Summary
Background
There is evidence linking the Metabolic Syndrome with an increased risk of developing cardiovascular disease, previously thought to be rare in Africa but now a major public health concern.
Objectives
To determine the frequency of occurrence of the Metabolic Syndrome among patients presenting with cardiovascular disease at the Korle Bu Teaching Hospital, Ghana.
Methods
This was a case-control study of 100 consecutive cardiovascular disease patients and 100 age- and sex- matched controls who underwent an interview and physical examination. Anthropometric measurements and fasting blood samples for plasma glucose and lipids were taken. The National Cholesterol Education Programme: Adult Treatment Panel III criteria were used for the diagnosis of the Metabolic Syndrome.
Results
The prevalence of Metabolic Syndrome among cases and controls was 54% and 18% respectively, with the prevalence increasing with advancing age. Hypertension and central obesity were the two components with the highest frequency among individuals with Metabolic Syndrome. The Metabolic Syndrome was associated with the development of cardiovascular disease (OR = 5.35, 95% CI: 2.81 – 10.18, p= 0.0001), with the odds ratio increasing with the number of components present.
Conclusion
The Metabolic Syndrome is prevalent among cardiovascular disease patients attending the Korle Bu Teaching Hospital, with a significant association between the number of components of the Metabolic Syndrome present and the probability of developing a cardiovascular disease. A policy to institute routine screening in clinical practice and provision of appropriate interventions for Metabolic Syndrome components among Ghanaian adults is needed.
Keywords: Metabolic Syndrome, cardiovascular disease, Syndrome X, Ghana
Introduction
The Metabolic Syndrome (MetS), which connotes the clustering of known cardiovascular disease (CVD) risk factors including abdominal obesity, dyslipidaemia, hyperglycaemia and systemic hypertension, is a major public health challenge worldwide.1 The syndrome, which has been described variously as Insulin Resistance Syndrome2, Deadly Quartet3, and Syndrome X4 was recognized at least 80 years ago.5 However, it was not until 1998 that an attempt was made by the World Health Organization (WHO) Diabetes Expert Group6 to provide an internationally recognized definition. Subsequently, several definitions have been proposed. Among these, the National Cholesterol Education Program: Adult Treatment Panel III (NCEP: ATPIII) criteria7 which was proposed in 2001, has good clinical relevance and application while avoiding sophisticated laboratory investigations, and is therefore the most practical for use in our setting in Ghana.
In earlier studies, using the WHO definition in the same population tended to give a higher prevalence rate of the MetS than the rate obtained with the NCEP: ATPIII definition.2 Using one criterion alone (the NCEP: ATPIII) in different parts of the world has demonstrated a variable prevalence of MetS from place to place. Prevalence rates of between 9.8%8 and 26.2%9 have been obtained for some European and Asian countries, while 24% of adult north Americans meet the diagnostic criteria for MetS.10 Very few population-based studies have been conducted in Africa to determine the prevalence of the MetS. However, studies conducted among Nigerian11,12 and Zimbabwean13 type 2 diabetes mellitus(DM) patients, using the WHO definition, puts the prevalence of MetS between 25.2% and 59.1%. The first population-based study in sub-Saharan Africa on the MetS using multiple criteria, namely, the WHO, NCEP: ATPIII as well as the International Diabetes Federation definitions, was recently undertaken in Cameroun.14 The highest prevalence (7.9% for men, 5.9% for women) was obtained with the WHO definition and the lowest (0.5% for men, 0.2% for women) with the NCEP: ATPIII criteria.
Insulin Resistance (IR) has been identified as the primary underlying cause for the MetS and it is currently recognized that visceral adiposity (central obesity) is a major contributor to the development of both IR and MetS1 through the production of large numbers of adipokines.15 There is also evidence to suggest a genetic basis for the MetS.16
The MetS is associated with an increased risk of developing CVD, and it serves as a simple clinical tool for identifying individuals with a relatively high long-term risk of CVD.17 While non-communicable diseases (NCD) are relatively more prevalent in developed countries, their impact is far more devastating in developing countries since they represent a significant burden on the already under-resourced public health service.18 A recent WHO report identifies CVDs as accounting for 9.2% of total deaths in the African region in 200119, and it is estimated that by the year 2015 the number of deaths in Africa due to NCDs in general will exceed that due to communicable diseases.20 This looming epidemic is adequate reason for the early institution of appropriate interventions to reduce the risk factors for CVD, including that related to MetS.
In Ghana and West Africa, the prevalence of hypertension21, DM22, IR23, hyperlipidaemia24 and obesity25 that are individual components of MetS are on the increase. There has also been a documented increase in CVD morbidity and case fatality in Ghana.26 Whereas these disorders have been reported on separately in Ghana, studies are yet to be done to ascertain the occurrence and collective impact of features of MetS on CVD morbidity. This study aimed at determining the frequency of occurrence of MetS and its relation to cardiovascular events at Korle Bu Teaching Hospital (KBTH), Accra, Ghana.
Methods
This case-control study was undertaken at the department of Medicine, (KBTH), Accra, Ghana, over a 12-month period. A case was any patient who was admitted to the medical ward with a stroke, acute coronary syndrome, peripheral arterial disease or heart failure related to hypertensive or ischaemic heart disease. Controls were apparently normal age (±2 years) - and sex- matched individuals, 65% of whom had come to the hospital to undergo routine pre-employment medical examination. The remaining 35% were attendants at general internal medicine out-patient clinics at the KBTH. Participants (cases and controls) with chronic kidney disease, cancer, connective tissue disease, rheumatic heart disease, infective endocarditis and sepsis were excluded from the study after history and clinical examination.
Sample size was calculated using the formula for hypothesis test of odds ratio for a case -control study30 (Power 80%, two-sided type 1 error 5%). Assuming an estimated prevalence for controls of 20% and Odds ratio of 2.5, a minimum sample size of 94 was obtained for both cases and controls. Consecutive patients meeting the inclusion criteria were recruited into the study after informed consent was obtained. The University of Ghana Medical School Research and Ethics Committee approved the study.
Data were collected through a questionnaire, clinical examination and the taking of anthropometric measurements. The questionnaire was administered to each participant(cases and controls) to obtain information on demographic characteristics and CVD risk factors (personal or family history of hypertension, DM or dyslipidaemia, tobacco use and physical activity). Physical activity was defined as being engaged in leisure time exercise at least 3 times a week for a minimum of 30minutes. Waist circumference was measured using a flexible measuring tape at the mid-point between the lower border of the rib cage and the iliac crest.27 The average of two blood pressure (BP) measurements taken 30-minutes apart on the day of examination was recorded. Venous blood samples were taken following a 12-hour overnight fast for measurement of glucose, total cholesterol, HDL cholesterol and triglycerides. Plasma glucose levels were measured using the glucose hexokinase method.28 Lipid levels were generated using ATAC 8000 random access clinical chemistry auto-analyzer (Elan Diagnostics, USA) and LDL cholesterol levels were estimated using the Friedewald formula.29
MetS was defined using the NCEP: ATPIII criteria of 3 or more of the following7:
Abdominal/ central obesity (waist circumference: ≥102cm in men, ≥88cm in women)
Hypertriglyceridaemia≥1.7mmol/L
(Low) HDL cholesterol (men ≤1.036mmol/L, women ≤1.295mmol/L)
(High) blood pressure ≥130/85mmHg or documented use of anti-hypertensive medication(s)
(High) fasting plasma glucose ≥ 6.1mmol/L or documented use of anti-diabetic medication(s)
Statistical analysis
Analysis of the data was done with SPSS 12.0 software. The Chi-square test and Student t-test were used respectively for comparison of categorical and continuous variables between the two groups. The odds ratio (OR) was used to determine the association between MetS and CVD.
Determination of the strength of association between MetS components and CVD was by multivariate multiple logistic regressions analysis. All statistical tests were two-sided and the level of significance was set at p< 0.05.
Results
One hundred cases were studied. These included 60 (60%) stroke patients (31cases of haemorrhagic stroke, 29 cases of infarctive stroke), 22 (22%) heart failure patients, 17 (17%) acute coronary syndrome patients and 1 (1%) patient with peripheral arterial disease. An equal number of age- and sex-matched controls were recruited.
Fifty-four (54) of the cases were male and 46 were female with a male to female ratio of 1.2:1. Similarly, a male to female ratio of 1.2: 1 was obtained for the controls. The mean age of the cases and controls were 55.6 ± 11.6 years and 54.9 ± 11.0 years respectively, with the difference not statistically significant. (p= 0.682).
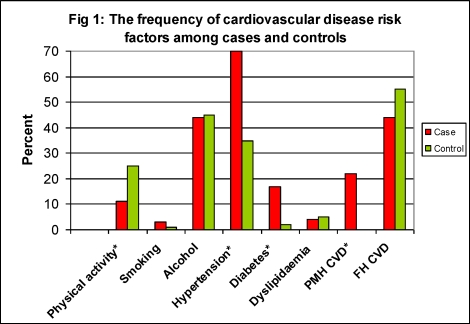
The frequency of cardiovascular risk factors for cases and controls is illustrated in Figure 1. Majority of both cases (89%) and controls (75%) were not engaged in any leisure-time physical activity. There was however a significant difference between cases and controls with respect to the engagement in leisure-time physical activity. Significant differences were also noted between cases and controls regarding the past medical history of hypertension, DM and CVD (p< 0.05).
Fig 1.
The frequency of cardiovascular disease risk factors among cases and controls
PMH Past medical history; FH Family history; *p<0.05
The anthropometric measurements and clinical chemistry parameters for cases and controls are shown in Table 1. The frequency of MetS components for cases and controls and the corresponding odds ratio are as shown in Table 2. Hypertension, the most frequently diagnosed component, was seen in 83% cases and 45% controls, followed by central obesity (54% cases and 33% controls). MetS components were found in significantly more cases than controls (p < 0.05).
Table 1.
Comparison of anthropometric and biochemical measurements of study and control subjects.
| Variable | Cases Mean (SD) |
Controls Mean (SD) |
p-value |
| Waist circumference (cm) Males Females |
98.6 (13.3) 96.9 (11.8) 100.5 (14.7) |
91.6 (10.8) 91.5 (10.2) 91.6 (11.6) |
0.0001 |
| SBP(mmHg) | 160 (26) | 132 (15) | 0.0001 |
| DBP(mmHg) | 99 (17) | 84 (9) | 0.0001 |
| FPG(mmol/l) | 7.0 (2.5) | 5.2 (0.9) | 0.0001 |
| Total Cholesterol (mmol/l) |
5.89 (1.79) | 5.39 (1.0) | 0.016 |
| HDL§(mmol/l) | 1.20 (0.42) | 1.52 (0.53) | 0.0001 |
| TGL(mmol/l) | 1.55 (0.82) | 1.31 (0.71) | 0.028 |
| LDL(mmol/l) | 3.99 (1.68) | 3.38 (1.09) | 0.002 |
Table 2.
Frequency of metabolic syndrome components for cases and controls and corresponding odds ratios (OR)
| MetS components | Cases n‡(%) |
Controls n‡(%) |
OR | 95% CI |
| Abdominal obesity |
54(54) | 33(33) | 5.38 | 3.22–8.98 |
| Hypertension | 83(83) | 45(45) | 4.50 | 2.29–8.85 |
| Low HDL | 48(48) | 26(26) | 3.63 | 2.42–5.43 |
| Hypertriglyceridaemia | 32(32) | 23(23) | 3.30 | 2.33–4.66 |
| Hyperglycaemia | 54(54) | 14(14) | 3.24 | 2.22–4.72 |
HDL-High density lipoprotein cholesterol
n-100 for cases and controls
The test of association between NCEP: ATPIII individual components and MetS resulted in central obesity having the highest Odds ratio of 5.38 (95% CI 3.22–8.98), followed by hypertension and low HDL. Hypertriglyceridaemia (OR= 3.30, 95% CI 2.33 – 4.66) and hyperglycaemia (OR=3.24, 95% CI 2.22 – 4.72) had the lowest values (p< 0.0001) Table 2.
Fifty-four percent of cases and 18% of controls had 3 or more MetS components, making the prevalence of the MetS among cases and controls 54% and 18%, respectively. Significantly more females (cases 69%, controls 28.3%) than males (40.7% cases, 9.3% controls) had MetS (p= 0.004).
The most common combination of the MetS for cases was hypertension, central obesity and hyperglycaemia, while that for controls were hypertension, central obesity and hypertriglyceridaemia. The prevalence of the MetS was found to increase with age. Prevalence rates of 3.7% and 66.7% were obtained for individuals aged 30 – 39 years and those older than 70 years, respectively. However this finding was not statistically significant. (p= 0.227).
The number of MetS components for cases and controls is as shown in Table 3. For cases, 32% had 2 components and a similar percentage was also found to have 3 of the MetS components. Nine percent of cases had all 5 components. Among the control subjects 27% had no MetS component, while 32% and 23% of controls had either 1 or 2 components respectively. There was a statistically significant difference between cases and controls with regardto the number of MetS components (p < 0.05).The odds ratio for the MetS was 5.35 [95% CI 2.81–10.18, p= 0.0001]. As the number of components increases the odds ratio for MetS also increases (Table 3).
Table 3.
Number of MetS components for cases and controls and corresponding odds ratio
| No. of MetS components |
Controls | Cases | Odds Ratio |
95% CI |
| 0 | 27 | 2 | 0.06 | 0.01–0.24 |
| 1 | 32 | 11 | 0.26 | 0.12–0.56 |
| 2 | 23 | 32 | 1.65 | 0.88–3.08 |
| 3 | 11 | 32 | 3.81 | 1.79–8.10 |
| 4 | 5 | 13 | 2.84 | 0.97–8.29 |
| 5 | 2 | 9 | 4.85 | 1.02–23.03 |
| Total | 100 | 100 | - | - |
Multivariate multiple logistic regression analysis done using NCEP: ATPIII cut-off values for the MetS components showed that hyperglycaemia (FPG ≥6.1mmol/L) and hypertension (BP≤130/85mmHg) were the only MetS components with a significant association with the development of CVD in this study (Table 4).
Table 4.
Association between MetS components and cardiovascular disease
| MetS Components |
p-value** | OR ‡‡ | 95% C.I. |
| TGL | 0.707 | 0.863 | 0.400 –1.862 |
| FPG | 0.000 | 6.555 | 3.039 –14.139 |
| HDL | 0.076 | 0.523 | 0.256 –1.071 |
| HPT | 0.000 | 5.755 | 2.708 –12.230 |
| WC | 0.109 | 0.565 | 0.281 –1.137 |
| Constant | 0.010 | 0.278 |
Significance for the Wald test
Predicted change in odds for a unit increase in co-variate
Discussion
This is the first study on frequency of MetS among CVD patients in Ghana and the findings could serve as a backdrop for further research on the MetS in Ghana. High levels of known CVD risk factors such as hypertension, obesity, physical inactivity and dyslipidaemia were seen in this study. Low proportions of both cases and controls were engaged in physical activity. Regular and sustained physical activity has been shown to improve all the risk factors of MetS.31 Low levels of physical activity have been shown to be associated with high rates of obesity in a previous study conducted in Ghana.32 Studies conducted in Cameroun found central obesity to be the key determinant of MetS.14 The finding of central obesity, which had a high frequency of occurrence, as the component with the strongest association with MetS is in keeping with these observations.
From this study, cases had a higher number of clusters of MetS components than controls. This is similar to findings from studies on CVD patients in the Netherlands.33 The syndrome is thought to follow a progressive course with the development of one risk factor, usually abdominal obesity, followed later over time, by the development and worsening of other risk factors.34
The prevalence of MetS among cases was three times higher than that for controls (54% vs 18%). Prospective studies on MetS and the risk of CVD have shown wide variations in the prevalence of the MetS among CVD patients, however, the prevalence ranged from 23% to 46% in majority of the studies.35 The prevalence of the MetS among the controls was comparable to rates in ‘population-based’ studies in Asia (15%– 18%) 8 but lower than rates in Europe9 and USA.10.
In Africa, the prevalence rate of MetS in population-based studies in Tunisia36 is comparable to that found among controls in this study, while lower rates have been reported in Mauritius37 and Cameroun.14 The relatively high prevalence of MetS in this study could be due to the high prevalence of MetS components especially hypertension and central obesity as well as the low levels of physical activity. In addition, all study subjects lived in urban areas where the prevalence rate of MetS has been observed to be higher than in the rural areas.14 In this study, MetS had a strong association with CVD [OR=5.35 95%CI, 2.81–10.18] and this conforms to what has previously been demonstrated in other studies11,35
In one study, it was found that MetS was at least as powerful as high LDL cholesterol in predicting risk of CVD.38 Hypertension and hyperglycaemia were the two components of the MetS with a significantly strong association with the development of CVD in this study. However, hypertriglyceridaemia and low HDL cholesterol were found to be strong predictors of CVD in studies conducted among Caucasians.38, 39 This brings to the fore the issue of racial differences with respect to the individual components of MetS. Indeed, it has been shown that hypertension carries a much greater CVD morbidity and mortality for people of African descent.40 In addition; preliminary studies have shown that people of African descent are more insulin resistant than their Caucasian counterparts.2
In view of this, researchers have proposed a framework for the reclassification of MetS in people of African descent, including the identification of different thresholds for the MetS parameters.41 In addition, it is recommended that these parameters are weighted differently in terms of their greater risk to predict CVD in different racial and ethnic populations. However more prospective studies are needed especially in sub-Saharan Africa to affirm this hypothesis.42
Conclusion
The presence of MetS is a significant predictor of CVD and serves as a simple clinical tool for identifying individuals with a relatively high risk of developing CVD. Individuals with MetS may have other metabolic risk factors that are not included in the standard diagnostic criteria. These risk factors, which impart independent risk for cardiovascular events, need to be identified and adequately treated to decrease CVD events.
A nationwide assessment of the impact of the MetS on adult morbidity and mortality is urgently needed together with a policy to institute routine screening in clinical practice and provision of appropriate interventions for MetS components among adults in Ghana.
Acknowledgement
The authors wish to express their gratitude to the technicians of Central Laboratory, KorleBu Teaching Hospital and Reverend Tom Ndanu for their technical assistance. We acknowledge the contribution of the late Prof J.O.M. Pobee, who passed away after the supervision of this work and the initial review of this article.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Defronzo RA, Ferrannini E. Insulin resistance. A multi-faceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan NM. The deadly quartet. Upper body obesity, glucose intolerance, hypertriglyceridaemia and hypertension. Arch Intern Med. 1989;149:1514–1520. doi: 10.1001/archinte.149.7.1514. PubMed. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. Banting Lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide population. Endocrinol Metabol Clin North Am. 2004;33:351–375. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Zimmet PZ. Definition, diagnosis, and classification of diabetes mellitus and its complication Part 1: diagnosis and classification of diabetes mellitus: provisional report of a WHO consultation. Diabetes Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Executive Summary of the Third Report of the National Cholesterol Education Program [NCEP] expert panel on detection, evaluation, and treatment of high blood cholesterol in adults [Adult Treatment Panel III] JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Gu D, Reynolds K, Wu X, Chen J. Prevalence of metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–1405. doi: 10.1016/S0140-6736(05)66375-1. PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Satter N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-Reactive Protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 11.Alebiosu CO, Odusan BO. Metabolic syndrome in-subjects with type 2 diabetes mellitus. J Natl Med Assoc. 2004;96(6):817–821. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 12.Isezuo SA, Ezunu E. Demographic and clinical correlates of metabolic syndrome in native African type 2 diabetic patients. J Natl Med Assoc. 2005;97:557–563. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 13.Makuyana D, Gomo Z, Munyombe T, Matenga JA, Hakim JG. Metabolic syndrome disorders in urban black Zimbabweans with type 2 diabetes mellitus. CentrAfr J Med. 2004;50(34):24–30. [PubMed] [Google Scholar]
- 14.Fezeu l, Balkau B, Kengne AP. Metabolic syndrome in a sub-Saharan African setting: Central obesity may be key determinant. Atherosclerosis. 2007;11:70–76. doi: 10.1016/j.atherosclerosis.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuzama Y. Adipocytes and metabolic syndrome. SeminVasc Med. 2005;5:34–39. [Google Scholar]
- 16.Hegele RA. Monogenic forms of insulin resistance: apertures that expose thecommon metabolic syndrome. Trends EndocrinolMetab. 2003;14:371–377. doi: 10.1016/s1043-2760(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 17.Isomea B, Almegren P, Tuomi T. Cardiovascular disease morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.cir.97.6.596. PubMed. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization, author. Reducing risk, promoting healthy life. Geneva: WHO; 2002. World Health Report 2002. http://www.who.int/whr/2002/en/index.html. [Google Scholar]
- 20.Bulato RA. Mortality by cause, 1970 to 2015. In: Gribble A, Preston S, editors. The Epidemiological Transition. Washington, DC: National Academy of Sciences Press; 1993. pp. 42–68. [Google Scholar]
- 21.Pobee JO. Community based high blood pressure programs in sub-Saharan Africa. Ethn dis. 1993;(Suppl):S38–S45. [PubMed] [Google Scholar]
- 22.Amoah AG, Owusu SK, Adjei S. Diabetes in Ghana: a community based prevalence study in Greater Accra. Diabetes Research and Clinical Practice. 2002;56:197–205. doi: 10.1016/s0168-8227(01)00374-6. [DOI] [PubMed] [Google Scholar]
- 23.Amoah AG, Schuster DP, Gaillard T, Osei K. Insulin resistance, beta cell function and cardiovascular risk factors in Ghanaians with varying degrees of glucose tolerance. Ethn Dis. 2002;12(4):S3–S10. PubMed. [PubMed] [Google Scholar]
- 24.Nyarko A, Adubofuor K, Ofei F, Kpodonu J, Owusu SK. Serum lipid and Lipoprotein levels in Ghanaians with diabetes mellitus and hypertension. JNatl Med Assoc. 1997;89:191–196. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 25.Amoah AG. Socio-demographic variations in obesity among Ghanaian adults. Public Health Nutrition. 2003;6(8):751–757. doi: 10.1079/phn2003506. [DOI] [PubMed] [Google Scholar]
- 26.Biritwum RB, Gulaid J, Amaning AO. Pattern of diseases or conditions leading to hospitalization at the Korle-Bu Teaching Hospital, Ghana in 1996. GMJ. 2000;34(4):197–205. [Google Scholar]
- 27.Lean MEJ, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. Bri Med J. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.A proposed method for determining blood glucose using Hexokinase and glucose-6-Phosphate dehydrogenase, Public Health Service, Center for Disease Control. 1976. [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipo-protein in plasma without use of preparative ultra-centrifuge. ClinChem. 1972;18:499–502. PubMed. [PubMed] [Google Scholar]
- 30.Stanley L. Adequacy of sample size in health studies. London: John Wiley& sons Ltd; 1990. Sample size for case-control studies; pp. 16–20. (For WHO Publishers) [Google Scholar]
- 31.Lakka TA, Laaksonen DE, Lakka HM. Sedentary lifestyle, poor cardio-respiratory fitness, and the metabolic syndrome. Med Sci Sports Exerc. 2003;(35):1279–1286. doi: 10.1249/01.MSS.0000079076.74931.9A. [DOI] [PubMed] [Google Scholar]
- 32.Biritwum RB, Gyapong J, Mensah G. The epidemiology of obesity in Ghana. Ghana Med J. 2005;39:82–85. [PMC free article] [PubMed] [Google Scholar]
- 33.Gorter PM, Olijhoek JK, van der Graaf Y, Algra A, Rabelink TJ. Prevalence of the metabolic syndrome in patients with coronary heart disease, cerebrovascular disease, peripheral artery disease or abdominal aortic aneurysm. Atherosclerosis. 2004;173:363–369. doi: 10.1016/j.atherosclerosis.2003.12.033. PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Meigs J, Nathan DM, Wilson PW, Cupples LA, Singer DE. Metabolic risk factors worsen continuously across the spectrum of non-diabetic glucose tolerance: The Framingham Offspring Study. Ann Intern Med. 1998;128:524–533. doi: 10.7326/0003-4819-128-7-199804010-00002. PubMed. [DOI] [PubMed] [Google Scholar]
- 35.Galasi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. PubMed. [DOI] [PubMed] [Google Scholar]
- 36.Bouguerra R, Ben Salem L, Alberti H, BenRayana C. Prevalence of metabolic abnormalities in the Tunisian adults: a population based study. Diabetes Metab. 2006;32:215–221. doi: 10.1016/s1262-3636(07)70271-9. PubMed. [DOI] [PubMed] [Google Scholar]
- 37.Cameron AJ, Shaw JE, Zimmet PZ, Cloitson P, Alberti KGGM. Comparison of WHO and NCEP: ATP III metabolic syndrome definitions over 5 years in Mauritius. Diabetologia. 2003;46:A3068.33. PubMed. [Google Scholar]
- 38.Jeppensen J, Hansen TW, Rasmussen S, Ibsen H, Pedersen CT. Metabolic syndrome, low-density lipoprotein cholesterol, and risk of cardiovascular disease: A population-based study. Atherosclerosis. 2006;189:369–374. doi: 10.1016/j.atherosclerosis.2005.12.010. PubMed. [DOI] [PubMed] [Google Scholar]
- 39.Millionis HJ, Rizos E, Goudevenos J, Seferiadis K, Mikhailidis DP. Components of the metabolic syndrome and risk for first-ever acute ischaemic non-embolic stroke in the elderly subjects. Stroke. 2005;36:1372–1376. doi: 10.1161/01.STR.0000169935.35394.38. PubMed. [DOI] [PubMed] [Google Scholar]
- 40.Lea J, Cheek D, Thornley-Brown D, et al. Progress of Chronic Kidney disease in Hypertensive African-American. Am J Kidn Dis. 2008;51:732–741. doi: 10.1053/j.ajkd.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Gaillard T, Schuster D, Osei K. Metabolic Syndrome in black people of the African diaspora: The paradox of current classification, definition and criteria. Ethn Dis. 19:S2-1–S2-7. [PubMed] [Google Scholar]