ABSTRACT
BACKGROUND:
Pancreatic ductal adenocarcinoma (PDAC) arising in intraductal papillary mucinous neoplasms (IPMN) may represent a different biologic entity than classic PDAC, and there is little evidence to inform adjuvant treatment decisions. The purpose of this study was to identify prognostic factors for PDAC arising in IPMN and determine the benefit of postoperative adjuvant therapy.
METHODS:
Forty-four patients without previous therapy who underwent surgery for invasive PDAC arising in association with IPMN at our institution were identified. Medical records were reviewed for clinical and pathologic features, adjuvant therapy, and outcomes.
RESULTS:
On univariate analysis, positive nodes (hazard rate [HR] 14, 95% confidence interval [CI] 4.2–44), CA 19-9 > 80 (HR 6.2, 95% CI 2.2–17), lymphovascular invasion (HR 4.7, 95% CI 1.5–15), perineural invasion (HR 3.9, 95% CI 1.5–10), and positive margins (HR 3.1, 95% CI 1.2–8.0) were associated with inferior cancer-specific survival. Patients with positive nodes who received adjuvant therapy had higher median cancer-specific survival (20 months) than those who received no adjuvant therapy (3.3 months).
CONCLUSIONS:
Patients with PDAC arising in IPMN presented at an earlier stage than is reported for classical PDAC. Adjuvant chemoradiotherapy was associated with improved overall and cancer-specific survival for patients with advanced disease. These hypothesis-generating results require validation in a larger prospective trial.
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is an increasingly recognized clinicopathologic entity that is characterized by mucin production, cystic dilation of the pancreatic ducts, and intraductal papillary growth.1 IPMN ranges in atypia from benign adenoma to pancreatic ductal adenocarcinoma (PDAC). PDAC arising in the setting of an IPMN appears to have a different natural history and molecular pathogenesis than classic PDAC.1–5 Furthermore, the presence of invasive carcinoma distinguishes this entity from noninvasive IPMN, which is felt to represent a premalignant condition with an excellent prognosis following surgical resection alone,6–9 with 5-year disease-specific survivals of up to 100%.7 In addition, adequate comparisons of malignant IPMN and classic malignant PDAC, controlling for stage, have not yet been done. Consequently, neither the PDAC literature nor the burgeoning data regarding the treatment of noninvasive IPMN serve as ideal guidance for the management of PDAC arising in IPMN.
To better characterize this unique disease, we identified patients with PDAC arising in IPMN from a prospectively collected database of patients treated with surgical resection for IPMN at the Massachusetts General Hospital between 1990 and 2005. Medical records were reviewed for clinicopathologic features, treatment parameters, and outcomes. Prognostic patient and treatment factors were identified to assist in treatment decisions for this increasingly recognized lesion.
PATIENTS AND METHODS
Between 1990 and 2005, data related to 200 patients undergoing resection for IPMN were prospectively recorded in a database in the Department of Surgery at the Massachusetts General Hospital. Tumors were coded in the database according to World Health Organization criteria as intraductal papillary mucinous adenoma, intraductal papillary mucinous tumor with moderate dysplasia or borderline, in situ intraductal papillary mucinous carcinoma, or infiltrating intraductal papillary mucinous carcinoma.
Records of patients who underwent resection for infiltrating intraductal papillary mucinous carcinomas were identified for the purposes of this study. Patients with prior surgery for pancreatic tumors and those who received neoadjuvant or intraoperative therapy were excluded from analysis. A retrospective review of clinical and pathologic features, adjuvant therapy, and outcome was conducted for the entire cohort.
Statistical Analysis
The Kaplan-Meier estimator, Cox proportional hazards model, and competing risks method were used to analyze the data and determine prognostic factors for overall survival and cancer-specific survival. Cancer-specific death was defined as a death occurring in the setting of recurrent cancer. The source documentation for date and cause of death were drawn from treating physicians' medical record and confirmed via communication with treating physicians' office or patients' families. For cancer-specific survival, death due to causes other than cancer represented a competing event. The equality of survivor functions was tested using the log-rank test. Two-sided P values less than .05 were considered statistically significant.
RESULTS
Patient Characteristics
Between 1990 and 2005, 200 patients underwent resection for IPMN at our institution. Of these, 151 (75.5%) had noninvasive disease, and 49 (24.5%) had an invasive component. Five patients were excluded from the analysis; 4 patients received preoperative radiation, and 1 patient had prior pancreatic surgery. Of the remaining 44 patients, 27 (61%) of these patients had resection alone. and 17 (39%) had adjuvant concurrent chemoradiotherapy (CRT).
CRT consisted of 37.8–60.4 Gy (median 50.4 Gy) given concurrently with infusional 5-fluorouracil (5-FU) in 11 (65%), bolus 5-FU in 4 (24%), capecitabine in 1 (6%), and 5-FU/gemcitabine in 1 (6%). Five patients in the CRT group also received 4–6 additional months of adjuvant chemotherapy (3 with 5-FU and 2 with gemcitabine), while 1 patient who did not receive adjuvant CRT received single-agent gemcitabine.
Median follow-up for all patients was 19 months (range 1–145) and 26 months for survivors (range 4–145). Median age was 72 years (range 37–84). Patient factors as analyzed by treatment cohort are shown in Table 1. Almost half of the patients (21/44) had stage I disease, and 30/44 (68%) patients had node-negative disease. Eleven patients had positive margins with invasive adenocarcinoma (pancreatic transection, 6 patients; bile duct, 2 patients; uncinate, 2 patients, and pancreatic transection and retroperitoneal, 1 patient). No patient had an R2 resection.
Table 1.
Patient characteristics by treatment cohort (P value from Fisher's exact test)
| Adjuvant chemoradiotherapy | Surgery alone | Total | P | ||
|---|---|---|---|---|---|
| Sex | Male | 8 | 15 | 23 | .76 |
| Female | 9 | 12 | 21 | ||
| Ethnicity | White | 17 | 23 | 40 | .17 |
| Nonwhite | 0 | 4 | 4 | ||
| Stage | I | 4 | 17 | 21 | .035 |
| II a | 4 | 5 | 9 | ||
| II b | 8 | 5 | 13 | ||
| III | 1 | 0 | 1 | ||
| Nodal disease | + | 9 | 5 | 14 | .024 |
| − | 8 | 22 | 30 | ||
| Margins | Positive | 6 | 5 | 11 | .29 |
| Negative | 11 | 22 | 33 | ||
| CA 19-9 | ≥ 80 | 7 | 10 | 17 | 1.0 |
| < 80 | 10 | 17 | 27 | ||
| IPMN type | Main duct | 5 | 9 | 14 | .39 |
| Side branch | 1 | 5 | 6 | ||
| Both | 11 | 11 | 22 | ||
| Uncertain | 0 | 2 | 2 | ||
| Lymphovascular invasion | Present | 6 | 6 | 12 | .49 |
| Absent | 11 | 21 | 32 | ||
| Perineural invasion | Present | 9 | 10 | 19 | .36 |
| Absent | 8 | 17 | 25 | ||
| Surgery | Total pancreatectomy | 4 | 6 | 10 | 1.0 |
| Partial pancreatectomy | 13 | 21 | 34 | ||
| Adjuvant chemotherapy | Yes | 5 | 1 | 6 | .025 |
| No | 12 | 26 | 38 | ||
Abbreviation: IPMN = intraductal papillary mucinous neoplasm.
Prognostic Factors
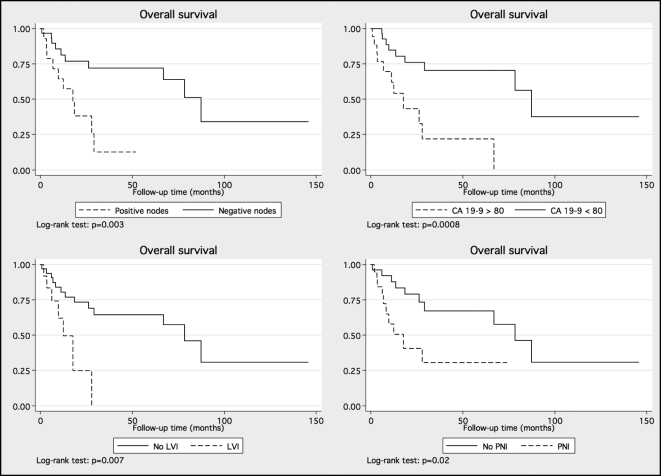
The results of univariate Cox regression analysis for overall and cancer-specific survival are shown in Table 2. Elevated CA 19-9, higher stage, presence of nodal disease, lymphovascular invasion (LVI), and perineural invasion (PNI) were associated with decreased cancer-specific and overall survival. Patients with positive nodes had significantly shorter cancer-specific (18 months vs. not reached) and overall survival (16 months vs. 78 months) than those with node-negative disease. Overall and cancer-specific survival curves stratified by the factors significant on the univariate Cox analysis are shown in Figures 1 and 2, respectively.
Table 2.
Hazard ratios, 95% confidence intervals (CI), and P values for univariate analysis for clinical and treatment factors related to overall and cancer-specific survival*
| Clinical covariates | Overall survival |
Cancer-specific survival |
||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |||
| Positive nodes | 5.5 | 2.3 | 13 | < .0001 | 14 | 4.2 | 44 | < .0001 |
| Lymphovascular invasion | 3.7 | 1.5 | 9.3 | .004 | 4.7 | 1.5 | 15 | .007 |
| Perineural invasion | 2.4 | 1.1 | 5.3 | .026 | 3.9 | 1.5 | 10 | .006 |
| CA 19-9 > 80 | 4.6 | 2.0 | 11 | < .001 | 6.2 | 2.2 | 17 | .001 |
| Adjuvant therapy | ||||||||
| Negative nodes | 0.79 | 0.24 | 2.7 | .7 | 2.1 | 0.35 | .4 | |
| Positive nodes | 0.13 | 0.029 | 0.56 | .006 | 0.10 | 0.018 | 0.59 | .01 |
| Total pancreatectomy (vs. partial) | 2.1 | 0.9 | 4.8 | .07 | 1.2 | 0.4 | 3.8 | .7 |
| Main duct IPMN (vs. others) | 0.9 | 0.4 | 2.0 | .8 | 0.9 | 0.3 | 2.4 | .8 |
| Age | 1.0 | 0.99 | 1.1 | .1 | 1.04 | 0.99 | 1.09 | .1 |
| Male sex | 0.6 | 0.3 | 1.4 | .2 | 0.7 | 0.3 | 1.7 | .4 |
| Positive margin | 1.8 | 0.8 | 4.2 | .1 | 3.1 | 1.2 | 8.0 | .02 |
All patients treated received chemoradiotherapy; 5 of 17 also received an additional 4–6 months of chemotherapy.
Abbreviation: IPMN = intraductal papillary mucinous neoplasm.
Figure 1.
Overall survival of patients segregated by nodal status (upper left), elevated CA 19-9 (upper right), lymphovascular invasion (bottom left), and perineural invasion (bottom right). LVI = lymphovascular invasion; PNI = perineural invasion.
Figure 2.
Cancer-specific survival of patients segregated by nodal status (upper left), elevated CA 19-9 (upper right), lymphovascular invasion (bottom left), and perineural invasion (bottom right). LVI = lymphovascular invasion; PNI = perineural invasion.
Treatment Effect
Chemoradiotherapy
For the entire cohort, there was no statistically significant difference in overall or cancer-specific survival between the patients who received adjuvant CRT and those who did not receive adjuvant CRT. However, patients treated with adjuvant chemoradiotherapy were more likely to be higher stage (p = .035) and have positive nodes (p = .024). When patients were stratified according to their nodal disease, the prognosis for patients with positive nodes was significantly better after adjuvant CRT (for cancer-specific survival, hazard ratio [HR] 0.10, 95% confidence interval [CI] 0.018–0.59; for overall survival, HR 0.13, 95% CI 0.029–0.56). The median cancer-specific survival for the node-positive group that received adjuvant therapy (n = 9) was 20 months; those who did not receive adjuvant therapy (n = 5) had a median cancer-specific survival of 3.3 months, suggesting some patients did not receive adjuvant therapy because of disease progression.
DISCUSSION
Much of the IPMN literature has sought to outline the natural history of the disease and determine prognostic factors for recurrence or distant metastasis.4,6–13 Overall survival is worse for malignant disease than for adenoma6–9,13 and worse for invasive tumors when compared to in situ disease.8 Nodal metastases,6,8 vascular invasion,6 and positive margins4 have also been associated with worse outcome. In addition to assessing prognostic factors associated with a carcinoma arising within an IPMN, this series addresses the use of adjuvant therapy in these patients.
PDAC arising within an IPMN appears to have a different prognosis compared with PDAC arising from pancreatic intraepithelial neoplasia (PanIN). Unfortunately, there are little data available to address the role for adjuvant therapy in patients with PDAC arising in IPMN. Further confounding treatment decisions is the fact that an adequate stage-by-stage evaluation of invasive IPMN and classical PDAC has not yet been done. For this reason, treatment decisions frequently extrapolate from the PDAC literature where three randomized control trials have produced conflicting results.
The GITSG and EORTC trials showed or suggested a benefit to adjuvant therapy for pancreatic adenocarcinoma, while ESPAC-1 suggested a discrepant effect of chemoradiotherapy and chemotherapy.14–17 CONKO showed a benefit to adjuvant gemcitabine chemotherapy without the use of radiotherapy.18 Growing evidence suggests, however, that IPMN is associated with markedly different genetic alterations than pancreatic adenocarcinoma and therefore may represent a biologically distinct entity.2,3,5,10 Thus, the extensive clinical experience with pancreatic adenocarcinoma may imperfectly guide the management of patients with PDAC arising in IPMN.
In our study, there were a number of factors that were found to be prognostic for overall and cancer-specific survival. Higher stage, nodal involvement, elevated CA 19-9, LVI, and PNI were all associated with higher risk of death from cancer. Overall, patients with PDAC arising in IPMN in our series presented at an earlier stage than those with classic PDAC, likely reflecting the natural history of the premalignant lesion, which may grow to a large size and become readily apparent on radiography prior to dissemination. This presentation at earlier stage was also shown in a series from Johns Hopkins University, with 64% of patients presenting with stage II disease and only one of 70 patients presenting with stage III disease.19
Patients receiving adjuvant CRT or chemotherapy were more likely to have nodal involvement and higher stage disease and, therefore, a worse prognosis. On univariate analysis, adjuvant therapy was not associated with improved survival, perhaps because of the excellent 5-year survival for patients with node-negative disease. Of the patients with positive nodes, the cohort that received adjuvant therapy had a 16.5-month median cancer-specific survival advantage. While the contribution of selection bias and small numbers complicate interpretation, this result is intriguing.
Our findings of an association of adjuvant radiation with poorer prognostic factors as well as the benefit of adjuvant CRT in patients with high-risk disease was also shown in the Johns Hopkins series.19 In that series, Swartz et al showed that for patients with positive margins or node positive disease, adjuvant CRT was associated with improved survival. However, as in our series, patients who received adjuvant treatment exhibited a significant selection bias, with more stage II–III disease or node positive disease.19 It is likely that this selection bias in the both series led to the finding of no significant benefit to adjuvant CRT in the overall cohorts.
If the patients who did not receive adjuvant therapy were “sicker” or more likely to die, they still required a rapid recurrence of disease to be counted as a cancer-specific death with a median survival of 3.3 months. Indeed, rapid disease progression is the most likely reason that these patients did not receive adjuvant therapy. However, it is impossible to draw any conclusions with only 14 node-positive patients. It is still striking, though, that the node positive patients who received adjuvant therapy had almost a 2-year median survival. Whether the favorable median survival is due to therapy, a favorable genotype, or a combination is unclear. Clearly, any definitive conclusions regarding the role of adjuvant therapy are limited by the small numbers and retrospective nature of this study. Furthermore, in any retrospective study, selection bias can play a significant role in showing benefit for an intervention.
PDAC arising in IPMN appears to represent a distinct neoplastic progression to pancreatic adenocarcinoma compared to PanIN. Our series suggests that patients with PDAC arising in IPMN present with earlier stage disease (30% with node-positive disease) than in classic PDAC series, for which ∼70% of resected patients have node-positive disease.14,16–18 Node-negative patients in the current series had an excellent cancer-specific and overall survival, while patients with more advanced stage disease had a much poorer prognosis, consistent with classic PDAC. Adjuvant therapy for patients with positive nodes may provide overall and cancer-specific survival benefits in patients with PDAC arising in IPMN. Future studies of pancreatic cancer should focus on clarifying the prognosis of invasive IPMN and PDAC in a stage-by-stage analysis, as well as further prognostic stratification by histologic subtype and genotyping.
ACKNOWLEDGMENTS
Deborah McGrath for database management and patient followup.
Footnotes
Supported in part by grant CA50628 from the National Institutes of Health (AN).
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Conlon KC: Intraductal papillary mucinous tumors of the pancreas. J Clin Oncol 23:4518–4523, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, et al. : Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol 157:755–761, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore PS, Orlandini S, Zamboni G, et al. : Pancreatic tumours: molecular pathways implicated in ductal cancer are involved in ampullary but not in exocrine nonductal or endocrine tumorigenesis. Br J Cancer 84:253–262, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sohn TA, Yeo CJ, Cameron JL, et al. : Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg 239:788–797, 2004; discussion 97–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terris B, Blaveri E, Crnogorac-Jurcevic T, et al. : Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol 160:1745–1754, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC: Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg 239:400–408, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez JR, Salvia R, Crippa S, et al. : Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology 133:72–79, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salvia R, Fernandez-del Castillo C, Bassi C, et al. : Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg 239:678–685, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wada K, Kozarek RA, Traverso LW: Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg 189:632–636, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Farrell JJ, Brugge WR: Intraductal papillary mucinous tumor of the pancreas. Gastrointest Endosc 55:701–714, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Jang JY, Kim SW, Ahn YJ, et al. : Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol 12:124–132, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Nakagohri T, Konishi M, Inoue K, Tanizawa Y, Kinoshita T: Invasive carcinoma derived from intraductal papillary mucinous carcinoma of the pancreas. Hepatogastroenterology 51:1480–1483, 2004 [PubMed] [Google Scholar]
- 13. Raut CP, Cleary KR, Staerkel GA, et al. : Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol 13:582–594, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Gastrointestinal Tumor Study Group Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer 59:2006–2010, 1987 [DOI] [PubMed] [Google Scholar]
- 15. Kalser MH, Ellenberg SS: Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 120:899–903, 1985 [DOI] [PubMed] [Google Scholar]
- 16. Klinkenbijl JH, Jeekel J, Sahmoud T, et al. : Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230:776–782, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neoptolemos JP, Stocken DD, Friess H, et al. : A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200–1210, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Oettle H, Post S, Neuhaus P, et al. : Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Swartz MJ, Hsu CC, Pawlik TM, et al. Adjuvant chemoradiotherapy after pancreatic resection for invasive carcinoma associated with intraductal papillary mucinous neoplasm of the pancreas. Int J Radiat Oncol Biol Phys 76:839–844, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]