscles). In limb and trunk muscles, satellite cells are derived from cells that express both Pax7 and its paralog, Pax3, during embryogenesis, whereas progenitors participating in development of many head muscles do not exhibit Pax3 expression (Epstein et al. 1996; Tajbakhsh et al. 1997; Gros et al. 2005; Relaix et al. 2005; Schienda et al. 2006; Harel et al. 2009; Relaix and Marcelle 2009; Sambasivan et al. 2009).
Although Pax7 is commonly expressed in satellite cells, the status of Pax3 in satellite cells is not entirely clear. Even among somite-derived muscles, Pax3 transcripts are expressed at a relatively higher level only by satellite cells in certain muscle groups (e.g., diaphragm), and likewise, Pax3-driven reporter expression is only detected in satellite cells of some adult muscles (Montarras et al. 2005; Relaix et al. 2006; Day et al. 2007). Satellite cell analysis with a well-characterized antibody for Pax3 (a mouse monoclonal antibody that does not cross-react with Pax7) has led researchers to conclude that Pax3 protein is not necessarily expressed by mouse satellite cells, even when they show a relatively high level of Pax3 transcript expression. Indeed, Pax3 protein expression during adult myogenesis appears to be repressed by a specific microRNA, thereby preventing inhibition of differentiation by Pax3 (Crist et al. 2009). However, the detection and specific degradation of Pax3 protein during satellite cell activation have also been described (Boutet et al. 2007). Different from the enigmatic status of Pax3 protein expression in mouse satellite cells, Pax3 protein is readily detected by immunostaining in some satellite cells (Pax7+) of chicken muscles, but there is also a decline with age in Pax3 detection (Kirkpatrick et al. 2009). It is thus possible that Pax3 expression by satellite cells in somite-derived muscles is a residual trait from embryogenesis, where both Pax3 and Pax7 are expressed by myoblasts during development. In contrast, satellite cells in some craniofacial muscles that develop without Pax3 gene activity do not demonstrate Pax3 transcripts or Pax3-driven reporter expression (Harel et al. 2009; Sambasivan et al. 2009; P. Stuelsatz, A. Shearer and Z. Yablonka-Reuveni, unpublished work).
In the original discovery of the satellite cell, it was detected in both intrafusal (Katz 1961) and extrafusal (Mauro 1961) myofibers; in both of these myofiber types satellite cells can be detected by Pax7 expression (Kirkpatrick et al. 2008; Kirkpatrick et al. 2009) (Fig. 2). Intrafusal myofibers are within special structures in skeletal muscles known as spindles, and they function in proprioception (Walro and Kucera 1999; Kirkpatrick et al. 2008; Osterlund et al. 2011). Some intrafusal myofibers extend beyond the spindle capsule (Walro and Kucera 1999), but because of their small diameter and rarity can be overlooked in routine inspection of cross-sections when not inside the capsule. Intrafusal myofibers make up a small subpopulation of the muscle myofibers, whereas the bulk of skeletal muscle myofibers are termed extrafusal myofibers. The diameter of intrafusal myofibers remains small from an early postnatal age, and they retain expression of developmental myosins as well as expression of LacZ in myofiber nuclei of Myf5nLacZ/+ knock-in mice (Kozeka and Ontell 1981; Walro and Kucera 1999; Zammit, Carvajal et al. 2004; Kirkpatrick et al. 2008; Kirkpatrick et al. 2009; Osterlund et al. 2011). It is unknown whether satellite cells associated with intrafusal versus extrafusal myofibers are functionally unique and/or derived from distinct myogenic populations. A better understanding of the distinctions, if any, between satellite cells in the two types of myofibers is important for regenerative medicine because a complete muscle recovery would require that intrafusal myofiber be present as well for proprioception.
Figure 2.
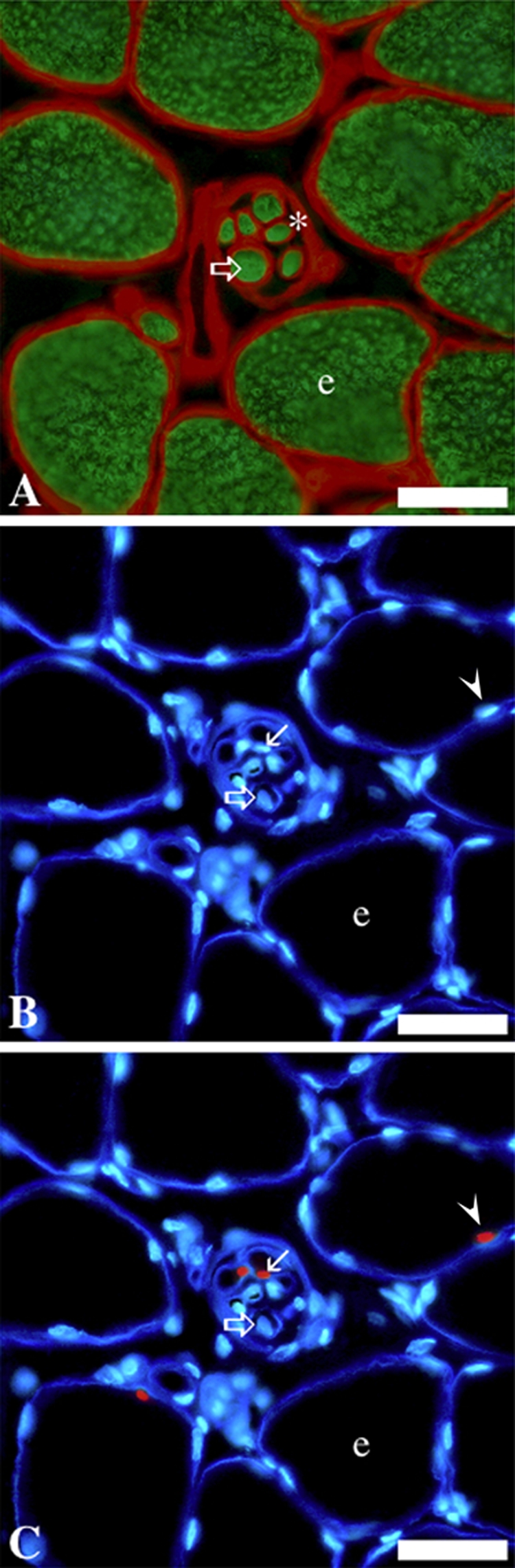
Immunohistochemical labeling of serial cross-sections of anterior latissimus dorsi muscle from a 2-month-old chicken, depicting Pax7+ satellite cells in intrafusal and extrafusal myofibers. The small intrafusal fibers (clustered within a spindle capsule) are near the center of each parallel image. A, Myosin (in green) highlights myofiber cross-sectional area; laminin (in red) highlights the myofiber basal lamina and the spindle capsule (identified with an asterisk). B and C, laminin (deep blue) and nuclei (light blue). C, Pax7+ nuclei (in red). e, extrafusal fiber; open/large arrow, intrafusal fiber; small arrow, Pax7+ cell associated with intrafusal fiber; arrowhead, Pax7+ cell associated with an extrafusal fiber. Scale bars = 30 µm. This figure is adapted from Kirkpatrick et al. (2009) with kind permission from Dr. Benjamin Rosser. Immunostaining regents and protocols are detailed in Kirkpatrick et al. (2009).
Detection of Satellite Cell Progeny by Temporal Expression Patterns of Myogenic-Related Transcription Factors
At the molecular level, myogenesis of satellite cells is highly orchestrated to ensure that specific genes are regulated in a temporally organized manner according to genetic blueprints, cell cycle requirements, and environmental factors (Yablonka-Reuveni et al. 2008). The resulting pattern of gene expression yields terminally differentiated myoblasts, capable of adding myonuclei to existing myofibers in addition to fusing together to form new myofibers during muscle growth and repair (Charge and Rudnicki 2004; Shefer and Yablonka-Reuveni 2008; Yablonka-Reuveni and Day 2011).
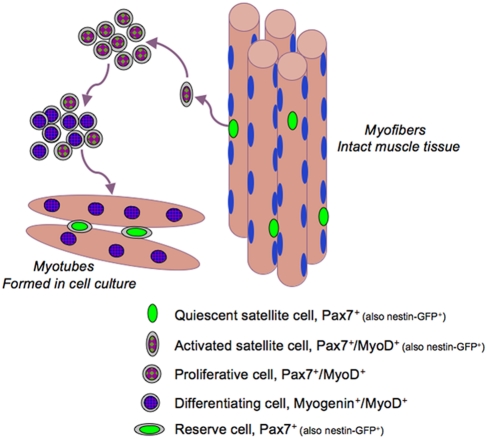
Satellite cell progeny can be distinguished from their quiescent progenitors based on distinctive gene expression patterns (Zammit et al. 2006; Yablonka-Reuveni et al. 2008; Yablonka-Reuveni and Day 2011). In particular, expression of MyoD and myogenin has been used extensively in conjunction with Pax7 (Yablonka-Reuveni and Rivera 1994; Zammit, Carvajal et al. 2004; Shefer et al. 2006; Day et al. 2009). Fig. 3 summarizes Pax7, MyoD, and myogenin expression patterns by satellite cells and their progeny. Proliferating progeny (myoblasts) continue to express Pax7 but, in contrast to their quiescent progenitors, also express MyoD. A decline in Pax7 along with the induction of the muscle-specific transcription factor myogenin marks myoblasts that have entered the differentiation phase and initiated cell cycle withdrawal. Coinciding with or occurring soon after the upregulation of myogenin, differentiating myoblasts initiate expression of various genes encoding structural proteins, such as sarcomeric myosin, and fuse into myotubes (Andres and Walsh 1996; Halevy et al. 2004; Shefer et al. 2006; Yablonka-Reuveni, Rudnicki et al. 1999; reviewed in Yablonka-Reuveni and Day 2011). Fig. 3 also presents the phases that express the nestin-GFP transgene: that is, quiescent and activated satellite cells and their renewing progeny (Day et al. 2007; Day et al. 2010); additional discussion about satellite cell renewal is detailed in the next section.
Figure 3.
The molecular signature of satellite cells and their progeny upon activation, proliferation, differentiation, and self-renewal. The model is based primarily on cell culture studies (Yablonka-Reuveni and Day 2011, with kind permission of Springer Science+Business Media; edited for nestin-GFP information). Such cell cultures have contributed to the identification of autocrine/paracrine factors that can regulate satellite cell activation and proliferation, demonstrating that hepatocyte growth factors and fibroblast growth factors are crucial growth factors involved in the process (e.g., Shefer et al. 2006; Wozniak et al. 2005; Yablonka-Reuveni and Rivera 1997; Yablonka-Reuveni, Seger et al. 1999; Yamada et al. 2009; Tatsumi et al 1998; Gal-Levi et al. 1998). The role of many other growth factors and extracellular matrix components has been studied extensively in cell culture as well; for a comprehensive review of these topics that are beyond the scope of this article, see Shefer and Yablonka-Reuveni (2008).
Both quiescent and proliferating satellite cells also express the myogenic regulatory factor Myf5 as determined by mRNA analysis (Kastner et al. 2000; Day et al. 2007; Day et al. 2010). Myf5 promoter activity can also be observed through β-gal detection in satellite cells and their proliferating progeny in myogenic cultures from the aforementioned Myf5nlacZ/+ mice (Beauchamp et al. 2000; Day et al. 2010). However, detection of the Myf5 protein has not been reported in quiescent satellite cells, although proliferating progenies do express it (Day et al. 2009), albeit at a higher level in myoblasts from mice lacking MyoD compared with Wild-type (Yablonka-Reuveni, Rudnicki et al. 1999). It is therefore possible that whereas the Myf5 promoter is active in quiescent satellite cells, Myf5 protein is not produced until cells begin to proliferate. Ultimately, Myf5 gene expression declines when myoblasts enter differentiation (i.e., withdraw from the cell cycle followed by fusion into myotubes), whereas MyoD expression persists well into the differentiation stage when satellite cells are maintained in our standard culture conditions (Shefer et al. 2006; Day et al. 2009; Yablonka-Reuveni and Day 2011).
The observation that Myf5 expression declines when myoblasts enter differentiation whereas MyoD expression persists suggests that these two myogenic regulatory factors have different roles during myogenesis of satellite cells (reviewed in Day et al. 2009). MyoD null mice contain functional satellite cells that can contribute to normal (albeit slightly delayed) muscle regeneration (Yablonka-Reuveni, Rudnicki et al. 1999; White et al. 2000). However, it was initially reported that MyoD is essential for muscle regeneration (Megeney et al. 1996). Indeed, the delayed differentiation observed in primary cell cultures from MyoD null mice (Yablonka-Reuveni, Rudnicki et al. 1999) and the impaired differentiation observed in single myofibers from such mice (Yablonka-Reuveni, Rudnicki et al. 1999; Cornelison et al. 2000) do suggest a cell autonomous defect that might affect the dynamics of muscle repair in vivo. Unpublished studies from our laboratory have also demonstrated that progeny of satellite cells from MyoD null mice differentiated poorly, if at all, when the progenitors were cultured at low or clonal density, but robust differentiation (although delayed) was observed with high-density cultures. Overall, it is possible that absence of MyoD may affect muscle regeneration only in certain types of injury protocols, and this may lead to the different conclusions stated in published in vivo regeneration studies of MyoD null mice (Megeney et al. 1996; McIntosh et al. 1998; White et al. 2000).
Early studies that developed several different Myf5 null mice demonstrated neonatal lethality, caused likely by compounded effect of the Myf5 mutations on a neighboring gene (Olson et al. 1996). However, more recent studies generated Myf5 null mice that survive to adulthood and are fertile, suggesting that Myf5 is not essential for satellite cell function in adult muscle (Gayraud-Morel et al. 2007; Ustanina et al. 2007). Myogenin expression is critical for muscle formation during embryogenesis. However, conditional inactivation of the myogenin gene in the adult muscle does not interfere with myogenesis and raises further questions (and new research directions) about the actual role of myogenin in adult life (Knapp et al. 2006; Meadows et al. 2008). The role of the fourth myogenic regulatory factor, MRF4, remains unknown in myogenesis of satellite cells, but it is the only MRF expressed at a high level in adult myofibers, whereas MyoD, Myf5, and myogenin expression levels are relatively lower (Hinterberger et al. 1991). Notably, MRF4 involvement in downregulating myogenin expression has been reported (Zhang et al. 1995). In two different studies on MRF4 mRNA expression in primary myogenic cultures, this factor was detected before, after, and concurrently with myogenin expression (Smith et al. 1993; Smith et al. 1994). In another study, a reporter gene in a targeted allele of MFR4 was expressed in the differentiated, but not proliferative, state of satellite cell progeny (Gayraud-Morel et al. 2007). Our unpublished work indicates differences between satellite cell cultures from limb and diaphragm muscle in the temporal expression of MRF4; relatively high levels of MRF4 transcripts were detected in limb cultures before the increase in the expression of the differentiation-linked marker myogenin, but in diaphragm cultures MRF4 expression coincided with myogenin expression. Hence, the kinetics and role of MRF4 expression be different depending on the age of the organism and the muscle from which myogenic progenitors are isolated.
Satellite Cell Self-renewal
Routine daily activity leads to subtle muscle injuries and requires nuclear replacement for localized myofiber repair throughout life. Therefore, without its renewal, the satellite cell pool would be depleted. However, very little is known about how the satellite cell pool is retained in vivo. Cell culture studies have demonstrated that coinciding with myoblast differentiation, a subpopulation of mononucleated cells downregulate MyoD expression and exit the cell cycle but maintain Pax7 expression, in contrast with neighboring cells that retain MyoD and upregulate myogenin expression (Halevy et al. 2004; Zammit, Golding et al. 2004; Shefer et al. 2006; Yablonka-Reuveni et al. 2008; Day et al. 2009; Day et al. 2010). BrdU labeling studies suggest that such Pax7+/MyoD– cells are derived from Pax7+/MyoD+ myoblasts that have downregulated MyoD expression and do not continue in the cell cycle or transit into differentiation, thus demonstrating the self-renewing population (Zammit, Golding et al. 2004; Day et al. 2007). These Pax7+/MyoD– nonproliferating cells define a reserve population that presumably reflects satellite cell self-renewal (Fig. 3). Indeed, following transplantation of donor satellite cells into host muscles, satellite cells are detected in vivo with donor reporter markers, indicating that at least some of the satellite cells underwent self-renewal (Collins et al. 2005; Sacco et al. 2008). It is sensible to suggest that the donor cells first go through an amplification phase before some of the cells enter the satellite cell niche based on engraftment of single satellite cells that produced both differentiated progeny and renewed satellite cells (Sacco et al. 2008).
There is some evidence that satellite cells self-renew through asymmetric cell divisions controlled by Notch and Wnt signaling pathways (Conboy and Rando 2002; Kuang et al. 2007; Ono et al. 2009; reviewed in Punch et al. 2009; Yablonka-Reuveni and Day 2011). The close association of satellite cells with capillaries residing in the surrounding interstitium also may provide satellite cells with direct signals from pathways traditionally shown to be involved in angiogenesis and vascular integrity (Christov et al. 2007). Interestingly, the angiopoeitin-1/Tie2 receptor-signaling pathway may directly control development of reserve cells and entry into quiescence (Abou-Khalil et al. 2009).
As detailed earlier in this article, our laboratory has found that nestin-GFP transgenic expression in mice allows monitoring of satellite cells in isolated myofibers. This transgene also appears to identify the self-renewing, Pax7+/MyoD– reserve progeny that develop in cell culture (Day et al. 2007; Day et al. 2010). Satellite cells express nestin-GFP, but this expression is diminished in proliferating and differentiating myoblasts and myotubes. However, cultures with dense myotube networks eventually develop mononuclear, nestin-GFP+ cells that do not proliferate (based on BrdU incorporation studies) and are Pax7+/MyoD–. Examination of satellite cells in clonal cultures showed that nestin-GFP+ reserve progeny develop only in dense clones, which accounts for only some of the clones (Day et al. 2007; Day et al. 2010). We also demonstrated an age-linked decline in such clones containing nestin-GFP+ reserve cells (Day et al. 2010). This age-linked decline in clones containing reserve cells is concomitant with a decline in satellite cell numbers on myofibers isolated from old mice (Day et al. 2010). Therefore, impairment in satellite cell renewal in old age may lead to the observed decline in satellite cells with age, which also results in a significant number of myofibers without satellite cells, as shown in myofibers from mouse and rat limb muscles (Shefer et al. 2006; Day et al. 2010; Shefer et al. 2010; ). It is possible that myofibers lacking satellite cells are more prone to age-associated atrophy, but this issue has not been studied further. In addition to secreted factors, contact with myotubes might provide a signal for self-renewal as also suggested in studies with isolated myofibers maintained in suspension where the satellite cells remain on the myofiber surface (Zammit, Golding et al. 2004; Kuang et al. 2007; Kuang et al. 2008).
Apart from the aforementioned evidence supporting the hypothesis that the self-renewing population is reserved from the pool of proliferating myoblasts, there is also the demonstration that satellite cells may include a minor subpopulation of stem cells that can self-renew while also providing committed satellite cells within a single proliferative round (Kuang et al. 2007; Kuang et al. 2008; Punch et al. 2009). According to this asymmetric cell division model, most of the satellite cells are “committed,” expressing both Pax7 and Myf5, and only provide committed progeny (i.e., characteristic myogenic cells). The minor “stem cell” population does not display Myf5 gene activity based on Myf5-Cre driven Rosa26-YFP reporter expression, whereas the committed cells do express YFP (Kuang et al. 2007). However, our studies with Myf5nLacZ/+ mice have suggested that essentially all satellite cells express the Myf5 reporter, but the cells with weaker expression may only be detected with increased sensitivity of β-gal detection methods (Beauchamp et al. 2000; Day et al. 2010).
Moreover, our unpublished studies with Myf5-Cre–driven reporter expression indicate that a minor population of satellite cells indeed does not show Cre-driven reporter (GFP) expression, and the proliferating/differentiated myoblasts and myotubes developed clonally from such satellite cells also do not express GFP. The animals used in these studies were generated by crossing Myf5-Cre mice (Tallquist et al. 2000; Kuang et al. 2007) with Rosa26-mT/mG mice (Muzumdar et al. 2007). The lack of GFP expression in both the progenitors and their committed progeny introduces a complication to the proposed model of asymmetric cell division when relying on the Myf5-Cre mouse. In contrast with the Myf5-Cre mice, a recent study with the MyoD-Cre/Rosa26-YFP mouse model suggests that all satellite cells are derived from cells that “historically” expressed MyoD and therefore are all committed to myogenesis (Kanisicak et al. 2009). Further studies are required to resolve questions about satellite cell functional heterogeneity and self-renewal based on different strains of reporter mice.
Isolation and Culture Approaches for Satellite Cell Analysis
Much of our understanding of satellite cell biology has arisen from analysis of satellite cells in culture. Satellite cells are routinely isolated from skeletal muscles by enzymatic digestion (Yablonka-Reuveni 2004). Depending on the enzymatic procedure used and the purpose for cell isolation, enrichment for satellite cells beyond the basic isolation protocol is often unnecessary; see for example our studies with Pronase digestion of skeletal muscle (Halevy et al. 2004; Shefer et al. 2006; Day et al. 2009; Yablonka-Reuveni 2004; reviewed in Danoviz and Yablonka-Reuveni 2012). Alternatively, satellite cells can be enriched from whole muscle cell suspensions by various approaches that remove myofiber debris present in the initial cell suspension and reduce the presence of fibroblastic cells. Such different enrichment/debris-cleaning approaches include (but are not limited to) (a) initial short-term plating on uncoated tissue cultures dishes that results in removal of fast adhering cells followed by culturing of the remaining non-adhering cells (i.e., differential plating) (Richler and Yaffe 1970; Rando and Blau 1994; Qu-Petersen et al. 2002); (b) fractionation on Percoll density gradients (Yablonka-Reuveni and Nameroff 1987; Yablonka-Reuveni et al. 1987; Morgan 1988; Kastner et al. 2000); and (c) cell sorting by forward and side scatter (Yablonka-Reuveni 1988).
In studies where further enrichment of satellite cells is warranted, cells can be isolated by FACS using antibodies that react with satellite cell surface antigens (Sacco et al. 2008; Ieronimakis et al. 2010). First, cells are released from the muscle tissue using collagenase–dispase, an enzyme mixture that preserves cell surface antigens more effectively than Pronase or trypsin digestion methods. Studies from various laboratories (performed mainly with mouse tissue) have established that satellite cells can be isolated based on being negative for CD45, CD31, and Sca1 and positive for CD34 and α7 integrin (Montarras et al. 2005; Sacco et al. 2008; Ieronimakis et al. 2010). Additional cell surface antigens, including CXCR4, β1 integrin, and syndecan-4, have also been used for isolation of myogenic progenitors from adult muscle (Cerletti et al. 2008; Tanaka et al. 2009). Fluorescence-based reporter systems in genetically manipulated mouse strains have also permitted reliable isolation and study of satellite cells. For example, we have isolated satellite cells from different muscle groups of transgenic nestin-GFP mice by FACS based on high GFP expression in satellite cells (Day et al. 2007; Day et al. 2010). Pax3- and Pax7-driven reporter expression have also been used to sort mouse satellite cells by FACS (Bosnakovski et al. 2008; Montarras et al. 2005). However, in contrast with the Pax7-driven reporter that is commonly expressed by satellite cells across different muscle groups, Pax3 expression is restricted to select satellite cells (Montarras et al. 2005; Relaix et al. 2006). In addition, mice with a GFP reporter gene targeted into the Myf5 locus have permitted isolation of myogenic cells by FACS (Biressi et al. 2007). However, not all satellite cells in this mouse exhibit detectable GFP expression, so efficiency of satellite cell isolation based on this report is reduced (Christov et al. 2007; Gayraud-Morel et al. 2007). Cre-Lox mouse models offer additional ways for isolating satellite cells based on permanent fluorescent reporter expression in cells derived from myogenic progenitor cells expressing Cre-recombinase driven by genes relevant to the myogenic lineage such as Pax3, Myf5, and MyoD (Schienda et al. 2006; Kuang et al. 2007; Kanisicak et al. 2009). When working with such Cre-Lox mouse models to sort satellite cells, one should be careful to ensure that the reporter is not expressed in additional cell types during embryogenesis. See for example the report on Myf5-Cre expression at nonmyogenic sites (Gensch et al. 2008).
The ability to isolate and culture single live myofibers (most commonly from rodent models) offers the advantage of following individual resident satellite cell activity in the presence of its parent myofiber (Bischoff 1986; Yablonka-Reuveni and Rivera 1994; Rosenblatt et al. 1995). In adherent myofiber culture models, satellite cells typically emanate from their position on the myofiber and give rise to proliferating myoblasts that differentiate and generate myotubes (Zammit et al. 2002; Shefer et al. 2006). Depending on culture and media conditions, rapid activation, proliferation, and differentiation of satellite cells can take place within the adherent parent myofiber unit (Yablonka-Reuveni and Rivera 1994, 1997; Yablonka-Reuveni, Rudnicki, et al. 1999; Yablonka-Reuveni, Seger, et al. 1999; Kastner et al. 2000; Shefer and Yablonka-Reuveni 2005) (Fig. 4). Alternatively, culture of myofibers in suspension permits the unique ability to observe the behavior of satellite cells as they proceed through multiple rounds of cell division in cell clusters while remaining in contact with the myofiber (Zammit, Golding et al. 2004).
Figure 4.
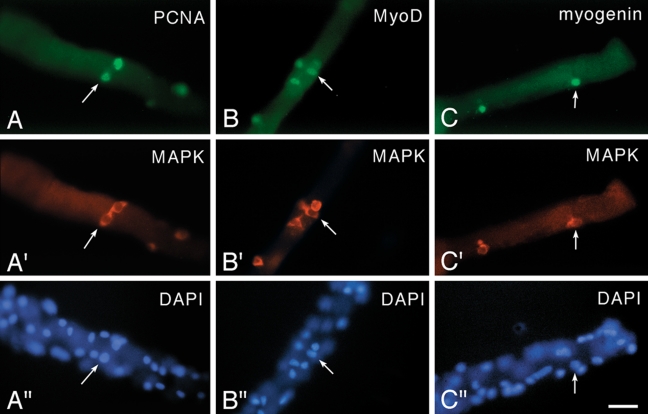
Micrographs of fiber cultures isolated from the flexor digitorum brevis muscle of an 8-week-old rat. Cultures were maintained in basal medium and reacted via double immunofluorescence with a rabbit polyclonal antibody against MAPK (anti-ERK1/2, highlights the cytoplasm of satellite cells as detailed in Yablonka-Reuveni, Seger et al. (1999) and mouse monoclonal antibodies against (A, A′) PCNA, which highlights proliferating cells; (B, B′) MyoD; and (C, C′) myogenin. For each antibody combination, the bottom panel (A′′–C′′) shows a parallel DAPI stain, which highlights both myofiber nuclei and satellite cell nuclei. Arrows in each set of adjacent panels point to the location of the same cell. Immunostaining with the anti-PCNA/anti-MAPK and the anti-MyoD/anti-MAPK is shown for Day 2 cultures, and immunostaining with the anti-myogenin/anti-MAPK is shown for Day 3 cultures. Not all positive nuclei or cells on the fibers are in the same focal plane. Bar = 34 µm. This figure was published first in Yablonka-Reuveni, Seger et al. (1999), where additional details regarding immunostaining reagents and protocols are provided. Note that before high-quality antibodies for satellite cell research were available, researchers investigated activation and proliferation of satellite cells on isolated myofibers by tracing triturated thymidine uptake, which required extra time (even several weeks) to obtain autoradiographic images of the cultures to identify the location of S-phase cells (Bischoff 1989; Schultz 1996; Yablonka-Reuveni and Rivera 1997). This approach provided valuable feedback about response of satellite cells to growth factors, regardless of the tedious method that was used to collect the data (Bischoff 1986).
Isolated single myofibers also allow tracing and recording of satellite cell numbers per myofiber based on specific marker expression, with endogenous Pax7 expression becoming a common and direct approach to monitor satellite cells by immunofluorescence (Beauchamp et al. 2000; Zammit et al. 2002; Shefer et al. 2006; Day et al. 2010). Individual progeny of satellite cells can also be analyzed in clonal studies. We have reported on such clonal analyses of satellite cells upon isolation of cells from whole muscles and also from triturated individual myofibers (Shefer et al. 2006; Yablonka-Reuveni et al. 1987; Day et al. 2010). Notably, some researchers have used an approach for satellite cell isolation that is based on bulk preparations of myofiber fragments. It should be noted that myofiber fragments retain “external” connective tissue cells and the preparation requires further enrichments; otherwise, myogenic purity of the resulting cells is drastically reduced.
Studies with myogenic cell lines (including rat L6 and L8 and mouse C2, C2C12, and MM14; (Yaffe 1969; Yaffe and Saxel 1977; Clegg et al. 1987; Yablonka-Reuveni et al. 1990; Graves and Yablonka-Reuveni 2000; Jones et al. 2005; Kwiatkowski et al. 2008) have permitted extensive biochemical and molecular analyses, although these models do not always fully adhere to the biology of satellite cells. We have previously published comprehensive summaries of the characteristics of the most commonly used myogenic cells lines (Shefer and Yablonka-Reuveni 2008; Yablonka-Reuveni and Day 2011).
Satellite Cell Transplantation: Hopes and Hypes for Muscle Regenerative Medicine
Satellite cell behavior has often been investigated by transplantation of cells into host muscles of experimental animals and assessment of donor cell contribution to myofiber formation shown by expression of donor-derived genes (Collins et al. 2005; Montarras et al. 2005; Collins et al. 2007; Cerletti et al. 2008; Sacco et al. 2008). The long-term goal of such studies has been the improvement of muscle quality and performance in cases of severe muscle wasting disorders such as Duchenne muscular dystrophy (Gussoni et al. 1997; Miller et al. 1997; Tremblay and Skuk 2008; Muir and Chamberlain 2009).
Donor satellite cells contribute myofiber nuclei while also establishing additional satellite cells through self-renewal in experimental mouse models, enabling further understanding of satellite cell biology in the in vivo environment (Collins et al. 2007; Sacco et al. 2008). However, limited migration of satellite cells and their progeny, donor–host histocompatibility issues, death of donor cells, and the need to deliver cells to many host muscles at once represent some of the major challenges that have not yet been resolved for cell-based therapies of skeletal muscle wasting disorders (Partridge 2004; Mouly et al. 2005; Lafreniere et al. 2009; Tremblay et al. 2009; Richard et al. 2010; Tedesco et al. 2010). Additionally, some reports have alluded to the failure of satellite cells injected into the circulation to reach target muscles for whole body treatment, although detailed evidence is not available (Sampaolesi et al. 2003; Dellavalle et al. 2007). Myoblasts expanded from satellite cells in culture to multiply the number of donor cells available also cannot be delivered systemically to target muscles and lose regenerative capacity compared with freshly isolated satellite cells upon direct intramuscular delivery (Morgan et al. 1993; Beauchamp et al. 1999; Montarras et al. 2005; Cossu and Sampaolesi 2007). It also appears that muscle injury or irradiation prior to donor cell delivery significantly enhances engraftment, as shown in experimental mouse models (Gross et al. 1999; Collins et al. 2005; Sacco et al. 2008). Therefore, cell-based therapy for skeletal muscle remains a challenge despite the availability and our understanding of satellite cells. Notably, certain cell culture conditions may better preserve the potential of satellite cell progeny to be used as donor cells for muscle repair upon intramuscular transplantation (Gilbert et al. 2010). Expanding human myogenic cell population under such conditions may allow the preparation of sufficient myogenic cells for cell-based muscle therapy of individual muscles or limited muscle groups (e.g., oculopharyngeal muscular dystrophy, Brais 2011; see also Negroni et al. 2011). However, genetic disorders that affect all muscles body-wide (e.g., Duchenne muscular dystrophy) will likely require delivery of donor cells via circulation and possibly combination of cell-based therapies with viral vector or other approaches to deliver therapeutic genes effectively (Muir and Chamberlain 2009).
Are Satellite Cells the Sole Source for Myogenic Progenitors in Adult Muscle?
The discovery of the satellite cell in 1961 established an obvious candidate for the source of new muscle growth and repair, and the satellite cell has remained the uncontested myogenic progenitor of skeletal muscle for many years based on cell culture and in vivo studies. More recent studies have demonstrated that new myofibers and new satellite cells can be generated from a few satellite cells resident on a single transplanted myofiber or even from individually transplanted satellite cells (Collins et al. 2005; Sacco et al. 2008). Additionally, lineage-tracing studies relying on inducible activation of Pax7-driven reporter expression in adult muscle have confirmed that satellite cells can repair damaged muscle tissue (Lepper et al. 2009).
However, other cell types isolated from skeletal muscle, such as mesoangioblasts (perivascular cells), myoendothelial cells, side population (SP) cells, interstitial PICs, and pericytes (i.e., contractile, smooth muscle-like cells engulfing the endothelium at the microvasculature wall), also seem to have some myogenic potency (Montanaro et al. 2004; Dellavalle et al. 2007; Zheng et al. 2007; Mitchell et al. 2010; Tedesco et al. 2010; Negroni et al. 2011). Identification of the aforementioned non–satellite cell myogenic sources has led some to question the classic view that satellite cells are the sole supply of myogenic precursors in postnatal and adult life. However, recent reports using novel genetic mouse models for specific ablation of satellite cells based on Pax7 expression (Lepper et al. 2011; McCarthy et al. 2011; Murphy et al. 2011; Sambasivan et al. 2011) provide once again strong, perhaps ultimate, evidence of the essential role of satellite cells in the regenerative biology of skeletal muscle. Whether the aforementioned non–satellite cell types participate in normal muscle maintenance and repair remains unclear, although these cells might offer alternative avenues for cell-based repair of muscle (especially in muscular dystrophy), because at least some of these cells can reach host muscles upon donor cell delivery via circulation (Gussoni et al. 1999; Dellavalle et al. 2007).
Pericytes (often referred to as “mural cells of the microvasculature”) are a particularly intriguing example of a possible non–satellite cell source of myogenic cells given their mesenchymal plasticity and proximity of the capillary networks to myofibers (Day et al. 2007; Diaz-Flores et al. 2009; Armulik et al. 2011). There is also a degree of resemblance between the pericyte and the satellite cell niche; similar to the satellite cell that is wedged between plasma and basal lamina of the myofiber, the pericyte is situated adjacent to the endothelium, sharing a common basement membrane that surrounds the capillary (Diaz-Flores et al. 1991; Armulik et al. 2005; Diaz-Flores et al. 2009; Armulik et al. 2011). Fig. 5 depicts the proximity of the microvasculature with its associated pericytes to the myofibers. The density of the capillary bed surrounding myofibers ranges widely between muscle groups. For example, extraocular muscles exhibit higher capillary density than the tibialis anterior muscle. We have shown smooth-to-skeletal muscle transition of vascular smooth muscle cell lines, where cells can spontaneously initiate MyoD expression followed by myogenin expression and fusion into myotubes (Graves and Yablonka-Reuveni 2000). In addition, we have shown that retinal derived pericytes can undergo myogenic reprogramming (i.e., express muscle specific genes and transgenes) upon their spontaneous fusion with host myotubes (Kirillova et al. 2007). Published studies on pericyte-like cells being able to contribute to muscle repair have defined cells isolated from skeletal muscle as being pericytes based on features of the cells after propagation in culture (Dellavalle et al. 2007). However, more direct approaches are needed to establish whether pericytes indeed function in routine myofiber maintenance and regeneration. We are presently using pericyte-specific reporter mice to develop means for isolating bona fide pericytes and their progenitors with the goal of evaluating the role of pericytes in skeletal myogenesis both in culture and in vivo.
Figure 5.
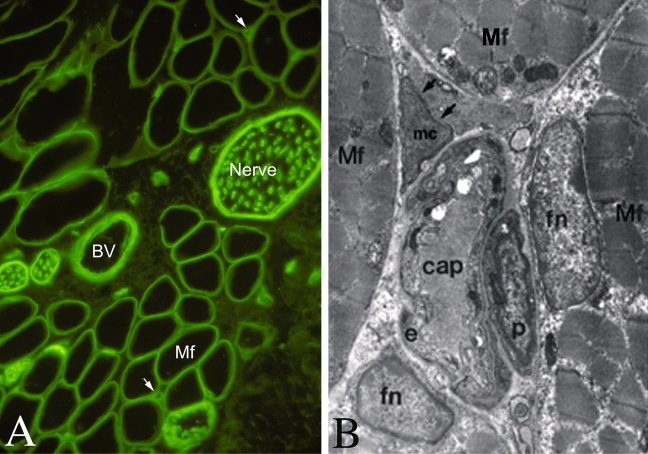
Low- and high-resolution images depicting capillaries of neighboring myofibers. A, immunofluorescent image of a cross-section from rat flexor digitorum brevis muscle reacted with an antibody against laminin (green), which highlights the basement membrane of the myofiber (Mf) and other structures in the muscle, including nerve bundles, blood vessels (BV), and capillaries (indicated by arrows). B, electron microscopy micrograph of adult chicken muscle (Yablonka-Reuveni 1995) demonstrating the fine details of a capillary surrounded by four myofibers. Mf, myofiber; fn, myofiber nucleus (myonucleus); cap, capillary; e, endothelial cell; p, pericyte; mc, an uncharacterized “mysterious cell,” for which a higher resolution EM revealed a large nucleus and some rER structures in the cytoplasm.
Concluding Remarks
The journey of the satellite cell began with its discovery in 1961. The identification of satellite cell molecular markers has enabled a more efficient analysis of this cell and its progeny both in vivo and in culture, permitting a more refined analysis of cell autonomous and environmental factors affecting satellite cell activation, proliferation, differentiation, and renewal. The development of genetic models for satellite cell analysis has moved satellite cell research to a level never possible before, including the ability to trace satellite cell progeny when the muscle architecture is destroyed upon injury and to study the outcome of satellite cell ablation. Major emphasis is further placed on developing a means for using satellite cells or alternative populations for muscle repair. Nevertheless, many classic aspects of satellite cell biology await future studies. These include the nature of the satellite cell niche and its role in regulating the quiescent and active states of the satellite cell, mechanisms of satellite cell renewal, the role of environmental and epigenetic factors, and the status of satellite cells in less optimal environments, for example, those caused by muscular dystrophies, aging, obesity, and diabetes. Happy 50th to the skeletal muscle satellite cell! We know you will continue to fascinate us for many more years to come.
Acknowledgments
The author thanks Lindsey Muir for her valuable comments on the manuscript and anonymous reviewers for their constructive comments.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support from funding agencies that have made possible the author’s studies on satellite cells is gratefully acknowledged, including past support from the American Heart Association and the USDA Cooperative State Research, Education and Extension Service. Current research in the author’s laboratory is funded by the National Institutes of Health (AG021566; AG035377; AR057794) and the Muscular Dystrophy Association (135908).
References
- Abou-Khalil R, Le Grand F, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, et al. 2009. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 5:298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguennouz M, Vita GL, Messina S, Cama A, Lanzano N, Ciranni A, Rodolico C, Di Giorgio RM, Vita G. 2011. Telomere shortening is associated to TRF1 and PARP1 overexpression in Duchenne muscular dystrophy. Neurobiol Aging. 32:2190–2197 [DOI] [PubMed] [Google Scholar]
- Allouh MZ, Yablonka-Reuveni Z, Rosser BW. 2008. Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem. 56:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE. 2006. The satellite cell as a companion in skeletal muscle plasticity: currency, conveyance, clue, connector and colander. J Exp Biol. 209:2276–2292 [DOI] [PubMed] [Google Scholar]
- Andres V, Walsh K. 1996. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 132:657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand O, Boutineau AM, Mauger A, Pautou MP, Kieny M. 1983. Origin of satellite cells in avian skeletal muscles. Arch Anat Microsc Morphol Exp. 72:163–181 [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. 2005. Endothelial/pericyte interactions. Circ Res. 97:512–523 [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. 2011. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 21:193–215 [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. 2000. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 151:1221–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. 1999. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 144:1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A, Betz W. 1977a. Properties of isolated adult rat muscle fibres maintained in tissue culture. J Physiol 271:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A, Betz WJ. 1977b. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol. 271:25–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bintliff S, Walker BE. 1960. Radioautographic study of skeletal muscle regeneration. Am J Anat. 106:233–245 [Google Scholar]
- Biressi S, Tagliafico E, Lamorte G, Monteverde S, Tenedini E, Roncaglia E, Ferrari S, Ferrari S, Cusella-De Angelis MG, Tajbakhsh S, et al. 2007. Intrinsic phenotypic diversity of embryonic and fetal myoblasts is revealed by genome-wide gene expression analysis on purified cells. Dev Biol. 304:633–651 [DOI] [PubMed] [Google Scholar]
- Biressi S, Rando TA. 2010. Heterogeneity in the muscle satellite cell population. Semin Cell Dev Biol. 21:845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. 1975. Regeneration of single skeletal muscle fibers in vitro. Anat Rec. 182:215–235 [DOI] [PubMed] [Google Scholar]
- Bischoff R. 1986. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol. 115:129–139 [DOI] [PubMed] [Google Scholar]
- Bischoff R. 1989. Analysis of muscle regeneration using single myofibers in culture. Med Sci Sports Exerc. 21:S164–S172 [PubMed] [Google Scholar]
- Blau HM, Webster C, Pavlath GK. 1983. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 80:4856–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin L, Muntoni F, Morgan JE. 2010. Are human and mouse satellite cells really the same? J Histochem Cytochem. 58:941-955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC, Kyba M. 2008. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells. 26:3194–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutet SC, Disatnik MH, Chan LS, Iori K, Rando TA. 2007. Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell. 130:349–362 [DOI] [PubMed] [Google Scholar]
- Brais B. 2011. Oculopharyngeal muscular dystrophy. Handb Clin Neurol 101:181–192 [DOI] [PubMed] [Google Scholar]
- Campion DR. 1984. The muscle satellite cell: a review. Int Rev Cytol 87:225–251 [DOI] [PubMed] [Google Scholar]
- Capers CR. 1960. Multinucleation of skeletal muscle in vitro. J Biophys Biochem Cytol. 7:559–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BM. 1973. The regeneration of skeletal muscle. A review. Am J Anat 137:119–149 [DOI] [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. 1989. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 256:C1262–C1266 [DOI] [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. 1996. The regeneration of noninnervated muscle grafts and marcaine-treated muscles in young and old rats. J Gerontol A Biol Sci Med Sci. 51:B43–B49 [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV, Conboy IM. 2009. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 8:676–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Hsu M, Conboy IM. 2008. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 454:528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. 2008. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 134:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. 2004. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 84:209–238 [DOI] [PubMed] [Google Scholar]
- Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, et al. 2007. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 18:1397–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciciliot S, Schiaffino S. 2010. Regeneration of mammalian skeletal muscle. basic mechanisms and clinical implications. Curr Pharm Des. 16:906–914 [DOI] [PubMed] [Google Scholar]
- Clegg CH, Linkhart TA, Olwin BB, Hauschka SD. 1987. Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 105:949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Gnocchi VF, White RB, Boldrin L, Perez-Ruiz A, Relaix F, Morgan JE, Zammit PS. 2009. Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS One. 4:e4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. 2005. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 122:289–301 [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. 2007. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 25:885–894 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 433:760–764 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. 2002. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 3:397–409 [DOI] [PubMed] [Google Scholar]
- Cooper WG, Konigsberg IR. 1961. Dynamics of myogenesis in vitro. Anat Rec 140:195–205 [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ. 2000. MyoD(-/-) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol. 224:122–137 [DOI] [PubMed] [Google Scholar]
- Cossu G, Eusebi F, Grassi F, Wanke E. 1987. Acetylcholine receptor channels are present in undifferentiated satellite cells but not in embryonic myoblasts in culture. Dev Biol 123:43–50 [DOI] [PubMed] [Google Scholar]
- Cossu G, Molinaro M. 1987. Cell heterogeneity in the myogenic lineage. Curr Top Dev Biol. 23:185–208 [DOI] [PubMed] [Google Scholar]
- Cossu G, Sampaolesi M. 2007. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 13:520–526 [DOI] [PubMed] [Google Scholar]
- Crist CG, Montarras D, Pallafacchina G, Rocancourt D, Cumano A, Conway SJ, Buckingham M. 2009. Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. Proc Natl Acad Sci U S A. 106:13383–13387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danoviz ME, Yablonka-Reuveni Z. 2012. Chapter 2. Skeletal muscle satellite cells: background and methods for isolation and analysis in a primary culture system. Methods Mol Biol. 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Paterson B, Yablonka-Reuveni Z. 2009. A distinct profile of myogenic regulatory factor detection within Pax7+ cells at S phase supports a unique role of Myf5 during posthatch chicken myogenesis. Dev Dyn. 238:1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. 2007. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 304:246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. 2010. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 340:330–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. 2007. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 9:255–267 [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr 2009. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 24:909–969 [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. 1991. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 6:269–286 [PubMed] [Google Scholar]
- Epstein JA, Shapiro DN, Cheng J, Lam PY, Maas RL. 1996. Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci U S A. 93:4213–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Stockdale FE. 1992. Temporal appearance of satellite cells during myogenesis. Dev Biol. 153:217-226 [DOI] [PubMed] [Google Scholar]
- Gal-Levi R, Leshem Y, Aoki S, Nakamura T, Halevy O. 1998. Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochim Biophys Acta. 1402:39-51 [DOI] [PubMed] [Google Scholar]
- Gayraud-Morel B, Chretien F, Flamant P, Gomes D, Zammit PS, Tajbakhsh S. 2007. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev Biol. 312:13–28 [DOI] [PubMed] [Google Scholar]
- Gensch N, Borchardt T, Schneider A, Riethmacher D, Braun T. 2008. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development. 135:1597–1604 [DOI] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. 2010. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 329:1078–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SD, Rando TA. 2008. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 7:590–598 [DOI] [PubMed] [Google Scholar]
- Graves DC, Yablonka-Reuveni Z. 2000. Vascular smooth muscle cells spontaneously adopt a skeletal muscle phenotype: a unique Myf5(–)/MyoD(+) myogenic program. J Histochem Cytochem. 48:1173–1193 [DOI] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thome V, Marcelle C. 2005. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 435:954–958 [DOI] [PubMed] [Google Scholar]
- Gross JG, Bou-Gharios G, Morgan JE. 1999. Potentiation of myoblast transplantation by host muscle irradiation is dependent on the rate of radiation delivery. Cell Tissue Res. 298:371–375 [DOI] [PubMed] [Google Scholar]
- Grounds MD. 1998. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci. 854:78–91 [DOI] [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z. 1993. Molecular and cell biology of skeletal muscle regeneration. Mol Cell Biol Hum Dis Ser. 3:210–256 [DOI] [PubMed] [Google Scholar]
- Gussoni E, Blau HM, Kunkel LM. 1997. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med. 3:970–977 [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. 1999. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 401:390–394 [DOI] [PubMed] [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. 2004. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 231:489–502 [DOI] [PubMed] [Google Scholar]
- Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimaraes-Camboa N, Evans SM, Tzahor E. 2009. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 16:822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RS, Bandman E, Yablonka-Reuveni Z. 1991. Myoblasts from fetal and adult skeletal muscle regulate myosin expression differently. Dev Biol. 148:249–260 [DOI] [PubMed] [Google Scholar]
- Hartley RS, Bandman E, Yablonka-Reuveni Z. 1992. Skeletal muscle satellite cells appear during late chicken embryogenesis. Dev Biol. 153:206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. 2001. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 91:534–551 [DOI] [PubMed] [Google Scholar]
- Hinterberger TJ, Sassoon DA, Rhodes SJ, Konieczny SF. 1991. Expression of the muscle regulatory factor MRF4 during somite and skeletal myofiber development. Dev Biol. 147:144–156 [DOI] [PubMed] [Google Scholar]
- Ieronimakis N, Balasundaram G, Rainey S, Srirangam K, Yablonka-Reuveni Z, Reyes M. 2010. Absence of CD34 on murine skeletal muscle satellite cells marks a reversible state of activation during acute injury. PLoS One. 5:e10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Tyner KJ, Nibarger L, Stanley HM, Cornelison DD, Fedorov YV, Olwin BB. 2005. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J Cell Biol. 169:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanisicak O, Mendez JJ, Yamamoto S, Yamamoto M, Goldhamer DJ. 2009. Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev Biol. 332:131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. 2000. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem. 48:1079–1096 [DOI] [PubMed] [Google Scholar]
- Katz B. 1961. The terminations of the afferent nerve fibre in the muscle spindle of the frog. Philos Trans Royal Soc Lond [Biol]. 243:221–240 [Google Scholar]
- Kelly R, Alonso S, Tajbakhsh S, Cossu G, Buckingham M. 1995. Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice. J Cell Biol. 129:383–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillova I, Gussoni E, Goldhamer DJ, Yablonka-Reuveni Z. 2007. Myogenic reprogramming of retina-derived cells following their spontaneous fusion with myotubes. Dev Biol. 311:449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick LJ, Allouh MZ, Nightingale CN, Devon HG, Yablonka-Reuveni Z, Rosser BW. 2008. Pax7 shows higher satellite cell frequencies and concentrations within intrafusal fibers of muscle spindles. J Histochem Cytochem. 56:831–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick LJ, Yablonka-Reuveni Z, Rosser BW. 2009. Retention of Pax3 expression in satellite cells of muscle spindles. J Histochem Cytochem. 58:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp JR, Davie JK, Myer A, Meadows E, Olson EN, Klein WH. 2006. Loss of myogenin in postnatal life leads to normal skeletal muscle but reduced body size. Development. 133:601–610 [DOI] [PubMed] [Google Scholar]
- Konigsberg UR, Lipton BH, Konigsberg IR. 1975. The regenerative response of single mature muscle fibers isolated in vitro. Dev Biol. 45:260–275 [DOI] [PubMed] [Google Scholar]
- Kozeka K, Ontell M. 1981. The three-dimensional cytoarchitecture of developing murine muscle spindles. Dev Biol. 87: 133–147 [DOI] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA. 2008. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2:22–31 [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. 2007. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 129:999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski BA, Kirillova I, Richard RE, Israeli D, Yablonka-Reuveni Z. 2008. FGFR4 and its novel splice form in myogenic cells: interplay of glycosylation and tyrosine phosphorylation. J Cell Physiol. 215:803–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere JF, Caron MC, Skuk D, Goulet M, Cheikh AR, Tremblay JP. 2009. Growth factor coinjection improves the migration potential of monkey myogenic precursors without affecting cell transplantation success. Cell Transplant. 18:719–730 [DOI] [PubMed] [Google Scholar]
- Leiter JR, Peeler J, Anderson JE. 2011. Exercise-induced muscle growth is muscle-specific and age-dependent. Muscle Nerve. 43:828–838 [DOI] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. 2009. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 460:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA, Fan CM. 2011. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 138:3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis WH, Lewis MR. 1917. Behaviour of cross striated muscle in tissue cultures. Am J Anat. 22:169–194 [Google Scholar]
- Lindstrom M, Pedrosa-Domellof F, Thornell LE. 2010. Satellite cell heterogeneity with respect to expression of MyoD, myogenin, Dlk1 and c-Met in human skeletal muscle: application to a cohort of power lifters and sedentary men. Histochem Cell Biol. 134:371–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom M, Thornell LE. 2009. New multiple labelling method for improved satellite cell identification in human muscle: application to a cohort of power-lifters and sedentary men. Histochem Cell Biol. 132:141–157 [DOI] [PubMed] [Google Scholar]
- Mauro A. 1961. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 9:493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Esser KA. 2007. Counterpoint: satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl Physiol. 103:1100–1102; discussion 1102–1103 [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, et al. 2011. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 138:3657–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh LM, Garrett KL, Megeney L, Rudnicki MA, Anderson JE. 1998. Regeneration and myogenic cell proliferation correlate with taurine levels in dystrophin- and MyoD-deficient muscles. Anat Rec. 252:311–324 [DOI] [PubMed] [Google Scholar]
- Meadows E, Cho JH, Flynn JM, Klein WH. 2008. Myogenin regulates a distinct genetic program in adult muscle stem cells. Dev Biol. 322:406–414 [DOI] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. 1996. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 10:1173–1183 [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. 2004. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 469:311–324 [DOI] [PubMed] [Google Scholar]
- Miller RG, Sharma KR, Pavlath GK, Gussoni E, Mynhier M, Lanctot AM, Greco CM, Steinman L, Blau HM. 1997. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 20:469–478 [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. 2010. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 12:257–266 [DOI] [PubMed] [Google Scholar]
- Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. 2004. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res. 298:144–154 [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. 2005. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 309:2064–2067 [DOI] [PubMed] [Google Scholar]
- Morgan JE. 1988. Myogenicity in vitro and in vivo of mouse muscle cells separated on discontinuous Percoll gradients. J Neurol Sci. 85:197–207 [DOI] [PubMed] [Google Scholar]
- Morgan JE, Pagel CN, Sherratt T, Partridge TA. 1993. Long-term persistence and migration of myogenic cells injected into pre-irradiated muscles of mdx mice. J Neurol Sci. 115:191–200 [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. 1971. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170:421–435 [DOI] [PubMed] [Google Scholar]
- Mouly V, Aamiri A, Perie S, Mamchaoui K, Barani A, Bigot A, Bouazza B, Francois V, Furling D, Jacquemin V, et al. 2005. Myoblast transfer therapy: is there any light at the end of the tunnel? Acta Myol. 24:128–133 [PubMed] [Google Scholar]
- Muir AR, Kanji AH, Allbrook D. 1965. The structure of the satellite cells in skeletal muscle. J Anat. 99:435–444 [PMC free article] [PubMed] [Google Scholar]
- Muir LA, Chamberlain JS. 2009. Emerging strategies for cell and gene therapy of the muscular dystrophies. Expert Rev Mol Med. 11:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. 2011. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 138:3625–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. 2007. A global double-fluorescent Cre reporter mouse. Genesis. 45:593–605 [DOI] [PubMed] [Google Scholar]
- Negroni E, Vallese D, Vilquin JT, Butler-Browne G, Mouly V, Trollet C. 2011. Current advances in cell therapy strategies for muscular dystrophies. Expert Opin Biol Ther. 11:157–176 [DOI] [PubMed] [Google Scholar]
- Noden DM, Francis-West P. 2006. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 235:1194–1218 [DOI] [PubMed] [Google Scholar]
- O’Connor RS, Pavlath GK. 2007. Point:Counterpoint: satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol. 103:1099–1100 [DOI] [PubMed] [Google Scholar]
- O’Connor RS, Pavlath GK, McCarthy JJ, Esser KA. 2007. Last word on Point:Counterpoint: satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol. 103:1107. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Yang Z, Tapscott SJ, Olwin BB. 2007. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol. 177:769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Arnold HH, Rigby PW, Wold BJ. 1996. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell. 85:1–4 [DOI] [PubMed] [Google Scholar]
- Ono Y, Boldrin L, Knopp P, Morgan JE, Zammit PS. 2010. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev Biol. 337:29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Gnocchi VF, Zammit PS, Nagatomi R. 2009. Presenilin-1 acts via Id1 to regulate the function of muscle satellite cells in a gamma-secretase-independent manner. J Cell Sci. 122:4427–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund C, Liu JX, Thornell LE, Eriksson PO. 2011. Muscle spindle composition and distribution in human young masseter and biceps brachii muscles reveal early growth and maturation. Anat Rec (Hoboken). 294:683–693 [DOI] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. 2004. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. Embo J. 23:3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge TA. 2004. Stem cell therapies for neuromuscular diseases. Acta Neurol Belg. 104:141–147 [PubMed] [Google Scholar]
- Punch VG, Jones AE, Rudnicki MA. 2009. Transcriptional networks that regulate muscle stem cell function. Wiley Interdiscip Rev Syst Biol Med. 1:128–140 [DOI] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. 2002. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 157:851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Blau HM. 1994. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 125:1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Marcelle C. 2009. Muscle stem cells. Curr Opin Cell Biol. 21:748-753 [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. 2006. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 172:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. 2005. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 435:948–953 [DOI] [PubMed] [Google Scholar]
- Richard PL, Gosselin C, Laliberte T, Paradis M, Goulet M, Tremblay JP, Skuk D. 2010. A first semi-manual device for clinical intramuscular repetitive cell injections. Cell Transplant. 19:67–78 [DOI] [PubMed] [Google Scholar]
- Richler C, Yaffe D. 1970. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 23:1–22 [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. 1995. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim. 31:773–779 [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. 2008. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 456:502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R, Gayraud-Morel B, Dumas G, Cimper C, Paisant S, Kelly RG, Tajbakhsh S. 2009. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev Cell. 16:810–821 [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. 2011. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 138:3647–3656 [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, et al. 2003. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 301:487–492 [DOI] [PubMed] [Google Scholar]
- Scharner J, Zammit PS. 2011. The muscle satellite cell at 50: the formative years. Skelet Muscle. 1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienda J, Engleka KA, Jun S, Hansen MS, Epstein JA, Tabin CJ, Kunkel LM, Kardon G. 2006. Somitic origin of limb muscle satellite and side population cells. Proc Natl Acad Sci U S A. 103:945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E. 1996. Satellite cell proliferative compartments in growing skeletal muscles. Dev Biol. 175:84-94 [DOI] [PubMed] [Google Scholar]
- Schultz E, Gibson MC, Champion T. 1978. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool. 206:451–456 [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. 2000. Pax7 is required for the specification of myogenic satellite cells. Cell. 102:777–786 [DOI] [PubMed] [Google Scholar]
- Seed J, Hauschka SD. 1988. Clonal analysis of vertebrate myogenesis. VIII. Fibroblasts growth factor (FGF)-dependent and FGF-independent muscle colony types during chick wing development. Dev Biol. 128:40–49 [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, McGeachie J, Grounds MD. 2010. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology. 11:363–366 [DOI] [PubMed] [Google Scholar]
- Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. 2010. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS One. 5:e13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. 2006. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 294:50–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Yablonka-Reuveni Z. 2005. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol Biol. 290:281–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Yablonka-Reuveni Z. 2008. Ins and outs of satellite cell myogenesis: the role of the ruling growth factors. In: Schiaffino S, Partridge T. editors. Skeletal Muscle Repair and Regeneration (Advances in Muscle Res, vol 3). Dordrecht, The Netherlands: Springer; p. 107–143 [Google Scholar]
- Smith CK, 2nd, Janney MJ, Allen RE. 1994. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol. 159:379–385 [DOI] [PubMed] [Google Scholar]
- Smith HK, Merry TL. 2011. Voluntary resistance wheel exercise during postnatal growth in rats enhances skeletal muscle satellite cell and myonuclear content at adulthood. Acta Physiol (Oxf). Published online ahead of print August 19. [DOI] [PubMed] [Google Scholar]
- Smith TH, Block NE, Rhodes SJ, Konieczny SF, Miller JB. 1993. A unique pattern of expression of the four muscle regulatory factor proteins distinguishes somitic from embryonic, fetal and newborn mouse myogenic cells. Development. 117:1125–1133 [DOI] [PubMed] [Google Scholar]
- Snow MH. 1978. An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res. 186:535–540 [DOI] [PubMed] [Google Scholar]
- Stockdale FE. 1992. Myogenic cell lineages. Dev Biol. 154:284–298 [DOI] [PubMed] [Google Scholar]
- Stockdale FE, Holtzer H. 1961. DNA synthesis and myogenesis. Exp Cell Res. 24:508–520 [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Buckingham M. 1996. Muscle progenitor cells failing to respond to positional cues adopt non-myogenic fates in myf-5 null mice. Nature. 384:266–270 [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. 1997. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 89:127–138 [DOI] [PubMed] [Google Scholar]
- Tallquist MD, Weismann KE, Hellstrom M, Soriano P. 2000. Early myotome specification regulates PDGFA expression and axial skeleton development. Development. 127:5059–5070 [DOI] [PubMed] [Google Scholar]
- Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. 2009. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 4:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. 1998. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 194:114–128 [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. 2001. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res. 267:107–114 [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. 2010. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 120:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LV. 2009. Age-related muscle dysfunction. Exp Gerontol. 44:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay JP, Skuk D. 2008. Another new “super muscle stem cell” leaves unaddressed the real problems of cell therapy for duchenne muscular dystrophy. Mol Ther. 16:1907–1909 [DOI] [PubMed] [Google Scholar]
- Tremblay JP, Skuk D, Palmieri B, Rothstein DM. 2009. A case for immunosuppression for myoblast transplantation in duchenne muscular dystrophy. Mol Ther. 17:1122–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustanina S, Carvajal J, Rigby P, Braun T. 2007. The myogenic factor Myf5 supports efficient skeletal muscle regeneration by enabling transient myoblast amplification. Stem Cells. 25:2006–2016 [DOI] [PubMed] [Google Scholar]
- Walro JM, Kucera J. 1999. Why adult mammalian intrafusal and extrafusal fibers contain different myosin heavy-chain isoforms. Trends Neurosci. 22:180–184 [DOI] [PubMed] [Google Scholar]
- Webster C, Blau HM. 1990. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet. 16:557–565 [DOI] [PubMed] [Google Scholar]
- White JD, Scaffidi A, Davies M, McGeachie J, Rudnicki MA, Grounds MD. 2000. Myotube formation is delayed but not prevented in MyoD-deficient skeletal muscle: studies in regenerating whole muscle grafts of adult mice. J Histochem Cytochem. 48:1531–1544 [DOI] [PubMed] [Google Scholar]
- White NK, Bonner PH, Nelson DR, Hauschka SD. 1975. Clonal analysis of vertebrate myogenesis. IV. Medium-dependent classification of colony-forming cells. Dev Biol. 44:346–361 [DOI] [PubMed] [Google Scholar]
- Wozniak AC, Kong J, Bock E, Pilipowicz O, Anderson JE. 2005. Signaling satellite-cell activation in skeletal muscle: markers, models, stretch, and potential alternate pathways. Muscle Nerve. 31:283–300 [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. 1988. Discrimination of myogenic and nonmyogenic cells from embryonic skeletal muscle by 90 degrees light scattering. Cytometry. 9:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. 1995. Development and postnatal regulation of adult myoblasts. Microsc Res Tech. 30:366–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. 2004. Isolation and culture of myogenic stem cells. In: R. Lanza R, Blau H, Melton D, Moore M, Thomas ED, Verfaillie C, Weissman IL, editors. Handbook of Stem Cells. San Diego (CA): Elsevier, Academic Press; p. 571–580 [Google Scholar]
- Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. 1990. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J Cell Biol. 111:1623–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Day K. 2011. Chapter 11: Skeletal muscle stem cells in the spotlight: the satellite cell. In: Cohen I, Gaudette G, editors. Regenerating the Heart: Stem Cells and the Cardiovascular System. New York: Springer, Humana Press; p. 173–200 [Google Scholar]
- Yablonka-Reuveni Z, Day K, Vine A, Shefer G. 2008. Defining the transcriptional signature of skeletal muscle stem cells. J Anim Sci. 86:E207–E216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Nameroff M. 1987. Skeletal muscle cell populations. Separation and partial characterization of fibroblast-like cells from embryonic tissue using density centrifugation. Histochemistry. 87:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Quinn LS, Nameroff M. 1987. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev Biol. 119:252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. 1994. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 164:588–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. 1997. Proliferative dynamics and the role of FGF2 during myogenesis of rat satellite cells on isolated fibers. Basic and Applied Myology (BAM). 7:176–189 . [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. 1999. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol. 210:440–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Seger R, Rivera AJ. 1999. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem. 47:23–42 [DOI] [PubMed] [Google Scholar]
- Yaffe D. 1969. Cellular aspects of muscle differentiation in vitro. Curr Top Dev Biol. 4:37–77 [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. 1977. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 270:725–727 [DOI] [PubMed] [Google Scholar]
- Yamada M, Tatsumi R, Yamanouchi K, Hosoyama T, Shiratsuchi SI, Sato A, Mizunoya W, Ikeuchi Y, Furuse M, Allen RE. 2009. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin. Am J Physiol Cell Physiol. 298:C465–C476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Carvajal JJ, Golding JP, Morgan JE, Summerbell D, Zolnerciks J, Partridge TA, Rigby PW, Beauchamp JR. 2004. Myf5 expression in satellite cells and spindles in adult muscle is controlled by separate genetic elements. Dev Biol. 273:454–465 [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. 2004. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 166:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Heslop L, Hudon V, Rosenblatt JD, Tajbakhsh S, Buckingham ME, Beauchamp JR, Partridge TA. 2002. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp Cell Res. 281:39–49 [DOI] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. 2006. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 54:1177–1191 [DOI] [PubMed] [Google Scholar]
- Zhang W, Behringer RR, Olson EN. 1995. Inactivation of the myogenic bHLH gene MRF4 results in up-regulation of myogenin and rib anomalies. Genes Dev. 9:1388–1399 [DOI] [PubMed] [Google Scholar]
- Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, et al. 2007. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 25:1025–1034 [DOI] [PubMed] [Google Scholar]