Abstract
The debate endures as to whether schizophrenia and bipolar disorder are separate entities or different manifestations of a single underlying pathological process. Here, we argue that this sterile argument obscures the fact that the truth lies somewhere in between. Thus, recent studies support a model whereby, on a background of some shared genetic liability for both disorders, patients with schizophrenia have been subject to additional genetic and/or environmental factors that impair neurodevelopment; for example, copy number variants and obstetric complications are associated with schizophrenia but not with bipolar disorder. As a result, children destined to develop schizophrenia show an excess of neuromotor delays and cognitive difficulties while those who later develop bipolar disorder perform at least as well as the general population. In keeping with this model, cognitive impairments and brain structural abnormalities are present at first onset of schizophrenia but not in the early stages of bipolar disorder. However, with repeated episodes of illness, cognitive and brain structural abnormalities accumulate in both schizophrenia and bipolar disorder, thus clouding the picture.
Keywords: schizophrenia, bipolar disorder, environmental factors, neurodevelopment
More than a century after Kraepelin’s distinction between dementia praecox (schizophrenia) and manic depression (bipolar disorder), the major classification systems continue to categorize them separately. However, clinicians frequently encounter patients who do not fall neatly into either category. Furthermore, antipsychotic drugs, once thought only to be useful in schizophrenia, are also effective in bipolar disorder, which is not surprising given that dopamine dysregulation is implicated in both disorders. Conversely, mood stabilizers are useful in treating certain schizophrenic patients, and similarly, antidepressants have a role in treatment of negative symptoms in schizophrenia.
In this article, we will argue that recent studies support a model 1,2 whereby, on a background of some shared genetic liability for both disorders, patients with schizophrenia have been subject to additional genetic and environmental risk factors that impair neurodevelopment.
Changing Genetic Perspectives
Over the last decade, the traditional view that schizophrenia and bipolar disorder are genetically distinct has been undermined. Thus, applying a nonhierarchical approach to diagnosis in Maudsley twin series, Cardno et al3 showed that 8.2% of the monozygotic (MZ) co-twins of probands with Research Diagnostic Criteria schizophrenia had met criteria for mania (8.2%), while 13.6% of the MZ co-twins of manic probands had met criteria for schizophrenia.
Family studies have also suggested genetic overlap between schizophrenia and bipolar disorder. Mortensen et al4 demonstrated that the risk of bipolar disorder was increased in those individuals whose parents or siblings had schizophrenia. Furthermore, a large Swedish family study showed increased risk for both schizophrenia and bipolar disorder in first-degree relatives of patients with either disorder.5 These findings were confirmed in a meta-analysis of family studies by van Snellenberg and Candia6 and by Dean et al7 who reported that individuals with a parental history of nonaffective psychosis have an increased risk of bipolar disorder and those with parental affective psychosis have an increased risk of schizophrenia. Thus, the coaggregation of the 2 disorders in MZ twin pairs and families defies the notion of distinct genetic disorders.
Genome-wide association studies (GWAS) indicate that a large number of genes exerting small individual effects are involved in the transmission of schizophrenia and bipolar disorder, and although there are inconsistencies, some genes have been implicated in both disorders. The International Schizophrenia Consortium8 which identified the most promising risk alleles from a large GWAS of schizophrenia, showed that these were found with excess frequency in bipolar disorder. Other studies comparing schizophrenia and bipolar GWAS datasets have demonstrated an overlap in susceptibility genes across both disorders.
The above might be taken to imply that the genetic contribution to schizophrenia and bipolar disorder is largely in common. However, the most recent genetic evidence suggests that there may yet be some life in the Kraepelinian distinction. Copy number variants (CNVs) are found in excess in schizophrenia and some appear to be in common between schizophrenia, autism, ADHD, and epilepsy. By contrast, CNVs are not found in excess in bipolar disorder.
Epidemiological and Environmental Similarites and Differences
Both schizophrenia and bipolar disorder have a typical onset between adolescence and mid twenties. Males become ill earlier than females by around 2.5 years in schizophrenia; a similar gender difference is also present for the first episode of mania. A male excess has often been reported in schizophrenia while most studies of bipolar patients have reported no sex difference in incidence.
The families of bipolar patients tend to be of a higher socioeconomic status than the general population, although we suspect that this finding may be confounded by general intelligence. Some studies have found an association between social deprivation at birth and schizophrenia.
One of the environmental factors that differentiate best between the disorders is urban upbringing, which has strongly been associated with the risk of schizophrenia, but not bipolar disorder.9 Further, studies focusing on the effect of neighborhood variation in city areas have demonstrated much more variation in rates of schizophrenia.
Migrants are at increased risk for schizophrenia. A meta-analysis estimated the risk for bipolar disorder in migrants also to be increased10 but this conclusion was based on only 5 studies, and the excess was attributable to African Caribbean migrants to the United Kingdom. When the contribution of the latter group was removed, the risk of bipolar disorder was no longer significantly increased in migrants. Subsequent individual studies including those from our group have reported increased rates of bipolar disorder among black and other minority ethnic groups, however, risk remains stronger for schizophrenia.
Obstetric events have been frequently reported to increase risk of schizophrenia; such factors do not seem to predispose to bipolar disorder. An excess of winter/spring births has consistently been reported in schizophrenia. While studies have observed associations of winter birth with bipolar disorder, most have not replicated this.
Recently, rekindled interest in the effects of early childhood trauma on developing psychosis led to observations that child abuse is highly prevalent in both. Particularly, strong association was observed between childhood trauma and auditory hallucinations in both disorders.
Both disorders are associated with cigarette and illicit drug use, particularly cannabis use, but the evidence is much stronger for schizophrenia. Furthermore, cannabis use is associated with earlier age of onset in both disorders.
Advanced parental age at conception is commoner than expected in schizophrenia. The fact that autism is also associated with increased paternal age and that both it and schizophrenia show an excess of CNVs has raised the possibility that such mutations may arise during the repeated mitosis in the progenitor sperm cells as men age. However, contrary to this, new evidence suggests that this may be an effect of older age at marriage rather than older age of having children, which can not be explained by de novo mutations in paternal germ cells (see Petersen et al11). Research concerning the relationship between paternal age and the risk of bipolar disorder is sparse.
Finally, individuals with schizophrenia have drastically reduced reproductive output. Bipolar disorder, however, is associated with normal reproductive success.
Neuroanatomical Commonalities and Specificities
Patients With Established Illness
People with established schizophrenia show brain structural abnormalities. Hallahan et al12 performed a collaborative mega-analysis of studies of bipolar disorder analyzing the individual volumetric ratings from 321 patients with bipolar disorder and 442 healthy subjects; the patients had increased right lateral ventricle volume, confirming previous findings. But this was to a lesser extent than in schizophrenia and the laterality difference was opposite to that in schizophrenia, where ventriculomegaly is bilateral but more prominent on the left.
Schizophrenia is additionally associated with extensive gray matter reductions. In bipolar disorder, generalized gray matter reductions have not been consistently reported. The hippocampus and amygdala are reduced in those with established schizophrenia. Adult patients with bipolar disorder do not show decrements in the volume of the hippocampus. Reports regarding amygdala volume in bipolar disorder have been inconsistent, but a recent meta-analysis found no change.13
Brain structural abnormalities are more severe in chronic than first episode schizophrenia. In their mega-analysis, Hallahan et al12 reported that cerebral volume was reduced significantly with increasing illness duration. Other studies also demonstrated reduced total gray matter volume with illness duration in bipolar disorder.
Patients With First Onset Illness
While lateral ventricular enlargement is present at first onset of schizophrenia, there is no convincing evidence that this is the case for bipolar disorder. Gray matter abnormalities are also evident in first-episode schizophrenia. A meta-analysis of magnetic resonance imaging studies conducted on first-episode patients with bipolar disorder demonstrated that the total gray matter volume appears to be unaffected at this stage.14
In summary, patients with established schizophrenia and also bipolar disorder show generalized brain structural abnormalities, to a much greater extent in the former than in the latter. At first episode, patients with schizophrenia but not those with bipolar disorder, already show some generalized abnormalities and decrements in the hippocampus.
There remains debate about the causes of the abnormalities. Some factors appear to operate before the onset of disorder; for example, hippocampal volume decrement is particularly marked in those schizophrenic patients who have been exposed to obstetric complications. However, further changes accumulate with length of illness. Stress-related increases in cortisol, cannabis and alcohol use, heavy smoking, and antipsychotics have all been proposed to contribute to these changes.
Cognitive Profiles
Patients With Established Illness
Patients with established schizophrenia exhibit lower IQ, memory and language impairment, and poor executive functioning when compared with controls. Deficits are also reported in those with established bipolar disorder but these tend to be milder. Consequently, Krabbendam et al15 showed that schizophrenia patients consistently performed more poorly than bipolar patients.
Patients With First Episode of Illness
Cognitive deficits are present in first onset schizophrenia while such deficits are either nonexistent or mild in first episode of bipolar disorder. Zanelli et al16 confirmed that those with schizophrenia demonstrated pervasive cognitive deficits, while those with bipolar disorder showed few; in particular, the schizophrenic patients showed premorbid cognitive deficits, which were absent in bipolar patients.
Prior to Illness Onset
Birth cohort studies consistently indicate that individuals with schizophrenia have premorbid cognitive impairments. These deficits appear absent or even reversed in children who will later develop bipolar disorder. Cannon and her colleagues17 reported that preschizophreniform children showed deficits in cognitive function and neuromotor development but children who went on to develop mania by 26 years performed significantly better than controls on motor performance. A subsequent analysis of data from the 32-year follow-up showed that higher childhood IQ predicted risk of later mania.18
Both Israeli and Swedish cohorts have shown no differences in childhood cognition between premanics and controls. Our group19 used the National Swedish registers to examine school performance in adolescents who later developed schizophrenia and bipolar disorder. The poorer the performance at school, the greater was the risk of schizophrenia. In contrast, children with excellent school performance showed increased risk of bipolar disorder. Furthermore, these results persisted after adjusting for socioeconomic group.
It is remarkable that high IQ and good school performance predict bipolar disorder. One possibility is that the genes, which increase susceptibility to mania, are also associated with good intellectual performance or even creativity. Another possibility is that certain genes predict liability to a continuum of serious mental illness but that those who are additionally neurodevelopmentally compromised express schizophrenic symptoms, while their cognitively unimpaired counterparts present a bipolar picture.
The divergence in premorbid functioning also extends to social functioning. While those destined to develop schizophrenia show abnormalities in premorbid social adjustment, Rietschel et al20 showed that 344 bipolar patients had better childhood social adjustment than 137 healthy controls.
Dimensions and Development
Some have advocated the replacement of the Kraepelinian diagnostic categories by symptom dimensions. Our group has examined clinical correlates of such dimensions in 2 different samples.21,22 In each of these studies, factor analysis produced 5 factors (mania, reality distortion, negative symptoms, depression, and disorganization). In the first study,21 the strongest correlations were between negative symptoms and poor premorbid functioning and insidious onset and poor outcome; in the second,22 the strongest association was between negative symptoms and soft neurological signs. In each case, we interpreted the correlations as suggesting that negative symptoms index neurodevelopmentally compromised individuals.
Conclusion
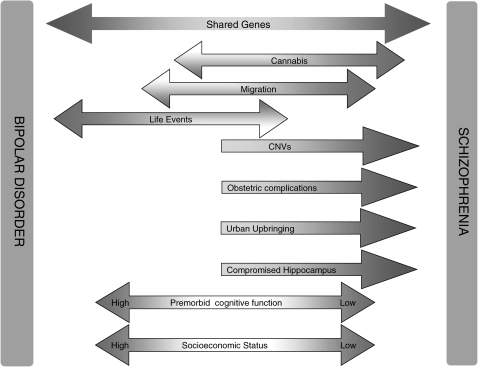
There exist both continuities and discontinuities between schizophrenia and bipolar disorder. Any model that aims to successfully represent psychosis needs to incorporate both of these (figure 1). The evidence we have reviewed suggests the following:
Fig. 1.
Diagram summarizing main differences and similarities between bipolar disorder and schizophrenia
The Effects of Illness
Following first diagnosis of either illness, individuals tend to accumulate cognitive deficits and brain structural abnormalities, particularly if the illness is recurrent or chronic; these deficits may be consequent upon illness or associated factors such as substance misuse, a sedentary lifestyle and antipsychotics. Consequently, in comparing the etiology of the 2 illnesses, it is important to concentrate on the picture at or before first onset of the disorders rather than that which evolves thereafter.
Genes in Common
Genetic studies indicate that a number of risk alleles of small effect predispose an individual to develop a functional psychosis and that a number of these genes are common to schizophrenia and bipolar disorder. This overlap is presumably the reason why schizophrenia and bipolar illness share certain pathophysiological similarities, have overlapping symptoms and a similar age of onset, and why symptoms of both illnesses benefit from dopamine blockade.
Schizophrenia but Not Bipolar Disorder is Associated With Neurodevelopmental Abnormality
At first onset of illness, many schizophrenia patients show neuroanatomical changes and cognitive deficits, which are absent in bipolar disorder. Diagnostic specific genetic and environmental risk factors must determine these differences. The excess of CNVs in schizophrenia and the lack of such an excess in bipolar disorder further supports our contention that the main difference between the 2 conditions is the additional neurodevelopmental component in schizophrenia. Such a view is consistent with the evidence that a proportion of what is inherited in schizophrenia is impaired intellect.
The impact of CNVs or risk alleles of genes involved in neurodevelopment results in the subtle neuromotor, cognitive, and behavioral impairment, which are seen in many preschizophrenic children. Similar deviations in neurodevelopment may occur following early environmental insults, such as hypoxic damage to the hippocampus. Then when exposed to further environmental stresses in adolescence or early adult life, there may be hypothalamic-pituitary-adrenal overactivity and the resultant raised cortisol induces further damage to the hippocampus. Such a “second hit” to the developmentally compromised hippocampus has been shown by Grace and colleagues23 to lead to dopamine dysregulation in their animal model of schizophrenia.
Two decades ago, we suggested that psychosis might be profitably divided into those with and without neurodevelopmental or congenital schizophrenia.24 More recently, Kaymaz and Van Os2 have suggested that “psychotic disorders appear to be originating from overlapping areas of risk, one more developmental associated with cognitive impairment and one more associated with affective dysregulation”. It is now clear that those who carry a major CNV or were exposed to severe hypoxia at birth fall into the former category. They are likely to show other indicators of neurodevelopmental impairment as demonstrated by Leask et al25 who found that indicators of neurodevelopmental risk were especially common in schizophrenia without affective symptoms.
The model we have outlined suggests several areas for further research. It is vital that the research effort in bipolar disorder is increased, particularly in areas where evidence is rich for schizophrenia but is scanty or absent for bipolar disorder. Further research is needed to clarify how genetic and environmental risk factors alter the neurodevelopmental trajectory of the individual, including the study of gene-gene and gene-environment interactions, and the epigenetic mechanisms by which environments may influence gene expression. The nature of the neurodevelopmental impairment that predisposes to schizophrenia, including its timing and its relationship to cognitive function, also requires further research. Finally, the association between enhanced cognitive function and predisposition to bipolar disorder requires explanation. Such research should improve our understanding of the etiology of both disorders and enable us to refine our classification systems using models that take etiological and biological factors into account.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
All our statements are referenced, but owing to publishing constraints it has not been possible to include all references in the text. Please refer to the supplementary material on line for access to additional references.
Acknowledgments
We acknowledge NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and (Institute of Psychiatry) King's College London. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Murray RM, Sham P, Van OJ, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;18:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Kaymaz N, Van Os J. Murray et-al. (2004) revisited: is bipolar disorder identical to schizophrenia without developmental impairment? Acta Psychiatr Scand. 2009;120:249–252. doi: 10.1111/j.1600-0447.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- 3.Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P. A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry. 2002;159:539–545. doi: 10.1176/appi.ajp.159.4.539. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen PB, Pedersen CB, Melbye M, Mors O, Ewald H. Individual and familial risk factors for bipolar affective disorders in Denmark. Arch Gen Psychiatry. 2003;60:1209–1215. doi: 10.1001/archpsyc.60.12.1209. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2009;66:748–755. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- 7.Dean K, Stevens H, Mortensen PB, Murray RM, Walsh E, Pedersen CB. Full spectrum of psychiatric outcomes among offspring with parental history of mental disorder. Arch Gen Psychiatry. 2010;67:822–829. doi: 10.1001/archgenpsychiatry.2010.86. [DOI] [PubMed] [Google Scholar]
- 8.International Schizophrenia Consortium. Purcell SM, Wray NR, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen CB, Mortensen PB. Urbanicity during upbringing and bipolar affective disorders in Denmark. Bipolar Disord. 2006;8:242–247. doi: 10.1111/j.1399-5618.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 10.Swinnen SG, Selten JP. Mood disorders and migration: meta-analysis. Br J Psychiatry. 2007;190:6–10. doi: 10.1192/bjp.bp.105.020800. [DOI] [PubMed] [Google Scholar]
- 11.Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. Am J Psychiatry. 2011;168:82–88. doi: 10.1176/appi.ajp.2010.10020252. [DOI] [PubMed] [Google Scholar]
- 12.Hallahan B, Newell J, Soares JC, et al. Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry. 2011;69:326–335. doi: 10.1016/j.biopsych.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Hajek T, Kopecek M, Kozeny J, Gunde E, Alda M, Höschl C. Amygdala volumes in mood disorders—Meta-analysis of magnetic resonance volumetry studies. J Affect Disord. 2009;115:395–410. doi: 10.1016/j.jad.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Vita A, De Peri L, Sacchetti E. Gray matter, white matter, brain, and intracranial volumes in first-episode bipolar disorder: a meta-analysis of magnetic resonance imaging studies. Bipolar Disord. 2009;11:807–814. doi: 10.1111/j.1399-5618.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 15.Krabbendam L, Arts B, Van OJ, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80:137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Zanelli J, Reichenberg A, Morgan K, et al. Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am J Psychiatry. 2010;167:78–85. doi: 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]
- 17.Cannon M, Caspi A, Moffitt TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. [see comment] Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 18.Koenen KC, Moffitt TE, Roberts AL, et al. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 2009;166:50–57. doi: 10.1176/appi.ajp.2008.08030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacCabe JH, Lambe MP, Cnattingius S, et al. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychol Med. 2008;38:1133–1140. doi: 10.1017/S0033291707002048. [DOI] [PubMed] [Google Scholar]
- 20.Rietschel M, Georgi A, Schmael C, et al. Premorbid adjustment: a phenotype highlighting a distinction rather than an overlap between schizophrenia and bipolar disorder. Schizophr Res. 2009;110:33–39. doi: 10.1016/j.schres.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Dikeos DG, Wickham H, McDonald C, et al. Distribution of symptom dimensions across Kraepelinian divisions. Br J Psychiatry. 2006;189:346–353. doi: 10.1192/bjp.bp.105.017251. [DOI] [PubMed] [Google Scholar]
- 22.Demjaha A, Morgan K, Morgan C, et al. Combining dimensional and categorical representation of psychosis: the way forward for DSM-V and ICD-11? Psychol Med. 2009;39:1943–1955. doi: 10.1017/S0033291709990651. [DOI] [PubMed] [Google Scholar]
- 23.Lodge DJ, Grace AA. Developmental pathology, dopamine, stress and schizophrenia. Int J Dev Neurosci. 2011;29:207–213. doi: 10.1016/j.ijdevneu.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray RM, O'Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319–332. doi: 10.1093/schbul/18.2.319. [DOI] [PubMed] [Google Scholar]
- 25.Leask SJ, Vermunt JK, Done DJ, Crow TJ, Blows M, Boks MP. Beyond symptom dimensions: schizophrenia risk factors for patient groups derived by latent class analysis. Schizophr Res. 2009;115:346–350. doi: 10.1016/j.schres.2009.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.