Summary
Background
Aqueous extracts of Tridax procumbens (TP) (Compositae) and Phyllanthus amarus (PA) (Euphorbiaceae) are used in traditional medicine in Ghana to treat malaria. Previous studies have demonstrated the anti-trypanosoma, anti-bacterial and anti-HIV effects of TP and PA.
Objective
To assess the antiplasmodial activity of extracts of TP and PA.
Method
Aqueous extracts of TP and PA were prepared. A portion of each was freeze-dried and the remaining extracted sequentially with ethyl acetate and chloroform. Ethanolic extracts were also prepared. The antiplasmodial activity of the extracts was assessed with the 3H-hypoxanthine assay using chloroquine-resistant (Dd2) Plasmodium falciparum parasites. Chloroquine was used as the reference drug. The modified tetrazolium-based colorimetric assay was also used to evaluate the red blood cell (RBC)-protective/antiplasmodial activities and cytotoxicities of the extracts.
Results
Results showed that TP and PA have antiplasmodial activities. The aqueous and ethanolic extracts of PA were the most active, yielding EC50 values of 34.9µg/ml and 31.2µg/ml, respectively in the tetrazolium-based assay. The TP and PA produced and IC50 values of 24.8µg/ml and 11.7µg/ml, respectively in the hypoxanthine assay. Protection of human RBCs against P. falciparum damage by the extracts highly correlated with their antiplasmodial activities. None of the extracts, within the concentration range (1.9–500µg/ml) studied produced any overt toxicity to human RBCs.
Conclusion
The results indicate that both PA and TP have activities against chloroquine-resistant P. falciparum (Dd2) parasites. The antiplasmodial principles extracted into water and ethanol but not chloroform or ethyl acetate.
Keywords: Antiplasmodial, Tridax procumbens, Phyllanthus amarus, tetrazolium-based assay, 3H-hypoxanthine uptake assays
Introduction
Malaria continues to be the most important parasitic disease in the world. Each year, up to three million deaths due to malaria are recorded globally, with Africa bearing more than 90% of the burden.1 Almost 3% and 10% of disability adjusted life years are due to malaria mortality globally and in Africa, respectively. In spite of various control strategies, malaria chemotherapy the world-over is confronted with the challenge of the emergence and spread of malaria parasites which are resistant to available drugs.2 This makes the search for new antimalarial drugs imperative.
In Ghana, malaria is the leading cause of morbidity, accounting for about 37.5% of all outpatients (OPD) attendance in the year 2006.3 It is also the leading cause of mortality in children under five years. Reports on a prevalence survey to profile the severity and seasonality of malaria and anemia performed in the Kassena Nankana district in the Northern region of Ghana indicated that 22% (n=2286) of cases were due to infections in the low transmission period and 61% during the high transmission period.4 Important pathophysiological features of malaria include parasite-mediated damage or loss of red blood cells (RBCs) and anaemia.5
Over the years, plants have been important sources of new drugs.6 Several herbal remedies continue to provide easily accessible alternatives to widely used antimalarials such as sulfadoxine-perimethamine (SP), chloroquine and mefloquine.7 Some of these plants may be sources of new antimalarial agents. In Ghana, medicinal plants used to treat malaria include Tridax procumbens (Compositae). (and Phyllanthus amarus (Euphorbiaceae, Schum. & Thonn.).
Similarly the latter is commonly used in Southeastern Nigeria for the treatment of malaria-related symptons.8 In vitro antiplasmodial activity of Phyllanthus amarus against chloroquine sensitive Plasmodium falciparum strain 3D7 has been reported.8 Results of a phase two clinical trial conducted on antiplasmodial herbal remedy, consisting of eight ingredients including Phyllanthus amarus and administered by Nigerian herbalists, confirmed the efficacy of the remedy.9 Tridax procumbens has been reported to have activity against some forms of Trypanosoma cruzi and bacteria, whilst Phyllanthus amarus has been shown to inhibit the DNA polymerase of hepatitis B virus and reverse transcriptase of HIV.10,11,12 However, these plants have not been investigated to establish their antimalarial properties particularly against drug-resistant parasite strains.
Various methods including P. falciparum culture using 3H-hypoxanthine uptake, have been employed in screening for new antimalarial drugs.13 Other methods like the tetrazolium assays which involve mitochondrial dehydrogenases and other dehydro- genases converting tetrazolium to formazan that indicates cell viability have been used to evaluate anti-microbial agents.14,15 The assay has also been used in models for screening cytocidal chemical agents.16,17 In this paper, we report the in vitro RBC protecting and antiplasmodial activities of Tridax procumbens and Phyllanthus amarus.
Materials and Methods
Parasite culture
Chloroquine-resistant strain of P. falciparum, Dd2, (Centre for Medical Parasitology, Copenhagen, Denmark) was maintained in continuous culture by the modified method of Jensen and Trager18 using RPMI 1640 medium containing Hepes, and NaHCO3 but without glutamine (Sigma Chemical Co. St. Louis, MO, USA). The medium was supplemented with 10% normal human serum (NHS), 1mg/ml L-Glutamine, 1mg/ml of D-Glucose (Sigma Chemical Co. St. Louis, MO, USA), and 80µg/ml of gentamicin (Gibco BRL Life Technologies, Paisley, Scotland). After informed consent, volunteers with blood group O Rh+ donated whole blood for the study. All other chemicals were of analytical grade and obtained from Sigma Chemical Co. St. Louis, MO, USA and Gibco BRL Life Technologies, Paisley, Scotland.
The proposal was reviewed and cleared by the appropriate institutional board of the Noguchi Memorial Institute for Medical Research (NMIMR), Legon, Ghana.
Plant materials
Phyllanthus amarus (PA) and Tridax procumbens (TP) were supplied by Papa Issah Yemoh, a Ghanaian traditional medicine practitioner. Phyllanthus amarus (voucher #GC47813) and Tridax procumbens (voucher #GC47814) were authenticated at the Ghana Herbarium, Department of Botany, University of Ghana, Legon, where voucher specimens have been deposited. Phyllanthus amarus belongs to the family Euphorbiaceae. The plant grows up to no more than 1½ feet tall and has small yellow flowers, small leaves, very small (2mm) fruits that burst open causing the seeds to be hurled way. Tridax procumbens, on the other hand belongs to the family Compositae. It is a very hairy plant with coarsely toothed leaves. It has six ray yellow composite flowers.
Extraction
The whole plant of Phyllanthus amarus and the stem, flowers and leaves of Tridax procumbens were picked air-dried and prepared as follows: 4g of each plant were boiled separately in 100ml of water for 5min. A portion of each of the aqueous extracts was freeze-dried and refrigerated for later use. The remaining aqueous extract was sequentially extracted with cold chloroform and ethyl acetate. A fresh sample of each plant was also extracted with ethanolic (cold maceration). Each of the organic extracts or fraction was evaporated under vacuum.
In vitro antiplasmodial assays Inhibition of 3H-hypoxanthine uptake
The antiplasmodial activities of the plant extracts were determined with the inhibition of 3H-hypoxanthine uptake assay as described by Desjardins et al. 13 Parasitized RBCs (5 × 108 cells/ml) at a parasitemia of 1.5% were added to wells of a 96-well microtitre plate, containing the test extracts or chloroquine at concentrations ranging between 1.9 – 500µg/ml and 0.1 – 100µg/ml, respectively. Parasite and RBC controls of infected and uninfected RBCs, respectively, were placed in wells with culture medium without plant extracts. The plates were incubated at 37°C in a humidified incubator at 5% of O2 and CO2 for 24h. This was followed by the addition of 20µl of 3H-hypoxanthine (40µCi/ ml) to each well and the plates incubated further for 24h.
Each plate was then kept in a refrigerator (4°C) to stop the reaction and store the cultures prior to cell harvesting. Cells were harvested unto glass fibre filters (Filter Mate 96 Cell Harvester, Model A960961, Packard A Canberra Company, USA).
The filters were dried in an incubator at 37°C for 24h after which radioactivity was counted (Direct Beta Counter matrix 96, Model A960961, Packard A Canberra company, USA), to determine the incorporation of radio-labelled hypoxanthine.
Tetrazolium based colorimetric (MTT) assay
A modified version of the tetrazolium based colorimetric assay was used to screen the plant extracts for their antiplasmodial activity and toxicity to RBCs.14 In this assay 100µl of each of the crude extract (concentration range 1.9 – 500µg/ml) was placed in separate wells of a 96-well microtitre plate in duplicate. This was followed by the addition of 100µl of Plasmodium falciparum infected RBCs (2.3 × 108 cells/ml) at a parasitemia of 1.5% to each well. The contributions of plant extracts, culture medium, infected and uninfected RBCs to the optical densities were excluded by setting up control experiments for each of these parameters separately alongside the main experiments.
All plates were incubated at 37°C for 5 days in a humidified incubator at 5% O2 and CO2 after which 20µl of MTT (7.5 mg/ml) were added to each well and the plates incubated again for 2h. Aliquots of culture media (150µl) were removed from each well and discarded. Triton X-100 in acidified isopropanol (200µl) was added to each well to dissolve any formazan formed. The plates were then kept at room temperature in the dark for 24h and the optical densities (OD) of the wells were read at 565 and 690nm on an ELISA plate reader (Molecular Devices Corporation, Menlo Park, California). The percent cell protection and survivals were computed using the modified formula of Ayisi et al (1991). These values were plotted against concentrations of extracts, and the 50% cell protection (effective concentration, EC50) and survival (cytotoxic concentration, CC50) were determined. The selective indices of the extracts were also computed as the ratio of the CC50 to the EC50.
Results
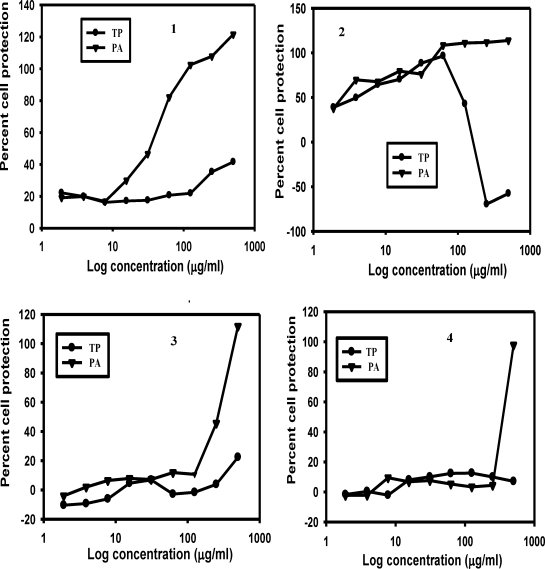
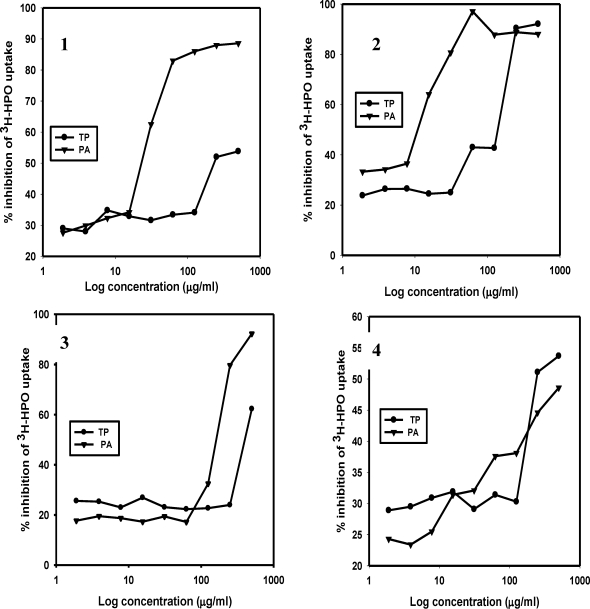
The results for the extract-mediated RBC protection and the antiplasmodial activities of the plant extracts are shown in Figures 1 and 2. Figure 1(1) shows that the aqueous extracts of PA protected the RBCs in a concentration-dependent fashion, starting at a concentration well below 100µg/ml. RBC protection by aqueous TP started at a concentration of 100µg/ml. Unlike the aqueous extracts, the ethanolic extracts of both PA and TP showed significant concentration-dependent cell protection (Figure 1(2)) but decreased sharply at a TP concentration of 100µg/ml and above. Neither the chloroform nor the ethyl acetate fractions of TP offered any significant cell protection (Figures 1(3) and 1(4)). However, the corresponding extracts of PA showed a considerable RBC protection at concentrations higher than 100µg/ml. Similar results were obtained when the antiplasmodial activity of the extracts were assessed with the inhibition of 3H-hypoxanthine uptake (Figure 2).
Figure 1.
Plots showing protection of RBCs by aqueous (1), ethanolic (2), chloroform (3) and ethyl acetate (4) extracts of TP and PA. Incubations were carried out using microtitre plates as described previously.14 Experimental well contained 2.3 × 107 of chloroquine-resistant P. falciparum infected RBCs at a parasitemia of 1.5%. Parasite infected RBCs were treated with different concentrations of the plant extracts ranging from 1.9 to 500µg/ml.
Figure 2.
Plots showing inhibition of 3H-hypoxanthine (3H-HPO) uptake by P. falciparum (strain Dd2) by aqueous (1), ethanolic (2), chloroform (3) and ethyl acetate (4) extracts of TP and PA. Incubations were performed as described by Desjardins et al13 with each well containing 2.3 × 107 of chloroquine-resistant P. falciparum infected RBCs at a parasitemia of 1.5%. Parasite infected RBCs were treated with different concentrations of the plant extracts ranging from 1.9 to 500µg/ml.
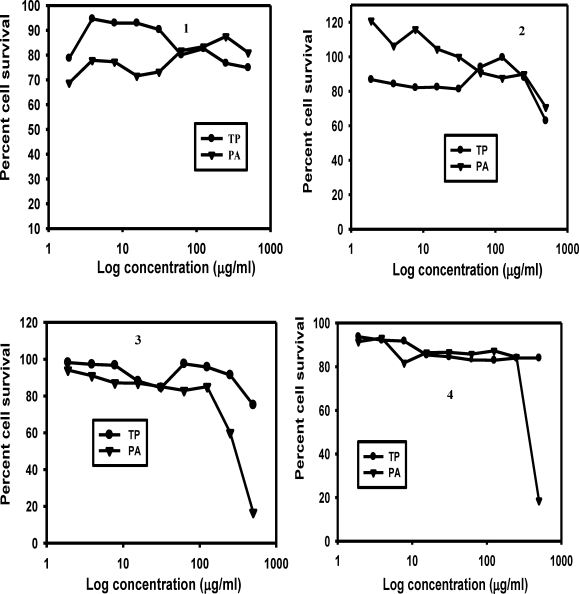
The results presented in Figure 3 indicate that survival of RBC exceeded 70% for all extracts over the concentration range (1.9–500 µg/ml) studied. At concentrations below 62.5µg/ml the aqueous extract of TP appeared to be relatively less toxic to the RBCs than that of PA (Figure 3(1)) whereas for the ethanolic extracts of PA appeared to be less (Figure 3(2)). Although the chloroform extracts of TP and PA also showed low toxicities to RBCs the toxicity of PA to the RBCs increased sharply at concentrations higher than 125µg/ml (Figures 2(3–4)).
Figure 3.
Plots showing RBCs survival in incubations containing aqueous (1), ethanolic (2), chloroform (3) and ethyl acetate (4) extracts of TP and PA. Incubations were carried out using microtitre plates as described previously.14 Each experimental well contained 2.3 × 107 of non-infected human RBCs. RBCs were treated with different concentrations of the plant extracts ranging from 1.9 to 500µg/ml.
The effective median concentrations for RBC protection (EC50) and RBC survival (cytotoxic concentration- CC50) determined from Figures 1 and 3 are shown in Table 1. The table shows that the aqueous and ethanolic extracts of PA yielded the lowest EC50. For TP, apart from the ethanolic extract that produced an EC50 of 121.3µg/ml, the EC50 for all the other fractions were higher than the highest concentration (500µg/ml) used in these studies. The CC50 values also could not be determined for the TP extracts because the percent cell survival values at all concentrations studied were greater than 50% (Figure. 2).
Table 1.
Median effective (EC50) and cytotoxic (CC50) concentrations and selective indices (SI) of extracts of PA and TP.
| EC50(µg/ml)1 | CC50(µg/ml)2 | SI3 | ||||
| Extracts | PA | TP | PA | TP | PA | TP |
| Aqueous | 34.9 | >500 | >500 | >500 | nd | nd |
| Ethanol | 31.2 | 121.3 | >500 | >500 | nd | nd |
| Chloroform | 263.9 | >500 | 302.6 | >500 | 1.1 | nd |
| Ethyl acetate | 368.4 | >500 | 389.7 | >500 | 1.1 | nd |
50% Antiplasmodial effective concentration.
50% Cytotoxic concentration.
Selective index. The SI was calculated as the ratio of CC50 to EC50
Data represents means for duplicate experiments.
nd: not determined as a result of determinants being out of range.
Similar results were obtained for the aqueous and ethanolic extracts of PA. The CC50 of the chloroform and ethyl acetate extracts of PA were below 500µg/ml thus enabling a computation of their selective indices (CC50/EC50). The median inhibitory concentrations (IC50) of the aqueous, ethanol, chloroform and ethyl acetate extracts of PA determined with chloroquineresistant P.falciparum strain (Dd2) in the 3Hhypoxanthine uptake assay were 24.8µg/ml, 11.7µg/ml, 173.6µg/ml and >500 µg/ml, respectively. For TP, the IC50 values were 225.0µg/ml, 143.4µg/ml, 430.6µg/ml and 250.0µg/ml, respectively for the aqueous, ethanol, chloroform and ethyl acetate extracts. The reference drug, chloroquine, demonstrated a concentrationdependent inhibition of parasite growth within the concentration range 0.4 – 100 µg/ml yielding an IC50 of 40µg/ml.
Discussion
Traditional healers in Ghana use Phyllanthus amarus (PA) and Tridax procumbens (TP) separately to manage various diseases including malaria. The present study shows that each of the plants has anti-plasmodial activities. Results from the tetrazolium-based colorimetric assay14 offered an added advantage in showing that the plants also protected RBCs against P. falciparum mediated damage. This is significant because RBC damage and hence loss are important pathophysiological features that precipitate the serious complications of anaemia associated with malaria infections.5 The viability of the RBCs in this study was confirmed by their ability to convert tetrazolium to formazan. The standard and direct method of Desjardins et al13, uses the inhibition of 3H-hypoxanthine uptake as an indicator of an agent's antiplasmodial potential.
Our results, therefore, show that in addition, to their RBC protective effects, the aqueous and ethanolic extracts of PA and TP inhibited growth of the chloroquine-resistant P. falciparum parasites used in the cultures. Ethyl acetate and chloroform extracts at high concentrations seemed to have adverse effects on the RBCs, demonstrated by a sharp drop in cell survival. The comparatively lower EC50 and IC50 values obtained for the ethanolic and aqueous extracts indicate that these solvents extracted more of the antiplasmodial agent. An in vivo study on antiplasmodial effect of aqueous extract of PA showed that it inhibits Plasmodium berghei growth in mice.20 Other studies have also reported in vitro antiplasmodial activity of aqueous and ethanolic extracts of PA.,20,21 These results and those of others showing that these plants have anti-microbial activities10,11,12 are consistent with claims of traditional medical practitioners that TP and PA are effective remedies.
In general, neither TP nor PA was overtly toxic to human RBCs in culture. As a result of their low toxicity to RBCs, the median cytotoxic concentration (CC50) could not be determined for most of the extracts. At higher extract concentrations of ethyl acetate and chloroform, however, PA appeared to affect the RBCs adversely (Figure 3(3 and 4)). Reports on toxicity studies on aqueous extract of PA on rats however revealed potential toxic effects of the extract.22 Thus, further investigations are required to establish the safety of PA in humans. At concentrations below 31µg/ml PA appears to exhibit stimulatory effects on the cells resulting in the observed high RBC survival (Figure. 3(2)). It is significant to note that the IC50 of 40 µg/ml for chloroquine (the standard drug) reported here, is far higher than the 1–64pmol/well reported for micro-tests.19 This affirms that the P. falciparum parasite (Dd2) strain used for the experiments described here are indeed chloroquine-resistant. Therefore, the aqueous and ethanolic extracts of PA and TP are effective against the chloroquine-resistant P. falciparum parasites.
Conclusion
We conclude from our results that the aqueous and ethanolic extracts of PA and TP have antiplasmodial activity against chloroquine-resistant P. falciparum parasites. The extracts have considerably low toxicities to human RBCs. These results lend support to claims of herbalists that decoctions of either TP or PA are useful medicines. These notwithstanding, more comprehensive animal toxicity studies need to be carried out on the plants, especially since humans are currently using them to treat malaria and other diseases.
This could be followed by pilot clinical trials for therapeutic dose range finding as well as proper documentation of effects of these plant medicines on humans. Evaluation of the antiplasmodial activities of the extracts in vivo would be helpful in assess the effects of the extracts in vivo. The plant extracts of TP and PA could be useful alternatives to antimalarial drug or useful in combination therapy, since they are much cheaper. This report demonstrates that the MTT assay which is relatively cheaper compared to the radiolabeled hypoxanthine assay will be a very useful assay system for screening for antimalarial agents.
Acknowledgements
These investigations were supported by a WHO Multilateral Initiative in Malaria (MIM) research grant awarded to Dr. K. A. Koram of the Noguchi Memorial Institute for Medical Research (NMIMR), Legon Ghana. We thank Professor David Ofori-Adjei, Former Director of NMIMR and Professor K. Bosompem for helpful discussion in relation to this work and Papa Issah Yemoh, a Traditional Medicine Practitioner, for providing the herbs. We also appreciate the technical support of Mr. Y. A. Akyeampon and staff of Clinical Pathology unit. We also gratefully acknowledge the Head and staff of the Immunology Unit, NMIMR, for allowing us the use of their culture facilities.
References
- 1.Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: What's new, What's needed: A summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- 2.Travassos MA, Laufer MK. Resistance to antimalarial drugs: Molecular, pharmacologic and clinical considerations. Pediatric Res. 2009;65:64–70. doi: 10.1203/PDR.0b013e3181a0977e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministry of Health, Republic of Ghana, author. Strategic plan for malaria control in Ghana 2008–2015. pp. 18–19.
- 4.Koram KA, Owusu-Agyei S, Fryauff DJ, Anto F, Atuguba F, Hodgson A, Hoffman AL, Nkrumah FK. Seasonal profiles of malaria infection, anaemia, and bednet use among age groups and communities in northern Ghana. Trop Med Int Health. 2003;8:793–802. doi: 10.1046/j.1365-3156.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- 5.Mohan K, Dubey ML, Gunguly NK, Mahajan RJ. Plasmodium falciparum Role of activated blood moncytes in erythrocyte membrane damage and red blood cell loss during malaria. Exptl Parasitol. 1995;80:54–63. doi: 10.1006/expr.1995.1007. [DOI] [PubMed] [Google Scholar]
- 6.Rates SMK. Plants as source of drugs. Toxicon. 2001;39:603–613. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 7.Mustofa VA, Benoit-Vical F, Pelissier Y, Kone-Bamba D, Mallie M. Antiplasmodial activity of plant extracts used in West African traditional medicine. J Ethnopharmacol. 2000;73:145–151. doi: 10.1016/s0378-8741(00)00296-8. [DOI] [PubMed] [Google Scholar]
- 8.Traore M, Diallo A, Nikiema JB, Tinto H, Dakuyo ZP, Ouedraogo JB, et al. In vitro and in vivo antiplasmodial activity of ‘saye’, an herbal remedy used in Burkina Faso traditional medicine. Phytother Res. 2008;22:550–551. doi: 10.1002/ptr.2308. [DOI] [PubMed] [Google Scholar]
- 9.Ajaiyeoba EO, Falade CO, Fawole OI, Akinboye DO, Gbotosho GO, Bolaji OM, et al. Efficacy of herbal remedies used by herbalists in Oyo State Nigeria for treatment of Plasmodium falciparum infections - a survey and an observation. Afr J Med Medical Sci. 2004;33:115–119. [PubMed] [Google Scholar]
- 10.Perumal SR, Ignacimuthu S, Raja DP. Preliminary screening of ethnomedicinal plants from India. J Ethnopharmacol. 1999;66:235–240. doi: 10.1016/s0378-8741(99)00038-0. [DOI] [PubMed] [Google Scholar]
- 11.Caceres A, Lopez B, Gonzalez S, Berger I, Tada I, Maki J. Plants used in Guatemala for the treatment of protozoal infections. I. Screening of activity to bacteria, fungi and American trypanosomes of 13 native plants. J Ethnopharmacol. 1998;62:195–202. doi: 10.1016/s0378-8741(98)00140-8. [DOI] [PubMed] [Google Scholar]
- 12.Mullen JE, O'shea S, Rostron T, Houghton PJ, Woldermariam TZ, Walker E, et al. Inhibition of HIV replication by the plant Phyllanthus amarus. Int Conf AIDS. 1996 Jul 7–12;11:67. [Google Scholar]
- 13.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semi automated micro dilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayisi NK, Gupta SV, Qualtiere LF. Modified tetrazolium-based colorimetric method for determining the activities of anti-HIV compounds. J Virol Methods. 1991;33:335–344. doi: 10.1016/0166-0934(91)90033-v. [DOI] [PubMed] [Google Scholar]
- 15.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 16.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 17.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofzifer TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumour cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 18.Jensen JB, Trager W. Cultivation of erythrocytic and exoerythrocytic stages of Plasmodium in malaria. Academic Press. 1980;2:280. [Google Scholar]
- 19.Dapper DV, Aziagba BN, Ebong OO. Antiplasmodial effect of the aqueous extract of Phyllanthus amarus Schumach and Thonn against Plasmodium berghei in Swiss albino mice. Nigerian J Physiol Sci. 2007;22:19–25. doi: 10.4314/njps.v22i1-2.54857. [DOI] [PubMed] [Google Scholar]
- 20.Musuamba CT, Cimanga RK, Dooghe L, Mesia GK, Luyindula N, Totté J, et al. In vitro antiplasmodial and cytotoxic activities of ethanol extracts of apical stem of Phyllanthus amarus Schum. et Thonn. (Ephorbiaceae) J Complementary Integrated Med. 2010;7(1) Article 55. [Google Scholar]
- 21.Adedapo AA, Adegbayibi AY, Emikpe BO. Some clinic-pathological changes associated with the aqueous extract of the leaves of Phyllanthus amarus in rats. Phytother Res. 2005;19:971–976. doi: 10.1002/ptr.1768. [DOI] [PubMed] [Google Scholar]
- 22.WHO, author. In vitro micro-test (Mark II) for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, sulfadoxine/pyrimethamine and amodiaquine. MAP. 1990;87(2):3–5. [Google Scholar]