ABSTRACT
BACKGROUND:
We report the epidemiologic features and the treatment experience of advanced gastric cancer (GC) at King Hussein Cancer Center (KHCC) in Jordan, and we retrospectively compare outcomes of two different regimens: DCF (docetaxel/cisplatin/5-fluorouracil) vs. ECF (epirubicin/cisplatin/5-fluorouracil).
METHODS:
Charts of 162 patients with inoperable GC treated between January 2004 and December 2008 were reviewed. A total 143 patients received chemotherapy (ECF = 113; DCF = 30). Choice of regimen was changed from ECF to DCF on January 2008 according to KHCC guidelines.
RESULTS:
The median patient age was 59 years, with a male:female ratio of 1.8:1. Lymph nodes (67.9%) and liver (49.4%) were the most common sites of metastasis. Primary disease site was stomach in 78.4%, gastroesophageal junction in 16.7%, lower esophagus in 4.9%. Poorly differentiated histology was predominant (46.9%). Anemia (53.7%), pain (48.1%), and reflux (44.4%) were the most common presenting symptoms. Helicobacter pylori infection was present in 79%. Average time between initial symptom and diagnosis was 6.0 months. The overall response rate (ORR) was 59.3% with DCF and 32.6% with ECF (P = .01). Time to tumor progression (TTP) was 6.9 months with DCF and 5.9 months with ECF (P = .005). Median survival was 11.0 months with DCF and 10.2 months with ECF (P = .17).
CONCLUSION:
Some epidemiologic features of GC in Jordan mimic those of high-risk areas. Our outcomes of chemotherapy are comparable to internationally reported data and suggest superiority of DCF over ECF in terms of ORR and TTP.
Gastric cancer (GC) is the fourth most common cancer worldwide and the second most common cause of cancer-related death in the world (700,000 deaths annually). Almost two thirds of cases occur in developing countries, with China alone accounting for 42%. The geographic distribution of GC is characterized by a wide international variation; high-risk areas include East Asia, Eastern Europe, and parts of Central and South America. The incidence in the Middle East countries is relatively low, with rates 5–15 times lower than in Japan.1,2 Major risk factors for stomach cancer are hypothesized to be nutritional and environmental, including Helicobacter pylori (H. pylori) infection, the prevalence of which ranges from 25% in developed countries to 80–90% in developing countries.3
Advanced GC patients have a poor prognosis, with a median survival of 3–5 months if untreated.4 Although the array of chemotherapy agents available for treating GC is increasing, no consensus has emerged regarding optimal palliative chemotherapy for advanced disease. It also remains unclear whether triplet regimens are superior to doublet regimens. Webb et al demonstrated that ECF (epirubicin/cisplatin/infusional 5-fluorouracil [5-FU]) was associated with a median survival of 8–9 months, with higher response rates, tolerable toxicity, and a better quality of life (QOL) compared with FAMTX (5-FU/doxorubicin/methotrexate).5,6
Although ECF would be regarded as standard of care in the United Kingdom, this regimen has not been widely accepted in North America. Van Cutsem et al showed that DCF (docetaxel/cisplatin/5-FU) is superior to CF (cisplatin/5-FU) in terms of response rate, time to tumor progression, and median survival.7 Ajani et al showed that DCF achieved better preservation of QOL compared with CF.8,9
The epidemiology of GC and treatment outcomes are not well characterized in the Middle Eastern population. GC in Jordan constitutes 3.2% of new cancer cases; one study from Jordan showed an age-adjusted incidence of 5.82/100,000 population/year.10
The goal of our study is to evaluate the epidemiologic and the clinicopathologic features of GC in Jordan, and to report the treatment outcomes of advanced GC at a single institution, King Hussein Cancer Center (KHCC), where approximately 70% of Jordanian oncology patients are treated. Also we retrospectively compare the outcomes of two different regimens (ECF and DCF) in the treatment of nonresectable GC. The choice of chemotherapy regimen was according to KHCC clinical practice guidelines, which were changed on January 2008 from ECF to DCF as first-line therapy for advanced GC.
PATIENTS AND METHODS
Charts of patients with gastric malignancies who were seen and treated at KHCC between January 2004 and December 2008 were retrospectively reviewed. A total of 294 patients were identified, 26 of whom were excluded from analysis because of a disease histology other than adenocarcinoma. Of the remaining 268 patients, only 162 had inoperable adenocarcinoma or undifferentiated carcinoma of the lower esophagus, gastroesophageal junction (GEJ), or stomach. Data collected from those 162 cases were included in our retrospective analysis. The primary tumor was classified as inoperable on the basis of either findings on laparotomy or computed tomography (CT) scan and endoscopic results.
Data extracted from charts included patient age, gender, Eastern Cooperative Oncology Group (ECOG) performance status (PS), stage at diagnosis (locally advanced vs. metastatic), sites of metastasis, site of the primary tumor, histology, symptoms at presentation, time between the appearance of first symptoms and the diagnosis of carcinoma, H. pylori status at presentation, and the type of chemotherapy received.
Histology was reviewed in all cases at KHCC pathology laboratories and was classified as well differentiated, moderately differentiated, or poorly differentiated adenocarcinoma, or undifferentiated carcinoma. Tumors of the lower esophagus were classified as esophageal when greater than 50% extended into the esophagus, and tumors with greater than 50% of their extent in the stomach were classified as GEJ carcinomas.
Chemotherapy
At KHCC, clinical practice guidelines are established by a multidisciplinary team for each type of cancer, according to evidence, international guidelines, cost effectiveness, and experience. These guidelines are followed strictly by all practicing oncologists and updated on a yearly basis. Between January 2004 and December 2007, the ECF regimen was first-line therapy for advanced GC according to guidelines, and all patients who were candidates for therapy were offered this regimen. In January 2008, this changed to the DCF regimen, and all new candidates for therapy are now offered this regimen.
Of the 162 inoperable GC patients, 113 received the ECF regimen (between January 2004 and December 2007), 30 received the DCF regimen (between January 2008 and December 2008), and 19 received best supportive care only (no chemotherapy group).
ECF Regimen
5-FU was given as a continuous intravenous (IV) infusion at a dose of 200 mg/m2/day using a portable pump for up to 6 months. Epirubicin (50 mg/m2 IV) and cisplatin (60 mg/m2 IV infusion with standard hydration)11 were given on day 1 every 3 weeks. Antiemetic prophylaxis was given according to standard KHCC procedure.
DCF Regimen
Docetaxel (75 mg/m2 IV infusion over 1 hr) and cisplatin (75 mg/m2 IV infusion over 1–2 hr with standard hydration)11 were given on day 1, followed by 5-FU (750 mg/m2/day as continuous IV infusion for 5 days) every 3 weeks. Again, antiemetic prophylaxis was given according to standard KHCC procedure.
Treatment was continued until disease progression, unacceptable toxicity, death, consent withdrawal, or a maximum of eight cycles. Chemotherapy was administered through a central venous catheter placed in the subclavian vein.
Assessment of Response and Survival
Responses were classified according to World Health Organization criteria.12 CT scan and endoscopy were performed every 8 weeks until progression in all patients. Time to tumor progression (TTP) was measured from the day of assignment to chemotherapy to the first evidence of progression or death occurring within 12 weeks of the last tumor assessment or to the last date of follow-up if the patient did not experience any event. Survival was defined from the date of assignment to chemotherapy to death from any cause or the last follow-up date if the patient was alive. The 143 patients who received chemotherapy were included in the outcome analysis.
Statistical Methods
The comparisons in characteristics and response rate between the patients in the two treatment arms were carried out using the chi-square test or Fisher's exact test, depending on the number of cases in the cells (<5 or ≥5, respectively). Survival and time to progression was evaluated using Kaplan-Meier survival methods. Comparisons in survival and TTP were performed using the log rank test. TTP and survival were calculated on the treated population (n = 143). Patients were considered assessable for response if they received two or more chemotherapy cycles (except in cases of early progression).
Multivariate analysis using logistic regression and Cox hazards model were performed to explore the effect of gender, PS, stage, site of primary tumor, and histology on response to therapy and survival, respectively.
RESULTS
A total of 162 patients with advanced GC were identified between January 2004 and December 2008. As shown in Table 1, male to female ratio was 1.8:1 with a median age of 59 years (range, 20–76). Most patients presented with a good PS (75.3% had ECOG-PS of 0). Metastatic disease was more common at presentation, and only 35.8% had locally advanced nonresectable cancer. The most common sites of metastasis were lymph nodes (67.9%), followed by the liver (49.4%) and the peritoneum (42.6%).
Table 1.
Patient characteristics (N = 162)
| No. (%) | ||
|---|---|---|
| Gender | Female | 58 (35.8) |
| Male | 104 (64.2) | |
| Age (years) | Median (range) | 59 (20–76) |
| Performance status (ECOG) | 0 | 122 (75.3) |
| 1 | 17 (10.5) | |
| 2 | 5 (3.1) | |
| 3 | 8 (4.9) | |
| 4 | 10 (6.2) | |
| Stage at diagnosis | Locally advanced | 58 (35.8) |
| Metastatic | 104 (64.2) | |
| Site of primary tumor | Stomach | 127 (78.4) |
| Lower esophageal | 8 (4.9) | |
| GEJ | 27 (16.7) | |
| Site of metastasis | Liver | 80 (49.4) |
| Lymph nodes | 110 (67.9) | |
| Peritoneum | 69 (42.6) | |
| Ovaries | 23 (14.2) | |
| Other | 17 (10.5) | |
| Histology | Well differentiated | 37 (22.8) |
| Moderately differentiated | 39 (24.1) | |
| Poorly differentiated | 76 (46.9) | |
| Unclassified | 1 (0.6) | |
| Undifferentiated | 9 (5.6) | |
| Presenting symptom(s) | Weight loss | 56 (34.6) |
| Anorexia | 25 (15.4) | |
| Pain | 78 (48.1) | |
| Reflux | 72 (44.4) | |
| Upper GI bleeding | 17 (10.5) | |
| Anemia | 87 (53.7) | |
| Nausea/vomiting | 61 (37.7) | |
| Gastric outlet obstruction | 13 (8.0) | |
| Dysphagia | 17 (10.5) | |
| None | 7 (4.3) | |
| Presence of HP at presentation | Yes | 128 (79.0) |
| No | 13 (8.0) | |
| Not available | 21 (13.0) | |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; GEJ = gastroesophageal junction; GI = gastrointestinal; HP = Helicobacter pylori infection.
Site of the primary tumor was predominantly in the stomach (78.9%), followed by GEJ (16.7%), with only a few cases of lower esophageal origin (4.9%). Almost half of the patients had poorly differentiated adenocarcinoma histology (46.9%), and a few patients had undifferentiated or unclassified histology. Most patients tested positive for the presence of H. pylori infection in their specimens at presentation (79%).
The most common presenting symptoms, as shown in Table 1, are anemia, pain, and reflux. In addition, 34.6% had weight loss of 5% or more 3 months before presentation, and 10.5% presented with upper gastrointestinal bleeding. Few patients were asymptomatic when disease was discovered incidentally (4.3%). The median time between initial symptoms appearance and time of diagnosis was 6.0 months (range, 1–12).
Chemotherapy Characteristics
A total 113 patients (69.8%) received the ECF regimen, 30 patients (18.5%) received the DCF regimen, and 19 patients (11.7%) received best supportive care only because of poor performance status or refusal of chemotherapy. Thus, only the 143 patients who received chemotherapy were assessable for response and survival. Median number of cycles received was 6 (range, 1–8) for both ECF and DCF. As shown in Table 2, the characteristics of the treated patients were well balanced between the two groups.
Table 2.
Characteristics of treated patients (N = 143)
| Total | DCF (n = 30) No. (%) | ECF (n = 113) No. (%) | P value | ||
|---|---|---|---|---|---|
| Age (years) | Median (range) | 143 | 58 (22–75) | 59 (20–76) | .40 |
| Gender | Female | 50 | 11 (36.7%) | 39 (34.5%) | .83 |
| Male | 93 | 19 (63.3%) | 74 (65.5%) | ||
| Performance status (ECOG) | 0 | 121 | 27 (90.0%) | 94 (83.2%) | .46 |
| 1 | 17 | 3 (10.0%) | 14 (12.4%) | ||
| 2 | 5 | 5 (4.4%) | |||
| Stage at diagnosis | Locally advanced | 46 | 8 (26.7%) | 38 (33.6%) | .47 |
| Metastatic | 97 | 22 (73.3%) | 75 (66.4%) | ||
| Site of primary tumor | Stomach | 113 | 23 (76.7%) | 90 (79.6%) | .75 |
| Lower esophageal | 7 | 1 (3.3%) | 6 (5.3%) | ||
| GEJ | 23 | 6 (20.0%) | 17 (15.0%) | ||
| Histology | Well differentiated | 33 | 6 (20.0%) | 27 (23.9%) | .97 |
| Moderately differentiated | 31 | 7 (23.3%) | 24 (21.2%) | ||
| Poorly differentiated | 70 | 15 (50.0%) | 55 (48.7%) | ||
| Unclassified | 1 | 1 (0.9%) | |||
| Undifferentiated | 8 | 2 (6.7%) | 6 (5.3%) | ||
| Presence of HP at presentation | Yes | 111 | 23 (76.7%) | 88 (77.9%) | .85 |
| No | 11 | 3 (10.0%) | 8 (7.1%) | ||
| Not available | 21 | 4 (13.3%) | 17 (15.0%) | ||
| Time between first symptom(s) and diagnosis (months) | Median (range) | 143 | 6.0 (2–12) | 6.0 (1–12) | .27 |
| Number of chemotherapy cycles | Median (range) | 143 | 6 (1–8) | 6 (1–8) | .56 |
Abbreviations: DCF = docetaxel, cisplatin, and 5-fluorouracil; ECF = epirubicin, cisplatin, and 5-fluorouracil; ECOG = Eastern Cooperative Oncology Group; GEJ = gastroesophageal junction; HP = Helicobacter pylori infection.
Response
Table 3 lists the overall objective response rates (ORR). The ORR for the whole population was 38.3%, with 3.1% complete response (CR) and 35.2% partial response (PR). A significant difference in ORR (P = .01) was shown between the DCF group, where ORR = 59.3% (11.1% CR and 48.2% PR), and the ECF group with ORR = 32.6% (1% CR and 31.6% PR).
Table 3.
Overall objective response rates for treated patients
| Response | ECF (n = 113) No. (%) | DCF (n = 30) No. (%) | P value |
|---|---|---|---|
| ORR (CR + PR) | 33 (32.6) | 16 (59.3) | .01 |
| CR | 1 (1.0) | 3 (11.1) | |
| PR | 32 (31.6) | 13 (48.2) | |
| SD | 34 (33.7) | 6 (22.2) | |
| PD | 34 (33.7) | 5 (18.5) | |
| Insufficient treatment | |||
| Early death | 5 | 1 | |
| Toxicity | 4 | 1 | |
| Patient request | 3 | 1 | |
Abbreviations: CR = complete response; DCF = docetaxel, cisplatin, and 5-fluorouracil; ECF = epirubicin, cisplatin, and 5-fluorouracil; ORR = overall response rate; PD = progressive disease; PR = partial response; SD = stable disease.
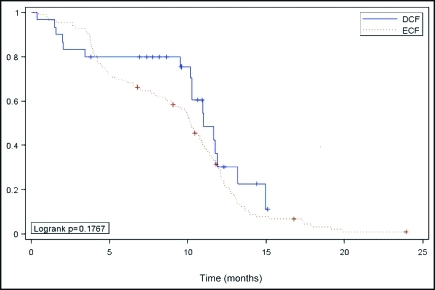
Survival data were assessed for the 143 treated patients. Follow-up evaluation was adequate, as 124 patients (87%) had died. The median survival time (MS) was 10.4 months (95% confidence interval [CI], 9.9–11.1) for the whole population. The MS was 11.0 months (95% CI, 10.3–13.2) with DCF and 10.2 months (95% CI, 9.1–10.9) with ECF (log-rank P = .17) as shown in Figure 1.
Figure 1.
Kaplan-Meier estimate of overall survival among advanced gastric cancer patients treated with DCF or ECF.
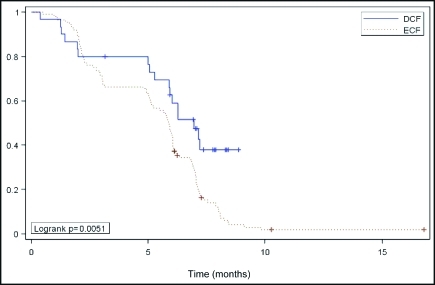
The median TTP for the whole population was 5 .9 months (95% CI, 5.6–6.1). The median TTP was significantly longer for DCF (6.9 months; 95% CI, 5.9–7.1 ) vs. ECF (5.9 months; 95% CI, 5.1–6.1; log-rank P = .0,051; Figure 2). Multivariate analysis showed that patient characteristics including gender, PS, stage, site of primary tumor, and histology have no effect on survival or response to chemotherapy.
Figure 2.
Kaplan-Meier estimate of time to tumor progression among advanced gastric cancer patients treated with DCF or ECF.
DISCUSSION
The epidemiology of GC has been widely studied in the West as well as in Japan.13–16 However, few reports from developing countries have been published.17–20 Good descriptive data on GC in Middle East countries are lacking. Our study showed that some epidemiologic features of GC in Jordan mimic those of high-risk areas but differ in others.
We observed that the male-to-female ratio was similar to that reported in the United States and Japan, but patients in Jordan were younger at presentation. This is similar to what was reported in another study from Jordan, where median age at presentation was 61.2 years.10 The median age in the United States is 70 years for men and 74 years for woman.21–23 This can probably be explained by a lower socioeconomic status, and a higher prevalence of H. pylori infection in Jordan and Middle East countries than in the United States and Western Europe.24
The association between chronic H. pylori infection and the development of GC is well established.25,26 Our study showed a 79% positivity rate for H. pylori among GC patients. In a population of Jordanian endoscopy patients, 82% prevalence was reported, but prevalence data for the general population are lacking.27 In the United States, the prevalence of H. pylori is <20% at the age of 20 years and 50% at 50 years.28
Our study showed that stomach was the most common site (78.4%) for GC, much more common than GEJ or lower esophageal tumors. In the United States, there is a rising trend in gastric cardia tumors, including lower esophageal and GEJ tumors, and a decreasing incidence of noncardia gastric tumors. This difference can also be attributed to the high prevalence of H. pylori in Jordan, as a meta-analysis of a prospective cohort studies showed that H. pylori infection was associated with the rise of noncardia, but not cardia, GC.29
Presentation of GC in Jordan and sites of metastasis are similar to other large series,30 as symptoms of anemia and indigestion appear to be paramount in making an early diagnosis. Our study showed a lower incidence of weight loss (34.6%) at presentation, compared with 57% in a study from the United States.7
Of the total number of patients with gastric adenocarcinoma (268 patients) who presented to KHCC over a 5-year period, 162 (60.5%) presented with advanced inoperable disease (21.6% locally advanced and 38.9% metastatic disease). This is similar to the western hemisphere, where 70% of patients have inoperable disease at diagnosis.31,32
Our data show that our patients are detected and treated after a relatively long delay, with 6.0 months median time between initial symptoms and diagnosis (range, 1–12). This delay is probably secondary to the difficult access to the healthcare system, and the delay in requesting the appropriate diagnostic tests by the primary care physicians. We noticed that there was a trend for a shorter delay over the last few years, as a previous study from Jordan published in the year 2004 showed an average of 10.1 months delay in diagnosis.10 No justification in favor of a possible GC screening effort in Jordan is supported by our study; rather, the need for earlier diagnosis and subsequent better care is clear.
The routine use of palliative chemotherapy in patients with advanced GC remains controversial. However, many studies suggest that chemotherapy for such patients improves the median survival from 3–4 months, with best supportive care alone, to 9–10 months.31–33 Some studies showed improved QOL and more cost effectiveness in favor of chemotherapy.33
Many regimens have been used in advanced GC, but no one regimen is considered the standard of care. Webb et al5 showed that ECF is superior to FAMTX regimen in terms of survival, response rate, and QOL measures, and it was associated with tolerable toxicity. Van Cutsem et al7 showed that DCF is superior to CF in terms of TTP, survival, and response rate, though with an expected increase in toxicities. Ajani et al8,9 showed that DCF resulted in better preservation of QOL compared with CF. Our retrospective study compared two well-established regimens in the treatment of advanced GC and showed superior TTP and overall response rate in patients receiving the DCF over patients receiving ECF. Although the difference in median survival was not statistically significant, there was a trend for better survival with the DCF regimen.
Our study did not attempt to evaluate the toxicity profile of each regimen, or the QOL measures because of its retrospective nature; however, no toxicity-related deaths were reported. To determine the preferred regimen, it will be important to evaluate toxicity, effect on QOL, and cost effectiveness prospectively in future studies in the Jordanian population. A potential drawback to the ECF regimen is the need for the prolonged use of a portable infusion pump compared to DCF (21 days vs. 5 days); this is an important issue in Jordan where pumps are expensive and not widely available.
Our study has some limitations. First, it is a retrospective study, which compares two different regimens given at two different time periods, although there was no bias in the regimen selection. Second, it included a small number of patients on the DCF regimen compared to the higher number of patients on the ECF regimen. Third, it included patients who were evaluated and treated at a single institution, which may not reflect the whole Jordanian population, despite the fact that approximately 70% of oncology patients in Jordan are managed at KHCC.
In spite of these limitations, our study does suggest the superiority of DCF over ECF, at least in the Jordanian population. This necessitates further follow-up for our patients and emphasizes the need for well-conducted prospective trials in our patient population. Such projects have already started in Jordan, including the evaluation of the burden of GC patients' care and management on their families and on the healthcare system; the comparison of survival and QOL measures between GC patients who are treated through a multidisciplinary approach and those who are not; and the evaluation of the prevalence of Her-2 expression using immuno-histochemistry and fluorescence in situ hybridization methods among Jordanian GC patients.
ACKNOWLEDGMENTS
We thank John Marshall MD from Lombardi Cancer Center at Georgetown University Hospital for his review and helpful suggestions, and Luna Zaru PhD and Dalia Al-Rimawi from the Office of Clinical Research and Cancer Registry at King Hussein Cancer Center for their help in statistical analysis.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
SA and HT wrote the protocol. AA provided additional scientific advice and guidance and reviewed the protocol. SA, AS, and AB coordinated the study. All authors reviewed and collected data from patients' charts. Data reviewed by SA, AS and HT. The report was written by SA and AB with contribution, review, and approval from all authors.
REFERENCES
- 1. Parkin DM: Global cancer statistics, 2002. CA Cancer J Clin 55:74–108, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Parkin DM, Pisani P, Ferlay J: Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer 80:827–841, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Paunder RE: The prevention of H. pylori in different countries. Aliment Pharmacol Ther 9:33–40, 1995 [PubMed] [Google Scholar]
- 4. Glimelius B, Ekstrom K, Hoffman K, et al. : Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 8:163–168, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Webb A, Cunningham D, Scarffe JH, et al. : Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15:261–267, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Ross P, Cunningham D, Scarffe H, et al. : Results of a randomized trial comparing ECF with MCF in advanced esophago-gastric cancer. Proc Am Soc Clin Oncol 18:261–272a, 1999. (abstr 1042) [Google Scholar]
- 7. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. : Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Ajani JA, Moiseyenko VM, Tjulandin S, et al. : Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol 25:3210–3216, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Ajani JA, Moiseyenko VM, Tjulandin S, et al. : Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol 25:3205–3209, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Bani-Hani KE, Yaghan RJ, Heis HA, et al. : Gastric malignancies in Northern Jordan with special emphasis on descriptive epidemiology. World J Gastroenterol 10:2174–2178, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Findlay M, Cunningham D, Norman A, et al. : A phase II study in advanced gastric cancer using epirubicin and cisplatin in combination with continuous 5-FU (ECF). Ann Oncol 5:609–616, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Miller AB, Hoogstraten B, Staquet M, et al. : Reporting results of cancer treatment. Cancer 47:207–214, 1981 [DOI] [PubMed] [Google Scholar]
- 13. Ekstrom AM, Hansson LE, Signorello LB, et al. : Decreasing incidence of both major histologic subtypes of gastric adenocarcinoma-a population-based study in Sweden. Br J Cancer 83:391–396, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaneko S, Yoshimura T: Time trend analysis of gastric cancer incidence in Japan by histological types, 1975–1989. Br J Cancer 84:400–405, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lauren PA, Nevalainen TJ: Epidemiology of intestinal and diffuse types of gastric carcinoma. A time-trend study in Finland with comparison between studies from high- and low-risk areas. Cancer 71:2926–2933, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Monferrer GR, Peiro GA, Galiana GR, et al. : Incidence of gastric cancer in the 02 health area of Castellon. An Med Int 13:68–72, 1996 [PubMed] [Google Scholar]
- 17. Johnson O, Ersumo T, Ali A: Gastric carcinoma at Tikur Anbessa Hospital, Addis Ababa. East Afr Med J 77:27–30, 2000 [PubMed] [Google Scholar]
- 18. Hamdi J, Morad NA: Gastric cancer in southern Saudi Arabia. Ann Saudi Med 14:195–197, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Al-Mofleh IA: Gastric cancer in upper gastrointestinal endoscopy population: prevalence and clinicopathological characteristics. Ann Saudi Med 12:548–551, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Al-Kayed S, Hijawi B: Cancer incidence in Jordan 1997 report. National Cancer Registry. The Hashemite Kingdom of Jordan, Amman (HKJ), Ministry of Health, 1999 [Google Scholar]
- 21. Parkin DM, Whelan SL, Ferlay J: Cancer Incidence in Five Continents, vol VII Lyon, France: International Agency for Research on Cancer, 822–823, 1997 [Google Scholar]
- 22. Yamamoto S: Stomach cancer incidence in the world. Jpn J Clin Oncol 31:548–471, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Nomura A: Stomach cancer, in Schottenfeld D: Cancer Epidemiology and Prevention, 2nd ed. New York, NY, Oxford University Press, 1996, pp 707–724 [Google Scholar]
- 24. Gilboa S, Gabay G, Zamir D, et al. : Helicobacter pylori infection in rural settlements (Kibbutzim) in Israel. Int J Epidemiol 24:232–237, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Parsonnet J, Friedman GD, Vandersteen DP, et al. : Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 325:1127–1131, 1991 [DOI] [PubMed] [Google Scholar]
- 26. EUROGAST Study Group An international association between Helicobacter pylori infection and gastric cancer. Lancet 341:1359–1362, 1993 [PubMed] [Google Scholar]
- 27. Bani-Hani KE, Hammouri SM: Prevalence of Helicobacter pylori in Northern Jordan. Endoscopy-based study. Saudi Med J 22:843–847, 2001 [PubMed] [Google Scholar]
- 28. Dooley CP, Cohen H, Fitzgibbons PL, et al. : Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med 321:1562–1566, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 49:347–353, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cassell P, Robinson JO: Cancer of the stomach: a review of 854 patients. Br J Surg 63:603–607, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Pyrhonen S, Kuitunen T, Kouri M: A randomized phase III trial comparing fluorouracil, epidoxorubicin and methotrexate (FEMTX) with best supportive care in non-resectable gastric cancer. Ann Oncol 3:603–12, 1992. (suppl 5; abstr 47) [Google Scholar]
- 32. Murad A, Santiago FF, Petroianou A, et al. : Modified therapy with 5-fluorouracil, doxorubicin and methotrexate in advanced gastric cancer. Cancer 72:37–41, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Glimelius B, Hoffman K, Graf W, et al. : Cost-effectiveness of palliative chemotherapy in advanced gastrointestinal cancer. Ann Oncol 6:267–274, 1995 [DOI] [PubMed] [Google Scholar]