Abstract
The tissue in the palatal region can be divided into the hard and the soft palates, each having a specialized function such as occlusion, speech, or swallowing. Therefore, an understanding of the mechanism of palatogenesis in relation to the function of each region is important. However, in comparison with the hard palate, there is still a lack of information about the mechanisms of soft palate development. In this study, the authors investigated the contribution of cranial neural crest (CNC) cells to development of both hard and soft palates. They also demonstrated a unique pattern of periostin expression during soft palate development, which was closely related to that of collagen type I (Col I) in palatine aponeurosis. Furthermore, organ culture analysis showed that exogenous transforming growth factor–β (TGF-β) induced the expression of both periostin and Col I. These novel patterns of expression in the extracellular matrix (ECM) induced by CNC cells suggest that these cells may help to determine the character of both the hard and soft palates through ECM induction. TGF-β signaling appears to be one of the mediators of Col I and periostin expression in the formation of functional structures during soft palate development.
Keywords: embryonic development, extracellular matrix, soft palate, periostin, TGF-β
Development of the mammalian palate is a multistep process that involves palatal shelf growth, elevation, and fusion (Chai and Maxson 2006). Cranial neural crest (CNC) cells derived from the lateral ridges of the neural plate compose the palatal mesenchyme that extends in an anterior to posterior direction during the early stages of palatogenesis (Chai et al. 2000). As CNC cells are pluripotent, they can differentiate into many cell types, such as osteoblasts, chondrocytes, or fibroblasts, thereby contributing to the development of bony and non-bony tissues in the craniofacial region.
Analyses of the molecular regulation of palatogenesis using gene mutation mice have elucidated the regional gene expression patterns in the anterior-posterior axis of the palate (Hilliard et al. 2005; Gritli-Linde 2007). Restricted expression of growth factors such as Bmp and Fgf, as well as transcription factors such as Msx1, Shox2, and Tbx22, has been demonstrated in the anterior-posterior domains of developing palatal mesenchyme (Zhang et al. 2002; Yu et al. 2005; Liu et al. 2008; Welsh and O’Brien 2009; Fuchs et al. 2010). However, the expression patterns of extracellular matrix (ECM) proteins in the anterior-posterior domain during palatogenesis have received little attention.
The mesenchyme of the craniofacial region contains two cell types originating from different lineages: myogenic cells derived from paraxial mesoderm and osteogenic or fibrogenic cells derived from neural crest cells. For the formation of skeletal muscle, reciprocal interaction between myogenic and fibrogenic cells is a critical requirement (Noden 1988). The soft palate mesenchyme also contains two types of cells to form palatine muscles and connective tissues. However, the distribution or interaction of these cells has not been focused on yet. It has been reported previously that paracrine regulation of skeletal muscle cells by neural crest cells expressing type I collagen gene (Col I) plays a very important role in tongue development (Hosokawa et al. 2010). Col I is the main structural protein of the ECM, being expressed ubiquitously in many kinds of tissue. Development of fibrous connective tissues such as tendons, ligaments, and blood vessels depends on the consistency and pattern of Col I expression. Furthermore, collagen fibrillogenesis is also regulated by a very complex process involving the expression of other collagen-associated proteins (Kadler 2004; Canty and Kadler 2005).
The expression of periostin plays an essential role in the regulation of connective tissue development (Norris et al. 2007). Periostin, originally known as osteoblast-specific factor 2, was first isolated from an osteoblastic cell line (Takeshita et al. 1993). It is a secreted 90-kDa ECM protein strongly expressed in collagen-associated tissue, periodontal ligament (PDL), periosteum, tendons, cornea, and cardiac valves (Horiuchi et al. 1999; Katsuragi et al. 2004; Litvin et al. 2006; Fortunati et al. 2010). Recently, several studies have shown that periostin in palatal mesenchyme is related to epithelial mesenchyme transformation of the medial edge epithelium during the process of palatal shelf fusion, although its relationship to fibrous connective tissues has not been discussed (Kruzynska-Frejtag et al. 2004; Kitase et al. 2011).
Members of the transforming growth factor–β (TGF-β) superfamily mediate a wide range of biological activities, including cell proliferation, differentiation, and ECM formation. Many recent studies have shown that TGF-β can induce Col I or periostin expression (Li et al. 2007; Snider et al. 2008; Norris et al. 2009; Sidhu et al. 2010; Zhou et al. 2010). In addition, important roles of TGF-β signaling during palatogenesis have been demonstrated using a variety of experimental approaches, including gene mutation mice (Ito et al. 2003; Xu et al. 2006; Iwata et al. 2011). However, it is still unclear about the role of TGF-β signaling for the expression of ECM during palatogenesis.
In the present study, we provide the evidence that periostin expresses characteristically in the soft palate mesenchyme, clearly overlapping Col I expression in palatine aponeurosis. Furthermore, we demonstrate that the possible role of TGF-β signaling regulates Col I and periostin expression during soft palate development.
Materials and Methods
Generation of Wnt1-Cre;R26R
All procedures for animal care were reviewed and approved by the Animal Experiment Committee of Fukuoka Dental College, Fukuoka, Japan. Wnt1-Cre and R26R transgenic mice were purchased from The Jackson Laboratory (Bar Harbor, ME). We crossed the Wnt1-Cre transgenic line with the R26R conditional reporter allele, which has been described previously (Danielian et al. 1998; Soriano 1999), to produce genetically labeled neural crest cells that were identifiable by their expression of β-galactosidase (Chai et al. 2000).
Cryostat Sectioning for X-Gal Staining
Mouse embryonic tissue was frozen, sectioned, and then stained according to standard procedures. Specifically, mouse tissue was dissected in PBS and fixed in a mixture of 0.2% glutaraldehyde and 5% neutral buffered formalin solution overnight at 4C. The tissue was then soaked in 15% sucrose in PBS for 2 hr at 4C, incubated in PBS plus 2 mM MgCl2 and 30% sucrose, and frozen in OCT compound. Sections 8 µm in thickness were cut and mounted on polylysine-coated slides. Thereafter, the slides were washed in PBS for 10 min and stained in X-gal staining solution overnight at 37C in the dark.
Immunofluorescence
Tissue was fixed with 10% neutral buffered formalin in PBS overnight at 4C, embedded in paraffin, serially sectioned, and mounted using standard procedures. Rabbit anti–collagen I (Abcam; Cambridge, UK), rabbit anti-periostin (Abcam), mouse anti-TGF-β2 (Abcam), or rabbit anti-TGF-β3 (Abcam) at 1:100 was used as the primary antibody. Mouse anti–collagen I (Abcam) was used for double staining with periostin. Sections were first treated with 4% normal goat serum to prevent nonspecific reactions and then incubated with each primary antibody for 1 hr at room temperature. After the sections had been washed in PBS, the immunoreaction was visualized on sections with anti-rabbit IgG antibody conjugated with Alexa Fluor 488 or 583 or with anti-mouse IgG antibody conjugated with Alexa Fluor 488 (Molecular Probes; Eugene, OR) at 1:500 for 30 min at room temperature. All tissue sections were pretreated with trypsin before immunofluorescence observation. Immunostained sections were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Labs; Burlingame, CA).
Organ Culture
Female mice with timed pregnancies were sacrificed on E14.5. The palatal shelves were dissected from the embryos under microscopic observation and organ-cultured according to external developmental characteristics. Affi-gel blue beads (Bio-Rad; Hercules, CA), diameter around 50 µm, were washed in PBS and then incubated with BSA and TGF-β2 (R&D; Minneapolis, MN) solution for 1 hr at room temperature. Each bead was implanted by fine-shaped tweezers on the left and right sides of palatal shelf mesenchyme. Palatal shelf explants were cultured for 24 hr in serum-free, chemically defined medium in accordance with standard methods (Ito et al. 2002). Five samples were prepared, fixed, embedded in paraffin, and serially sectioned. One section, including both BSA and TGF-β2 beads, was chosen from every sample, and hematoxylin and eosin (H&E) staining was performed. Immunohistochemical analysis with Col I and periostin antibodies was performed using an adjacent section of H&E staining.
Results
Histological Appearance of Hard and Soft Palate Formation
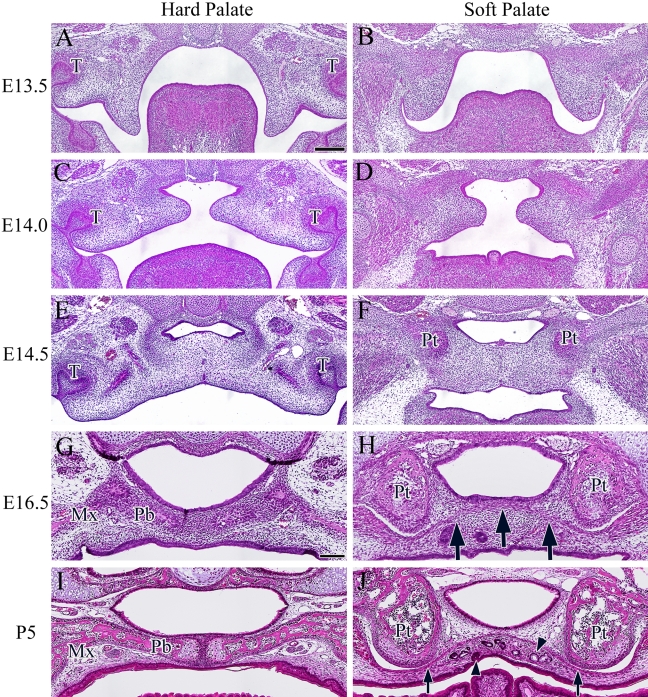
Dynamic morphological changes in the palatal shelves started from E13.5 in both hard and soft palates. Hard palate sections were chosen to include maxillary tooth germs and the maxillary bone. Soft palate sections were chosen to include the pterygoid process. At E13.5, the palatal shelves were evident on both sides of the tongue (Fig. 1A,B). Around E14.0, the palatal shelves were located above the tongue and became elongated horizontally to meet each other on the midline (Fig. 1C,D). At E14.5, the bone matrix of the maxilla in the hard palate and the sphenoid bone of the soft palate were each detectable, representing the characteristic components of each palate. Disappearance of the medial edge epithelium (MEE) started at the midline (Fig. 1E,F). At E16.5, palatine aponeurosis started to form in the soft palate and was clearly evident as a layer of aggregated cells between the medial pterygoid processes of the sphenoid bone (Fig. 1H, arrow). At P5, the ECM was clearly deposited around the aggregated cell layer of palatine aponeurosis (Fig. 1J, arrow). On the oral side of the soft palatal mesenchyme, palatine glands and taste buds were evident (Fig. 1J, arrowhead). These H&E staining observations showed that palatal mesenchymal cells in the hard and soft palates presented no histological differences until E14.5. However, after E14.5, each of the osteogenic and fibrogenic components of the hard and soft palates was clearly identifiable.
Figure 1.
Histological appearance of the hard and soft palates revealed by hematoxylin and eosin (H&E) staining during development at E13.5 (A and B), E14 (C and D), E14.5 (E and F), E16.5 (G and H), and P5 (I and J). Arrow indicates the aggregated cell layer. Arrowhead indicates the salivary glands or taste bud in the soft palate. T, tooth germ; Mx, maxillary bone; Pb, palatal process of palatine bone; Pt, pterygoid process. Bar = 100 µm.
CNC Cells Are Major Players in the Organization of Mesenchyme in Both Hard and Soft Palates
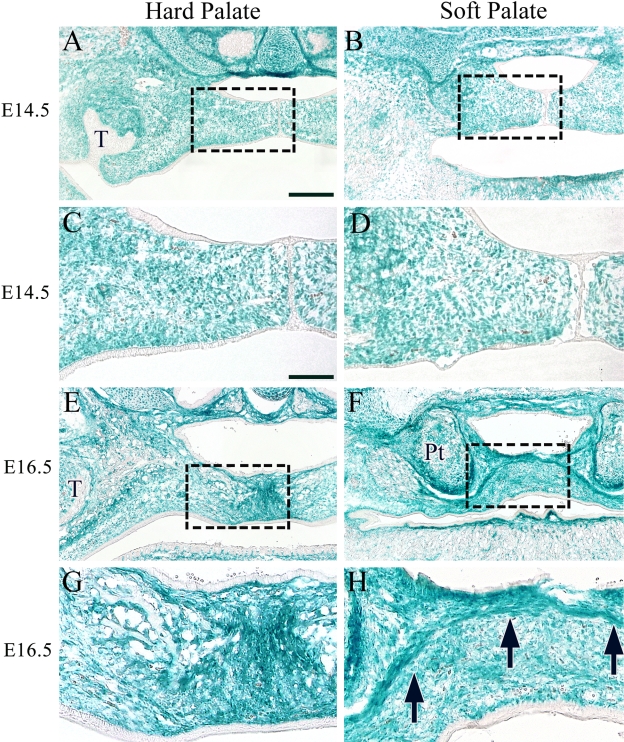
To confirm the distribution of CNC cells during soft palate development, we performed X-gal staining of specimens from Wnt1-cre;R26R mice. After sectioning and staining at E14.5, we found that most of the palatal mesenchyme of the hard and soft palates was composed primarily of CNC-derived cells (Fig. 2A–D). In addition, we verified that the maxillary and palatine bones of the hard palate, as well as the connective tissue and palatine aponeurosis of the soft palate at E16.5, were also mostly composed of CNC cells (Fig. 2E–H, arrow). These observations suggested that the several distinctive features of hard and soft palates such as bone and palatine aponeurosis are attributable to the differentiation of CNC cells in developing palatal mesenchyme.
Figure 2.
The contribution of cranial neural crest cells to the hard and soft palates of Wnt1-Cre;R26R mice. X-gal staining at E14.5 (A–D) and E16.5 (E–H). Higher magnifications of boxed areas in A, B, E, and F are shown in C, D, G, and H, respectively. Arrow indicates the aggregated cell layer. T, tooth germ; Pt, pterygoid process. Bar = 50 µm.
Distribution of Type I Collagen Deposition in the Hard and Soft Palates
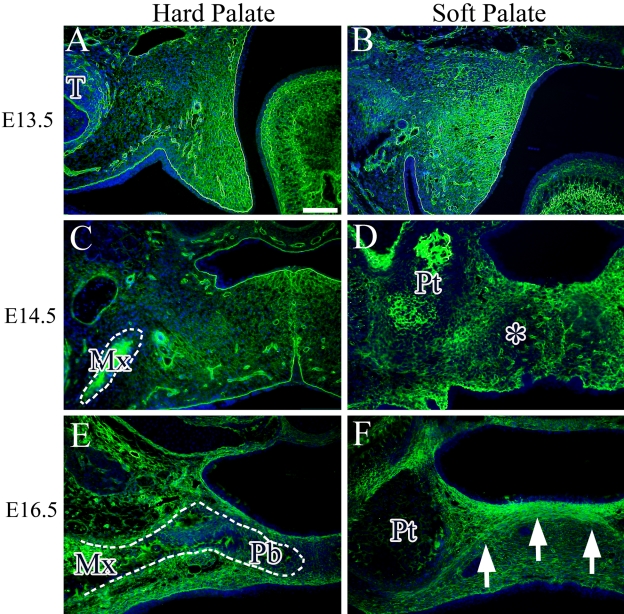
To observe ECM expression in the hard and soft palates, we performed immunohistochemical analysis of Col I, which is a major component of mesenchymal tissue. At E13.5, abundant expression of Col I was observed in the palatal mesenchyme, including the basal membrane and blood vessel wall (Fig. 3A,B). At E14.5, Col I was also expressed widely in the hard and soft palates and also showed strong expression in the bone matrix of the maxilla and the area of the pterygoid process. In the soft palate, faint Col I expression was detected in the midline region (Fig. 3D, asterisk). At E16.5, Col I expression was also observed in bone and connective tissue of the hard palate (Fig. 3E). In the soft palate, the area of palatine aponeurosis showed a high intensity of Col I expression (Fig. 3F, arrow).
Figure 3.
Immunohistochemical expression of Col I (green) in the hard and soft palates at E13.5 (A and B), E14.5 (C and D), and E16.5 (E and F). Cell nuclei were stained with DAPI (blue). Asterisk indicates faint expression of Col I in the soft palate. Arrow indicates the aggregated cell layer. Dotted line indicates the maxillary bone (C) and palatal process of palatine bone (E). T, tooth germ; Mx, maxillary bone; Pb, palatal process of palatine bone; Pt, pterygoid process. Bar = 50 µm.
Distribution of Periostin Deposition in the Hard and Soft Palates
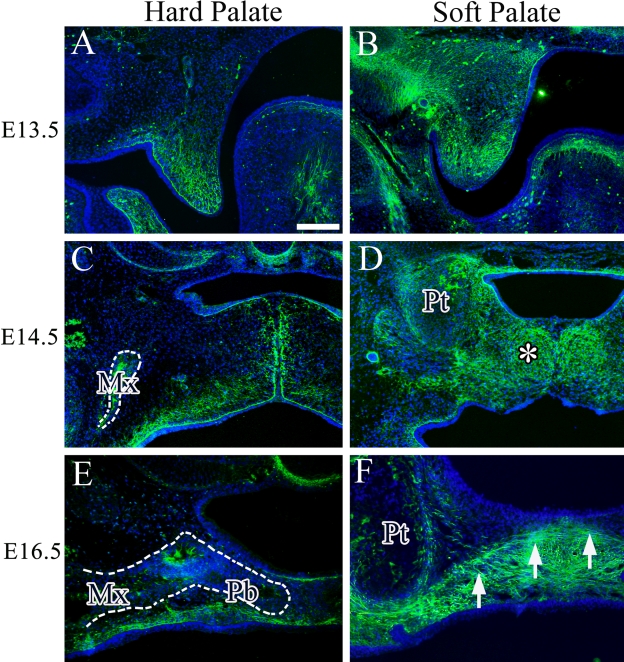
We then studied the expression of periostin in the hard and soft palates during palatal development. At E13.5, there was a clear difference in the intensity and range of periostin expression between the hard and soft palates. Periostin was weakly expressed in the mesenchyme and part of the basal membrane in the hard palate at E13.5, and this expression became gradually stronger on the presumptive oral aspect side in comparison with the nasal aspect side (Fig. 4A). At E14.5, the expression pattern of periostin in the hard palate also became stronger gradually on the oral side, and periostin expression was also seen in the bone matrix of the maxilla (Fig. 4C). At E16.5, periostin expression had almost disappeared in the hard palate mesenchyme (Fig. 4E), whereas in the soft palate, the range and intensity of periostin expression were greater than in the hard palate at each stage (Fig. 4B,D,F). At E14.5, almost entire mesenchymal tissue in the soft palate expressed periostin (Fig. 4D, asterisk). Interestingly, periostin was strongly expressed on the oral side from the boundary line of palatine aponeurosis at E16.5 (Fig. 4F, arrow).
Figure 4.
Immunohistochemical expression of periostin (green) in the hard and soft palates at E13.5 (A and B), E14.5 (C and D), and E16.5 (E and F). Cell nuclei were stained with DAPI (blue). Asterisk indicates strong expression of periostin in the soft palate. Arrow indicates the aggregated cell layer. Dotted line indicates the maxillary bone (C) and palatal process of palatine bone (E). Mx, maxillary bone; Pb, palatal process of palatine bone; Pt, pterygoid process. Bar = 50 µm.
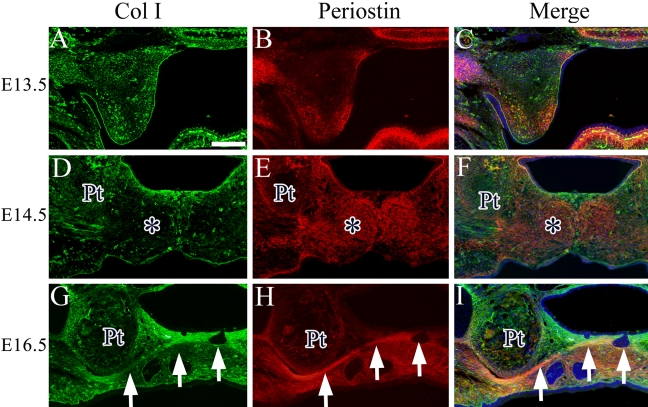
Type I Collagen and Periostin Are Co-expressed in Palatine Aponeurosis of the Soft Palate
In view of the unique expression of periostin in the soft palate, we next investigated whether possible colocalization of Col I and periostin is related to any particular type of structure during soft palate development. We performed immunohistochemical analysis using antibodies against Col I and periostin and found a region that was stained a yellowish color where Col I and periostin were colocalized. At E13.5, periostin and Col I were partially colocalized in the tip of the mesenchyme of soft palate (Fig. 5A–C). At E14.5, the difference in the expression pattern of Col I and periostin became clearer. In the middle of the soft palate mesenchyme, mainly periostin, but not Col I, was expressed (Fig. 5D–F, asterisk). At E16.5, colocalization of Col I and periostin was observed only in palatine aponeurosis layer, and their expressions were separated on the nasal and oral sides from palatine aponeurosis (Fig. 5G–I, arrow).
Figure 5.
Colocalization of Col I (green) and periostin (red) in the soft palate at E13.5 (A–C), E14.5 (D–F), and E16.5 (G–I). Cell nuclei were stained with DAPI (blue). Asterisk indicates the opposing expression region of Col I and periostin in the soft palate. Arrow indicates the aggregated cell layer. Pt, pterygoid process. Bar = 50 µm.
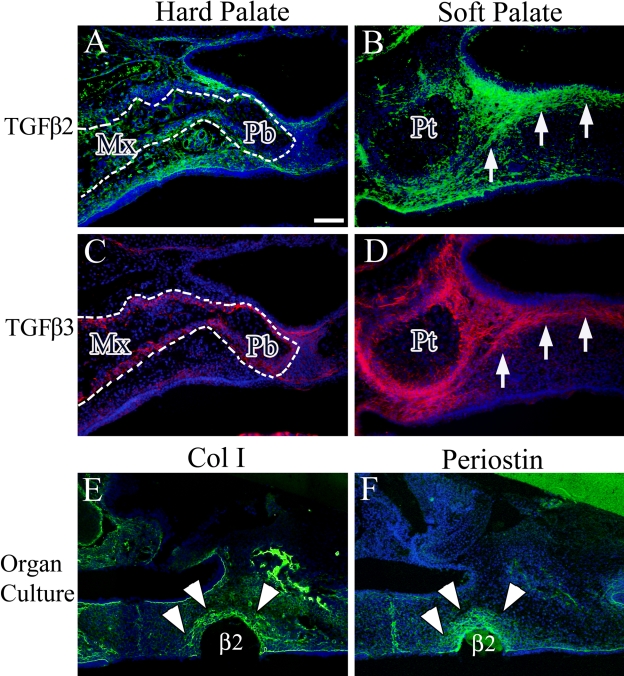
Exogenous TGF-β Protein Induces the Expression of Both Periostin and Col I
We then tried to find the inducer of Col I and periostin expression in the palatal mesenchyme. Several studies have shown that TGF-β induces the expression of Col I and periostin (Teekakirikul et al. 2010; Wen et al. 2010) and that this growth factor plays an important role in palatogenesis (Iwata et al. 2011). Therefore, immunohistochemical analysis was performed to clarify the expression patterns of TGF-β2 and -β3 in the soft palate at E16.5. TGF-β2 was expressed in the maxillary and palatine bones and connective tissue of the hard palate (Fig. 6A), as well as in the palatine aponeurosis region of the soft palate (Fig. 6B, arrow). TGF-β3 was expressed in perimaxillary and palatine bones and also weakly in connective tissue of the hard palate (Fig. 6C). The expression pattern of TGF-β3 in the soft palate was similar to that of TGF-β2 (Fig. 6B,D, arrow). We then examined the effect of exogenous TGF-β in inducing expression of both Col I and periostin in the palatal mesenchyme using an organ culture experiment. All sections of organ-cultured samples were stained by H&E to confirm that the experiment succeeded (data not shown). This revealed induction of Col I and periostin expression around the TGF-β2-soaked beads (Fig. 6E,F, arrowhead). Around the BSA-soaked beads, we detected the endogenous expression of Col I and periostin (data not shown).
Figure 6.
Immunohistochemical expression of transforming growth factor (TGF)–β2 (A and B) and TGF-β3 (C and D) in the hard and soft palates at E16.5. (E) Exogenous TGF-β2 induces Col I expression around the implanted beads. (F) Exogenous TGF-β2 induces periostin expression around the implanted beads. Cell nuclei were stained with DAPI (blue). Arrow indicates the aggregated cell layer. Arrowhead indicates region of protein expression around the beads. Dotted line indicates the maxillary and palatal process of palatine bone. Mx, maxillary bone; Pb, palatal process of palatine bone; Pt, pterygoid process; β2, TGF-β2-soaked beads. Bar = 50 µm.
Discussion
Many genes have been investigated for their roles in palatogenesis. Recently, a unique expression pattern of genes in the anterior-posterior axis of the palatal shelf has been demonstrated (Hilliard et al. 2005; Gritli-Linde 2007). However, ECM-related genes have received little attention. We hypothesized that regional ECM expression patterns might be present in the anterior-posterior domains during palatogenesis, thus contributing to the formation of the bony hard palate and non-bony soft palate composed of CNC cells.
In the craniofacial region, mesenchymal cells are derived from two types of cell lineage: the paraxial mesoderm and CNC. CNC cells play an important role in ECM deposition during skeletal muscle development in the craniofacial region. It has been shown previously that neural crest cells have a role in tongue development, where in the early stage, most of the mesenchyme cells are CNC-derived cells, and the mesoderm-derived skeletal muscle cells migrate later into the CNC-populated tongue mesenchyme. These two cell populations cooperate to form skeletal muscle tissue (Hosokawa et al. 2010), and thus ECM expression induced by CNC cells is a critical requirement for skeletal muscle tissue. It is also well known that CNC can differentiate into several cell types, such as osteoblasts, chondrocytes, or fibroblasts. Therefore, it is generally considered that most structures in the hard palate are composed of CNC cells. In the soft palate region, however, two different cell lineages make up the mesenchyme structure: Mesoderm-derived cells differentiate into palatine muscles such as the tensor and levator veli palatini and compose the skeletal muscle structures along with connective tissues derived from CNC cells.
In this study, we demonstrated the contribution of CNC cells to palatogenesis using Wnt1-Cre;R26R mice. CNC cells were found to be the major components of the palatal mesenchyme, not only in the hard palate but also in the soft palate during the early stage of palatogenesis. The soft palate is made up of the palatal muscles, palatine aponeurosis, and connective tissue. Therefore, we hypothesized that CNC cells would express a specific ECM protein in the posterior domain of the palatal shelves to ensure functional organization of the soft palate.
Periostin has been shown to affect the migration and differentiation of various cells (Inai et al. 2008; Norris et al. 2009). Our observations of periostin expression in the soft palate have suggested that periostin has a critical role in soft palate development. At E13.5 and E14.5, periostin was expressed at the tip of the posterior palatal shelf, where the cells are highly proliferative (data not shown). Periostin was thus investigated as an inducer of cell proliferation in the soft palatal mesenchyme. However, at E16.5, few proliferative cells were evident in palatine aponeurosis where periostin was expressed. In this case, periostin expression obviously overlapped that of Col I in palatine aponeurosis. This suggested that CNC cells stopped proliferating in the region where Col I and periostin were colocalized at the boundary of expression of each protein and then induced further expression of periostin and Col I to form palatine aponeurosis. It has been reported that periostin is able to bind directly to Col I and promotes fibril cross-linking (Katsuragi et al. 2004; Suzuki et al. 2004; Kii et al. 2010). Colocalization of these two proteins may have a critical role in the formation of palatine aponeurosis, which becomes an important structure for palatine muscle formation, because not only is it the insertion of the tensor and levator veli palatini muscles, but it also forms the boundary of the nasal and oral sides of the soft palate.
During palatogenesis, TGF-β signaling is involved in the appropriate growth of the palatal shelves and disappearance of the midline epithelial seam (Xu et al. 2006; Iwata et al. 2011). Several reports have indicated that TGF-β signaling is required for the proliferation of CNC cells (Ito et al. 2003; Iwata et al. 2010). Loeys-Dietz syndrome caused by TGFBR1 and TGFBR2 mutation is characterized by craniofacial anomalies, including bifid uvula in addition to other connective tissue defects such as joint laxity (Loeys et al. 2005). The human phenotype of the TGFBR mutation suggests that TGF-β signaling has a critical function in the development of the soft palate. We observed that TGF-β2 and -β3 were expressed in palatine aponeurosis. These results suggested that TGF-β signaling regulated palatine aponeurosis formation through Col I and periostin expression. The TGF-β2 antibody that we used detects the latent form of TGF-β2, but it is unknown about active-form detection. The TGF-β3 antibody has been confirmed to detect both the latent and active forms of TGF-β3. Because the present study had the possibility of detecting both latent and active forms of TGF-βs, TGF-βs might be stored as the latent form, not inducing Col I and periostin expression in palatine aponeurosis. To address this question, we performed the organ culture experiment and showed that exogenous TGF-β protein was able to induce Col I and periostin expressions in the palatal mesenchyme. In organ culture experiments, we selected the TGF-β2 protein because the gene expression pattern of TGF-β2 or the phenotype of TGF-β2 and TGF-β3 mutant mice suggested that TGF-β2 was functional in mesenchyme tissue (Fitzpatrick et al. 1990; Sanford et al. 1997). TGF-β3 has been shown to play an important role in the disappearance of the medial edge epithelium during the process of fusion (Kaartinen et al. 1995; Taya et al. 1999). However, further investigations of the biological function of each ligand of TGF-β during palatogenesis will be needed.
In conclusion, our results suggest that the expression pattern of ECM components such as Col I and periostin in CNC cells is related to differentiation of the hard and soft palates along the anterior-posterior axis during palatogenesis and that TGF-β signaling may be a key factor regulating the expression of these two proteins.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology [KAKENHI (19890156 and 20791581) to KO] and the Advanced Science Research Center, Fukuoka Dental College [C12 to KO].
References
- Canty EG, Kadler KE. 2005. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 118:1341–1353 [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. 2000. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 127:1671–1679 [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr 2006. Recent advances in craniofacial morphogenesis. Dev Dyn. 235:2353–2375 [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. 1998. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 8:1323–1326 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DR, Denhez F, Kondaiah P, Akhurst RJ. 1990. Differential expression of TGF beta isoforms in murine palatogenesis. Development. 109:585–595 [DOI] [PubMed] [Google Scholar]
- Fortunati D, Reppe S, Fjeldheim AK, Nielsen M, Gautvik VT, Gautvik KM. 2010. Periostin is a collagen associated bone matrix protein regulated by parathyroid hormone. Matrix Biol. 29:594–601 [DOI] [PubMed] [Google Scholar]
- Fuchs A, Inthal A, Herrmann D, Cheng S, Nakatomi M, Peters H, Neubuser A. 2010. Regulation of Tbx22 during facial and palatal development. Dev Dyn. 239:2860–2874 [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A. 2007. Molecular control of secondary palate development. Dev Biol. 301:309–326 [DOI] [PubMed] [Google Scholar]
- Hilliard SA, Yu L, Gu S, Zhang Z, Chen YP. 2005. Regional regulation of palatal growth and patterning along the anterior- posterior axis in mice. J Anat. 207:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. 1999. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 14:1239–1249 [DOI] [PubMed] [Google Scholar]
- Hosokawa R, Oka K, Yamaza T, Iwata J, Urata M, Xu X, Bringas P, Jr, Nonaka K, Chai Y. 2010. TGF-beta mediated FGF10 signaling in cranial neural crest cells controls development of myogenic progenitor cells through tissue-tissue interactions during tongue morphogenesis. Dev Biol. 341:186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inai K, Norris RA, Hoffman S, Markwald RR, Sugi Y. 2008. BMP-2 induces cell migration and periostin expression during atrioventricular valvulogenesis. Dev Biol. 315:383–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Bringas P, Jr, Mogharei A, Zhao J, Deng C, Chai Y. 2002. Receptor-regulated and inhibitory Smads are critical in regulating transforming growth factor beta-mediated Meckel’s cartilage development. Dev Dyn. 224:69–78 [DOI] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. 2003. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 130:5269–5280 [DOI] [PubMed] [Google Scholar]
- Iwata J, Hosokawa R, Sanchez-Lara PA, Urata M, Slavkin H, Chai Y. 2010. Transforming growth factor–beta regulates basal transcriptional regulatory machinery to control cell proliferation and differentiation in cranial neural crest-derived osteoprogenitor cells. J Biol Chem. 285:4975–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata J, Parada C, Chai Y. 2011. The mechanism of TGF-beta signaling during palate development. Oral Dis. 17:733–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. 1995. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 11:415–421 [DOI] [PubMed] [Google Scholar]
- Kadler K. 2004. Matrix loading: assembly of extracellular matrix collagen fibrils during embryogenesis. Birth Defects Res C Embryo Today. 72:1–11 [DOI] [PubMed] [Google Scholar]
- Katsuragi N, Morishita R, Nakamura N, Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T, Sugimura K. 2004. Periostin as a novel factor responsible for ventricular dilation. Circulation. 110:1806–1813 [DOI] [PubMed] [Google Scholar]
- Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A. 2010. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem. 285:2028–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitase Y, Yamashiro K, Fu K, Richman JM, Shuler CF. 2011. Spatiotemporal localization of periostin and its potential role in epithelial-mesenchymal transition during palatal fusion. Cells Tissues Organs. 193:53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S, Markwald RR, Conway SJ. 2004. Periostin is expressed within the developing teeth at the sites of epithelial-mesenchymal interaction. Dev Dyn. 229:857–868 [DOI] [PubMed] [Google Scholar]
- Li P, Oparil S, Novak L, Cao X, Shi W, Lucas J, Chen YF. 2007. ANP signaling inhibits TGF-beta-induced Smad2 and Smad3 nuclear translocation and extracellular matrix expression in rat pulmonary arterial smooth muscle cells. J Appl Physiol. 102: 390–398 [DOI] [PubMed] [Google Scholar]
- Litvin J, Blagg A, Mu A, Matiwala S, Montgomery M, Berretta R, Houser S, Margulies K. 2006. Periostin and periostin-like factor in the human heart: possible therapeutic targets. Cardiovasc Pathol. 15:24–32 [DOI] [PubMed] [Google Scholar]
- Liu W, Lan Y, Pauws E, Meester-Smoor MA, Stanier P, Zwarthoff EC, Jiang R. 2008. The Mn1 transcription factor acts upstream of Tbx22 and preferentially regulates posterior palate growth in mice. Development. 135:3959–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, et al. 2005. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 37:275–281 [DOI] [PubMed] [Google Scholar]
- Noden DM. 1988. Interactions and fates of avian craniofacial mesenchyme. Development. 103(Suppl):121–140 [DOI] [PubMed] [Google Scholar]
- Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, et al. 2007. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 101:695–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Potts JD, Yost MJ, Junor L, Brooks T, Tan H, Hoffman S, Hart MM, Kern MJ, Damon B, et al. 2009. Periostin promotes a fibroblastic lineage pathway in atrioventricular valve progenitor cells. Dev Dyn. 238:1052–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. 1997. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 124:2659–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. 2010. Roles of epithelial cell–derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 107:14170–14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, et al. 2008. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 102:752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 21:70–71 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Amizuka N, Kii I, Kawano Y, Nozawa-Inoue K, Suzuki A, Yoshie H, Kudo A, Maeda T. 2004. Immunohistochemical localization of periostin in tooth and its surrounding tissues in mouse mandibles during development. Anat Rec A Discov Mol Cell Evol Biol. 281:1264–127 [DOI] [PubMed] [Google Scholar]
- Takeshita S, Kikuno R, Tezuka K, Amann E. 1993. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 294:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya Y, O’Kane S, Ferguson MW. 1999. Pathogenesis of cleft palate in TGF-beta3 knockout mice. Development. 126:3869–3879 [DOI] [PubMed] [Google Scholar]
- Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, et al. 2010. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest. 120:3520–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh IC, O’Brien TP. 2009. Signaling integration in the rugae growth zone directs sequential SHH signaling center formation during the rostral outgrowth of the palate. Dev Biol. 336:53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Chau E, Jackson-Boeters L, Elliott C, Daley TD, Hamilton DW. 2010. TGF-ss1 and FAK regulate periostin expression in PDL fibroblasts. J Dent Res. 89:1439–1443 [DOI] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P, Jr, Urata MM, Chai Y. 2006. Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Dev Biol. 297:238–248 [DOI] [PubMed] [Google Scholar]
- Yu L, Gu S, Alappat S, Song Y, Yan M, Zhang X, Zhang G, Jiang Y, Zhang Z, Zhang Y, et al. 2005. Shox2-deficient mice exhibit a rare type of incomplete clefting of the secondary palate. Development. 132:4397–4406 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. 2002. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 129:4135–4146 [DOI] [PubMed] [Google Scholar]
- Zhou HM, Wang J, Elliott C, Wen W, Hamilton DW, Conway SJ. 2010. Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal. 4:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]