Summary
Introduction
In West Africa, the prevalence of sickle cell disease (SCD) is 2%. The disease adversely affects growth, development and organ function including the kidneys. There is however a dearth of information about the renal status of SCD children in Ghana.
Objectives
To assess the renal status of children with SCD in steady state.
Design
A cross-sectional case-control study.
Setting
Paediatric Sickle Cell Clinic, Korle Bu Teaching Hospital, Accra.
Participants
Cases-357 SCD cases and 70 of their HbAA siblings as controls.
Methods
Documentation of their socio-demographic data, clinical data and dipstick urinalysis findings, and renal ultrasonography on selected participants.
Results
The mean [SD] age was 7.18 [3.15]yrs for cases and 5.16[3.28]yrs for controls. The genotypes were Hb SS (76.7%), Hb SC (21.8 %), and Hb Sβthal (1.4%). Urinalysis showed leucocyturia in 12.6% versus 5.7% (χ2=62.5 and the p=0.000)), isolated proteinuria in 2.8% versus 1.43% (χ2=10.01 and p=0.001) haematuria in 2.6% versus 0% (χ2=9.233, p=0.002) and nitrites in 2.2% versus 1.4% (χ2 =16.3,p=0.02) of cases and controls respectively. The youngest SCD case with proteinuria was 2yrs. old. Proteinuria prevalence increased with age, , occurring in 5.7% of cases aged 9–11yrs. and 20.6% of cases aged 12yrs. Two-thirds of the proteinuria cases were aged 9–12yrs., of whom 50% were aged 12yrs. Renal ultrasound findings were normal in all those examined.
Conclusion
Urinary abnormalities suggesting nephropathy occur early in SCD patients in Ghana. Routine dipstick screening at clinic visits countrywide would help early detection and prompt intervention to limit renal impairment.
Keywords: Kidney, Sickle Cell Disease, Children, Ghana
Introduction
Extensive research and understanding of SCD in the last century has informed management for better health outcomes.1,2 Early screening with appropriate follow-up and support has lead to prolonged and improved quality of life. SCD prevalence in Ghana has remained at 2% and the trait 25% in the population.3,4 Glomerulosclerosis has been identified as the commonest renal pathology in SCD patients. Chronic renal failure (CRF), a known cause of death in adults with SCD is often the end stage manifestation of asymptomatic nephropathy in childhood.5,6
Among the challenges in the management of renal complications of SCD are identifying early indicators, reducing glomerular damage and progression to end stage renal failure. Studies have been carried out in children in the sub region and beyond to assess renal status with varied outcomes depending on the indicator used.6,7 The relatively higher glomerular filtration rate (GFR) of children with SCD together with tubular secretion of creatinine result in low serum creatinine levels especially in sickle cell anemia patients. Thus biochemical indices like creatinine, urea and electrolytes have been essentially within the normal range in SCD children with renal complications and imminent renal failure, while proteinuria has been identified to be persistent and to increase progressively with severity of renal damage.7–9
Ghana's national neonatal screening program is yet to start, therefore children with the disease are diagnosed late. This means that organ impairment including renal, may already have set in before diagnosis, increasing the risk for development of disease complications leading to increased morbidity and mortality. However, there is a dearth of information on this in Ghana, hence the need for this study. This study sought to find the extent to which renal damage and impaired renal function occur in children with SCD in Ghana, using non-invasive methods.
It also sought to establish the impact, if any, of early diagnosis of sickle cell disease on renal status of patients who are on regular clinic follow-up, and the reason for the diagnosis being made in the first place.
Materials, Subjects and Methods
This was a hospital-based cross-sectional study, conducted over a 4 month period, July to November 2008 at the Department of Child Health, (DCH) Paediatric Sickle Cell Clinic (SCC), Korle Bu Teaching Hospital (KBTH), Accra. A control group was selected to provide a case control comparison for patient's selected. All patients who visited the sickle cell clinic during the study period were eligible for recruitment into the study. Those who were in steady state (defined as reporting to SCC without a fever or any acute illness) and whose parent/guardians gave written informed consent and older (>7years) children who assented to participate in the study were recruited. A sample of their siblings living in the same household within the same age range with confirmed HbAA genotype was recruited to serve as control. Following informed written consent from parents or guardians, 357 children with SCD as cases aged 1–12 yrs and 70 of their genotype Hb AA siblings as controls within the same age range were recruited by purposive sampling.
A structured questionnaire capturing demographic data, genotype, past medical history including age at diagnosis and reason for Hb electrophoresis being done was completed by one of the investigators (CO-Y). Dipstick urinalysis was conducted on freshly voided midstream urine of all recruited participants at the project site using the Combi-Screen 10SL dipstick. Renal ultrasound studies were conducted for all patients who had proteinuria on dipstick testing and a selected sample of patients who had normal urinalysis findings. The sample was selected using the first patient and subsequently every tenth patient identified with normal urinalysis results. All the ultrasound examinations were done by the same radiologist and with the same ultrasound machine. Dipstick measured protein, blood, leucocytes and nitrites in urine. Abdominal ultrasound focused on kidney texture and corticomedullary differentiation. Ethical Clearance for the study was obtained from the Ghana Health Service Ethics Review Committee.
Data analysis
Data was entered into Access database. Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS) for Microsoft Windows, 14th students' edition. Continuous variables like age were reported using mean and standard deviation. Bivariate analysis was reported using Chi-squared (χ2) and correlation coefficients were calculated for variables of interest and the significance level set at 0.05.
Results
The age and sex distribution of the 427 participants, 357 cases and 70 controls, is shown in Table 1. The genotypes identified were ‘SS’ (76.7%), ‘SC’ (21.8%), ‘SD’ (15.4%) and S βthal (1.4%). All the controls were genotype Hb AA. Of the cases 48.6% were diagnosed with SCD before their 2nd birthday, and 78.5% before their 5th birthday.
Table 1.
Age and sex distribution of 427 participants
| Age in years |
Sickle Cell Disease Cases n=357 |
HbAA Controls n=70 |
Total | |||
| Male n (%) | Female n (%) | Male n (%) | Female n % | Male n (%) | Female n (%) | |
| 1<2 | 7 (2.0) | 2 (0.6) | 10 (14.3) | 1 (1.4) | 17 (4.0) | 3 (0.7) |
| 2-<5 | 48 (13.4) | 32 (9.0) | 11 (15.7) | 13 (18.6) | 59 (13.8) | 45 (10.5) |
| 5-<9 | 67 (18.8) | 63 (17.6) | 9 (12.9) | 15 (21.4) | 76 (17.8) | 78 (18.3) |
| 9–12 | 66 (18.7) | 72 (20.1) | 8 (11.4) | 3 (4.3) | 74 (17.3) | 75 (17.6) |
| Total | 188 (52.7) | 169 (47.3) | 38 (54.3) | 32 (45.7) | 226 (52.9) | 201 (47.1) |
| χ2 = 5.3, p=0.147 | χ2 = 12.3, p=0.006 | χ2 = 12.29,p=0.006 | ||||
Most of them (85.7%) were diagnosed as part of investigations for an acute illness, 10.0% on a health worker's recommendation due to suggestive symptoms and 5.3% by voluntary testing. There were statistically significant differences in dipstix urinalysis findings between cases and controls (Table 2).
Table 2.
Dipstick urinalysis findings in participants
| Urinalysis findings | Cases n=357 | Controls n=70 | p- value | ||
| No | (% of total) | No | (% of total) | ||
| Only proteinuria | 10 | 2.8 | 1 | 1.43 | 0.001 |
| Proteinuria + blood only | 4 | 1.12 | 0 | 0 | 0.024 |
| Proteinuria + leucocytes only | 3 | 0.84 | 3 | 4.29 | 0.000 |
| Proteinuria + nitrite only | 1 | 0.28 | 2 | 2.86 | 0.001 |
| Proteinuria+blood+leucocytes | 3 | 0.84 | 0 | 0 | 0.014 |
| Leucocytes only | 33 | 9.24 | 0 | 0 | 0.000 |
| Blood + leucocytes only | 2 | 0.56 | 0 | 0 | 0.145 |
| Nitrite + leucocytes only | 4 | 1.12 | 0 | 0 | 0.024 |
| Nitrite only | 3 | 0.84 | 0 | 0 | 0.014 |
| No abnormality | 285 | 79.83 | 64 | 91.43 | 0.000 |
| Total | 357 | 100.00 | 70 | 100.00 | |
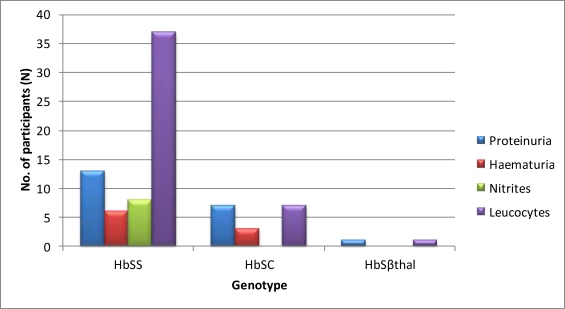
Patients with Hb SS phenotype were more likely to present with proteinuria, and to have leucocytes and haematuria. The intergenotype differences however were not statistically significant. Analysis showed that HbSS cases accounted for 13/21 (57%), 37/45(71%), 6/9(66%) and 8/8 (100%) of the total cases of proteinuria, leucocyturia, haematuria and nitrites respectively among children with haemoglobinopathy.
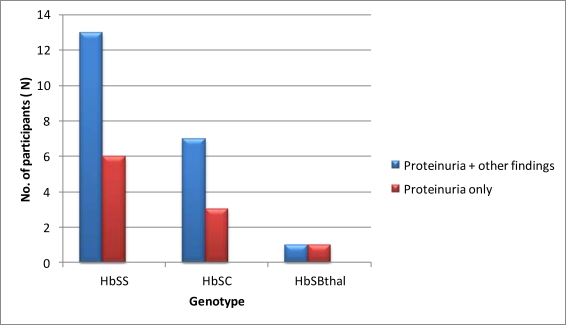
Participants who presented with only proteinuria had no urinary tract infection by their urine culture report. Figure 1 shows the Dipstix urinalysis by genotype. The prevalence of proteinuria in SCD cases increased with age; the youngest case with proteinuria was 2 years old, and two-thirds of cases with proteinuria were aged 9 to 12 years. Of these, 50% were aged 12years. The distribution of proteinuria according to genotype is show in Figure 2There were 21 cases and 6 controls with proteinuria on dipstix testing.
Figure 1.
Dipstix urinalysis findings by genotype
Figure 2.
Distribution of proteinuria by genotype of participants
Regarding the amount of proteinuria, of the cases, 11 (52.4%) had trace proteinuria, 7 (33.3%) had 30mg/dl of proteinuria, 2 (9.5%) had 100mg/dl of proteinuria and 1(4.8%) had 500mg/dl of proteinuria. Thus 95.2% of cases with proteinuria had microalbuminuria (20–100 mg/dl); while 4.8% had macroalbuminuria(>200mg/dl). All the controls had only trace proteinuria. There was a statistically significant difference in the amount of proteinuria between the cases and controls. The study found 47.6% of cases compared with 0% controls having between 30mg and 500mg of proteinuria (p = 0.001).
In addition, 5(83.3%) of the six controls with proteinuria had other findings suggestive of urinary tract infection; three had leucocyturia, and two had nitrites. Their urine cultures confirmed urinary tract infections for which appropriate treatment was given. All the participants who had renal ultrasound had normal kidney sizes and corticomedullary differentiation. There was no correlation between age at diagnosis of sickle cell disease and proteinuria (r = 0.02, p = 0.76).
Discussion
A number of studies have shown that proteinuria is the earliest sign of renal disease in SCD patients.5,9,10 Sickle cell nephropathy is known to start in children as microalbuminuria progressing to macroalbuminuria and then subsequent decline of renal function and creatinine clearance.11,12 Our finding that proteinuria was more common among genotype HbSS than HbSC cases is in keeping with that of other studies.5,12 The prevalence of proteinuria in the SCD patients in this study was observed to increase with age and the increase was significant after 9 years of age as others have found 12,14,15 . Our prevalence 9.4% was lower than the 14.3% found by Datta et al 2003 12 or the 24% found by McBurney in 2002. 14However, our highest prevalence was 21.1 % among children aged 12years, and this age actually accounted for one third of the children with proteinuria. We therefore speculate that including older children in the study may have increased the prevalence of proteinuria to within the higher range recorded by these workers.
Severity of Proteinuria in SCD patients
In terms of severity of proteinuria the majority had microalbuminuria. This is consistent with the findings of Alvarez and colleagues.11 They however had a higher proportion (17.4%) of their patients with macroalbuminuria and the upper- age- limit was 18 years. Our lower upper- age- limit of 12 years may account for the disparity in proportions since proteinuria from sickle cell nephropathy, like diabetic nephropathy, worsens with age. The younger the age-group, the more likely it is that one will observe more microalbuminuria. Our finding that proteinuria in the controls was only trace and that all but one of the controls who had proteinuria also had other signs of urinary tract infection suggests that proteinuria in these controls was due to infection and not intrinsic renal pathology, unlike the SCD cases.
Haematuria
It was not surprising that haematuria was encountered in a similar proportion of HbSS 3.3 % of the total number of patients and HbSC (2.2 %) of the total number of cases and no controls, as unlike proteinuria, this manifestation tends to appear in equal proportions in both genotypes.5,19 This is understandably so since the inner medullary sickling and papillary necrosis that is the cause of this phenomenon occurs in all who carry the Hb S gene. As expected none of the participants with genotype AA presented with haematuria. Interestingly none of the patients with Sβ thal had haematuria. This could be because of the small number of cases patients with Sβ thal in the study.
Leucocytes and nitrites
Asymptomatic urinary tract infection may be cause for concern among our patients. Leucocytes and nitrites, known indicators of potential urinary tract infection, were present in more SCD patients than the controls. It is worrying that while none of these children had complained of urinary symptoms and were in fact well and in steady state at the time of the study, as many as 12.6% of them had leucocyturia compared with only 5.7% of controls, and 2.2% of them compared to 1.4% of controls had nitrites. These findings support those of other workers that asymptomatic urinary tract infection is more common among SCD patients than the general population.9,17,18 Asymptomatic UTI if not identified and treated early may eventually cause more renal complications.. Routine dipstick urinalysis is the key to early detection and treatment to minimize these complications.
Ultrasound findings in children with SCD
All the participants who had renal ultrasound had normal kidney sizes and corticomedullary differentiation. The findings of all SCD patient kidneys duly examined by ultrasound conformed with the findings of Papadaki and colleagues19 who reported that none of their patients aged below 15 years presented with renal enlargement or poor corticomedullary differentiation. Poor corticomedullary differentiation was observed only in those older than 15 years in that study.
Awareness of SCD in the population
It is cause for concern that a high proportion (94.7%) of patients were diagnosed as part of investigations for an acute illness or following health worker's recommendation based on suggestive symptoms. While this demonstrates that Ghanaian health workers are well sensitized about the disease, it also implies that the general populace is not. The findings highlight the need for a review of the current advocacy efforts by health and religious bodies to the population. Priority should be placed on intensified public education with emphasis on voluntary Hb electrophoresis for all and opportunities for voluntary testing provided nationwide.
Though it is noteworthy that almost 50% of children with SCD who attend our clinic were diagnosed before 2 years of age, only very early diagnosis and follow up reduce the morbidity and mortality associated with the disease as workers worldwide such as Rahimy and colleagues in Benin20 and other researchers elsewhere have shown 21, 22 Thus the value of newborn screening cannot be over emphasized. Since 85% of patients were diagnosed as part of diagnostic investigations for illness. Ghanaian children like those in USA, Nigeria, and Jamaica1,23,24 are presenting with their first symptoms of SCD early in life and are unfortunately suffering morbidity before diagnosis. While plans are being put in place for a national neonatal screening program for sickle cell disease in Ghana, it is important to increase the populace awareness and knowledge of sickle cell disease to empower parents to opt for early voluntary testing.
Health workers should be sensitized to recommend Hb electrophoresis instead of sickling test at every opportunity patients have to do haematological investigations, as a negative sickling test result is not synonymous with genotype Hb AA as many in the population wrongly believe. Aggressive testing will go a long way to ensure early diagnosis and early initiation of preventive measures that have been proven to improve quality of life of sickle cell disease patients.
Conclusion
Proteinuria occurs at an early age in the Ghanaian child with SCD. Routine dipstick urinalysis should be in the follow-up protocol drawn for the sickle cell clinic especially for children aged 9 years and above. The implementation of the proposed newborn screening programme would help reduce the morbidity associated with renal abnormalities in these children.
Acknowledgement
We wish to thank. Dr. (Mrs) Klenam Dzefi Tetteh for performing the abdominal ultrasound scans.
References
- 1.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in Sickle Cell Disease-life expectancy and risk factors for early death. The New England J of Medicine. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 2.McKerrel DH, Cohen HW, Billet HH. The older sickle cell patient. Am J Hematol. 2004;76:101–106. doi: 10.1002/ajh.20075. [DOI] [PubMed] [Google Scholar]
- 3.Ohene-Frempong K, Oduro J, Tetteh H, Nkrumah F. Screening newborns for sickle cell disease in Ghana. Paediatrics. 2008;121:S120–S121. [Google Scholar]
- 4.Konotey-Ahulu FID. The Sickle Cell Disease Patient. London and Basingstoke: Macmillan Publsihing Company; 1991. p. xv. [Google Scholar]
- 5.Ataga KI, Orringer EP. Renal abnormalities in sickle cell disease. Am JHematol. 2000;63:201–211. doi: 10.1002/(sici)1096-8652(200004)63:4<205::aid-ajh8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Thompson J, Reid M, Hambleton I, Serjeant GR. Albuminuria and renal function in homozygous sickle cell disease. Observations from a cohort study. Arch Intern Med. 2007;167:701–708. doi: 10.1001/archinte.167.7.701. [DOI] [PubMed] [Google Scholar]
- 7.Wigfall DR, Ware RE, Burchinal MR, Kinney TR, Foreman JW. Prevalence and clinical correlates of glomerulopathy in children with sickle cell. J Pediatrics. 2000;136:749–753. [PubMed] [Google Scholar]
- 8.Okoro BA, Onwuameze IC. Glomerular filtration rate in healthy Nigerian children and in children with sickle cell anaemia in a steady state. Ann Trop Paediatr. 1991;11:47–50. doi: 10.1080/02724936.1991.11747477. [DOI] [PubMed] [Google Scholar]
- 9.Gausch A, Cua M, Mitch WE. Early detection and the course of glomerular injury in patients with sickle cell anaemia. Kidney Int. 1996;49:786–791. doi: 10.1038/ki.1996.109. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt F, Martinez F, Brillet G, et al. Early glomerular dysfunction in patients with sickle cell anaemia. Am J of Kidney Dis. 1998;32(2):208–214. doi: 10.1053/ajkd.1998.v32.pm9708603. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez O, Lopez-Mitnik G, Zilleruelo G. Short-term follow-up of patients with sickle cell disease and albuminuria. Pediatr Blood Cancer. 2008;50:1236–1239. doi: 10.1002/pbc.21520. [DOI] [PubMed] [Google Scholar]
- 12.Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr. 2003;70(4):307–309. doi: 10.1007/BF02723586. [DOI] [PubMed] [Google Scholar]
- 13.Marsenic O, Couloures KG, Wiley JM. Proteinuria in children with sickle cell disease. Nephrol Dial Transplant. 2008;23:715–720. doi: 10.1093/ndt/gfm858. [DOI] [PubMed] [Google Scholar]
- 14.Sesso R, Almeida MA, Fitgueiredo MS, Bordin JO. Renal dysfunction in patients with sickle cell anaemia or sickle cell trait. BrazJ Med Biol Res. 1998;31:1257–1262. doi: 10.1590/s0100-879x1998001000004. [DOI] [PubMed] [Google Scholar]
- 15.McBurney PG, Hanevald CD, Hernandez CM, Waller JL, McKie EM. Risk factors for microalbuminuria in children with sickle cell anaemia. J Pediatr Hematol Oncol. 2002;24(6):473–477. doi: 10.1097/00043426-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Pham PT, Pham PC, Willinson AH, Lew SQ. Renal abnormalities in sickle cell disease. Kidney Int. 2000;57(1):1–8. doi: 10.1046/j.1523-1755.2000.00806.x. [DOI] [PubMed] [Google Scholar]
- 17.Asinobi AO, Fatunde OJ, Brown BJ, Osinusi K, Fasina NA. Urinary tract infection in febrile children with sickle cell anaemia in Ibadan, Nigeria. Annals of Tropical Paediatrics: International Child Health. 2003;23(2):129–134. doi: 10.1179/027249303235002198. (6) [DOI] [PubMed] [Google Scholar]
- 18.Cumming V, Ali S, Forrester T, Roye-Green K, Reid M. Asymptomatic bacteriuria in sickle cell disease: a cross sectional study. BMC Infectious Diseases. 2006;6:46. doi: 10.1186/1471-2334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadaki MG, Kattamis AC, Papadaki IG, et al. Abdominal ultrasonographic findings in patients with sickle cell anaemia and thalassemia intermedia. Pediatr Radiol. 2003;33:515–521. doi: 10.1007/s00247-003-0950-5. [DOI] [PubMed] [Google Scholar]
- 20.Rahimy MC, Gangbo A, Ahouignan G, et al. Effect of comprehensive clinical care program on disease course in severely ill children with sickle cell anaemia in a sub- Saharan African setting. Blood. 2003;102:834–838. doi: 10.1182/blood-2002-05-1453. [DOI] [PubMed] [Google Scholar]
- 21.Bergman S, Zheng D, Barredo J, Abboud MR, Jaffa AA. Renal kallekrein: a risk marker in children with sickle cell disease. J Pediatr Hematol Oncol. 2006;28:147–153. doi: 10.1097/01.mph.0000203722.91189.9d. [DOI] [PubMed] [Google Scholar]
- 22.Malinauskas BM, Gropper SS, Kawachak DA, Zemel BS, Ohene-Frimpong K, Stallings VA. Impact of acute illness on nutritional status of infants and young children with sickle scell disease. J Am Diet Assoc. 2000;100:330–334. doi: 10.1016/S0002-8223(00)00103-6. [DOI] [PubMed] [Google Scholar]
- 23.Oyedije GA. The health, growth, and educational performance of sickle cell disease children. East Africa Med J. 1991;68(3):181–189. [PubMed] [Google Scholar]
- 24.Thomas AN, Pattison C, Serjeant GR. Outside Europe. Causes of death in sickle cell disease in Jamaica. Br Med J. 1982;285:633–633. doi: 10.1136/bmj.285.6342.633. [DOI] [PMC free article] [PubMed] [Google Scholar]