Abstract
Background:
Abnormal connectivity of the anticorrelated intrinsic networks, the task-negative network (TNN), and the task-positive network (TPN) is implicated in schizophrenia. Comparisons between schizophrenic patients and their unaffected siblings enable further understanding of illness susceptibility and pathophysiology. We examined the resting-state connectivity differences in the intrinsic networks between schizophrenic patients, their unaffected siblings, and healthy controls.
Methods:
Resting-state functional magnetic resonance images were obtained from 25 individuals in each subject group. The posterior cingulate cortex/precuneus and right dorsolateral prefrontal cortex were used as seed regions to identify the TNN and TPN through functional connectivity analysis. Interregional connectivity strengths were analyzed using overlapped intrinsic networks composed of regions common to all subject groups.
Results:
Schizophrenic patients and their unaffected siblings showed increased connectivity in the TNN between the bilateral inferior temporal gyri. By contrast, schizophrenic patients alone demonstrated increased connectivity between the posterior cingulate cortex/precuneus and left inferior temporal gyrus and between the ventral medial prefrontal cortex and right lateral parietal cortex in the TNN. Schizophrenic patients exhibited increased connectivity between the left dorsolateral prefrontal cortex and right inferior frontal gyrus in the TPN relative to their unaffected siblings, though this trend only approached statistical significance in comparison to healthy controls.
Conclusion:
Resting-state hyperconnectivity of the intrinsic networks may disrupt network coordination and thereby contribute to the pathophysiology of schizophrenia. Similar, though milder, hyperconnectivity of the TNN in unaffected siblings of schizophrenic patients may contribute to the identification of schizophrenia endophenotypes and ultimately to the determination of schizophrenia risk genes.
Keywords: schizophrenia, unaffected sibling, default mode network, functional connectivity, resting-state
Introduction
The pathophysiology of schizophrenia is believed to involve abnormal activity and connectivity of distributed brain networks.1,2 Recently, 2 anticorrelated intrinsic networks, the task-negative network (TNN) and task-positive network (TPN), have been implicated in schizophrenia.3–5 The TNN, also called the default mode network, is most active in the resting-state human brain and is deactivated by goal-directed tasks.6–8 The TNN is relevant to internally generated stimulus-independent thoughts, self-monitoring, and baseline monitoring of the external world.7–9 The TPN, by contrast, is most active during the performance of goal-directed tasks that require focused attention and supports active exploration of the external world.3,4,10 The TNN and TPN are functionally complementary and reciprocally competitive such that TNN activity corresponds to TPN attenuation and vice versa.8,10 Proper communication and coordination between these 2 intrinsic anticorrelated networks is thought to be crucial for optimal information integration and cognitive functioning.10 Finally, these networks are evident not only during task performance but also in resting-state functional connectivity analysis, which examines the temporal correlation of spontaneous fluctuations of the blood oxygen level–dependent (BOLD) signal.10,11
Given the relevance of the intrinsic network functions to schizophrenia, there has been increasing interest in the role that altered connectivity of these networks may play in the illness.3,4 For example, it has been proposed that deficits in self-monitoring, a function of the TNN, may be implicated in many of the positive symptoms of schizophrenia.12 The TNN is involved in differentiating between internal and external sources of information, and abnormalities of this network may contribute to the generation of hallucinations.13 Indeed, neuroimaging studies of patients with active hallucinations have demonstrated activity in regions of the TNN including the orbitofrontal cortex and the cingulate cortex.14 Cognitive deficits, such as impaired working memory, attention allocation, and central executive function, are not only core symptoms of schizophrenia15 but also are functions ascribed to the TPN.16,17 Finally, numerous studies in schizophrenia have identified abnormalities in the regions that make up both the TNN and the TPN.18–20 Our early studies using BOLD signal analysis detected abundant connectivity and activity abnormalities in schizophrenic patients, with widespread decreased connectivity as the most consistent finding.18,21,22 When we focused our analysis on the intrinsic networks, however, we found increased connectivity in the TNN, both increased and decreased connectivity in the TPN, and increased anticorrelation between the TNN and the TPN.5 These findings suggest that examining the connectivity and coordination of both the anticorrelated intrinsic networks may improve our understanding of their roles in schizophrenia susceptibility and pathophysiology.
Strong evidence for a genetic contribution to schizophrenia includes concordance rates of 41–65% in identical twin pairs and heritability estimates of 80–85%,23 although the precise genes involved remain unknown. It is hypothesized that the identification of endophenotypes, or biological markers, that are more proximal to the genes than the illness itself will contribute to elucidating the genes involved in schizophrenia risk.24 Unaffected siblings of schizophrenic patients share a genetic background with schizophrenic patients and have an approximately 8-fold higher risk to develop schizophrenia than the general population.25 Moreover, relatives of schizophrenic patients exhibit a pattern of brain abnormalities and cognitive deficits that is similar to, but milder than, that of schizophrenic patients.26–31 Because unaffected siblings are comparatively free of confounding factors, such as antipsychotic medications, substance abuse, and institutionalization, that can complicate findings from schizophrenic patients, data from unaffected siblings may both elucidate illness endophenotypes and provide insights into the pathophysiology of schizophrenia.
In the present study, we sought to examine the resting-state functional connectivity differences in the anticorrelated intrinsic networks between schizophrenic patients, their unaffected siblings, and healthy controls. Using our previously published methods,5 we identified the intrinsic networks and compared their interregional connectivity between the 3 subject groups. We hypothesized that schizophrenic patients would demonstrate increased connectivity in the intrinsic networks and that similar, but less pronounced, hyperconnectivity would be evident in the intrinsic networks of unaffected siblings of schizophrenic patients.
Materials and Methods
Participants
Twenty-five schizophrenic patients were recruited from the Department of Psychiatry, Second Xiangya Hospital of Central South University, Changsha, China. All the patients were evaluated by the Structured Clinical Interview for DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition), Patient version (SCID-I/P)32 and met the DSM-IV diagnostic criteria for schizophrenia. The patients had no history of neurological disorder, severe medical disorder, substance abuse, or electroconvulsive therapy. In addition, each patient had at least one unaffected sibling. In the schizophrenia sample, 6 of the patients were drug naive, while the remainder were receiving antipsychotic medications at the time of image acquisition (risperidone [n = 10, 2–6 mg/day], clozapine [n = 4, 200–350 mg/day], quetiapine [n = 4, 400–600 mg/day], and sulpiride [n = 1, 200 mg/day]).
Twenty-five unaffected siblings of the schizophrenic patients were recruited such that each patient had a sibling in the present study. The inclusion and exclusion criteria were the same as those for the patients except that the siblings did not meet the DSM-IV criteria for any Axis-I psychiatric disorders. Twenty-five healthy controls were recruited from the Changsha City area. The inclusion and exclusion criteria were the same as those for the siblings except that the controls had no first-degree relatives with a history of psychiatric disorders. The schizophrenic patients, their unaffected siblings, and the healthy controls were well matched for sex, age, and education (table 1).
Table 1.
Demographic and Clinical Profiles of the Schizophrenic Patients, Unaffected Siblings, and Healthy Controls (Mean ± SD)
| Characteristics | Schizophrenic Patients (n = 25) | Unaffected Siblings (n = 25) | Healthy Controls (n = 25) | |
| Age (y) | 25.36 ± 6.32 | 25.56 ± 6.78 | 25.48 ± 5.45 | |
| Education (y) | 12.28 ± 2.57 | 12.48 ± 2.52 | 13.68 ± 2.85 | |
| Sex (male/female) | 13/12 | 15/10 | 14/11 | |
| Duration of illness (mo) | 18.32 ± 15.84 | — | — | |
| PANSS | Total Score | 87.24 ± 12.23 | — | — |
| Positive Scale Score | 21.92 ± 4.74 | — | — | |
| Negative Scale Score | 23.36 ± 5.7 | — | — | |
| General Psychopathology Scale Score | 41.96 ± 6.39 | — | — | |
Note: PANSS: Positive and Negative Syndrome Scale.
All participants gave their written informed consent to participate in the study after the risks and benefits were discussed in detail. The study was approved by the ethics committee of the Second Xiangya Hospital, Central South University.
Instruction to Participants
Before scanning, the participants were explicitly instructed to lie supine, stay relaxed with their eyes closed, and move as little as possible.
Image Acquisition
Images were acquired on a 1.5-T GE Signa Twinspeed scanner (General Electric Medical System, Milwaukee, Wisconsin). A standard head coil was used for radio frequency transmission and reception of magnetic resonance signal. Foam pads and earplugs were used to minimize head motion and scanner noise, respectively. Functional images were acquired by using a gradient-echo echo-planar imaging sequence sensitive to BOLD signal (repetition time [TR]/echo time [TE] = 2000/40 ms, flip angle = 90°, field of view = 24 × 24 cm2). Whole-brain volumes were acquired with 20 contiguous 5-mm-thick transverse slices with a 1 mm gap and 3.75 × 3.75 mm2 in-plane resolution. For each participant, the functional magnetic resonance imaging (fMRI) scanning lasted for 6 min. T1-weighted images (TR/TE = 2045/9.6 ms, flip angle = 90°) were acquired at the same location as the functional images in order to acquire anatomical information.
Image Preprocessing
The SPM2 software (Wellcome Department of Imaging Neuroscience, London, UK) was used for image preprocessing. The first 10 volumes of each functional time series were discarded for scanner calibration and for participants to get used to the circumstances. The remaining 170 volumes were corrected for the acquisition delay between slices and for head motion. All the images of each participant met the following 2 conditions: (1) maximum displacement in x, y, or z was less than 2 mm and (2) angular rotation about each axis was less than 2°. Because correlation analysis is sensitive to gross head motion effects, we further characterized the peak displacements as a measure of head motion for each participant.33 No significant difference in the peak displacements was found among the 3 groups (ANOVA, P > 0.05). Further preprocessing included spatial normalization, resampling to 3 × 3 × 3 mm3, and spatial smoothing (full width at half-maximum = 4 × 4 × 4 mm3). To reduce the effects of confounders, 6 motion parameters, linear drift, and the mean time series of all voxels in the whole brain were removed from the functional data through linear regression. Then the functional data were band-pass filtered (0.01–0.08 Hz) using AFNI software (http://afni.nimh.nih.gov/).10,33
Identification of the Anticorrelated Intrinsic Networks
We identified the intrinsic networks (TPN and TNN) according to our previous methods5 and as described below.
Definition of the Initial Seed Regions.
The WFU_PickAtlas software (http://www.ansir.wfubmc.edu)34 was used to generate the initial seed regions necessary to constitute the intrinsic networks. The R.DLPFC refers to Broadmann area 46 (BA46) in the right middle frontal gyrus, and the PCC/PCu refers to BA31 in the bilateral posterior cingulate cortices and the adjacent precuneus. These 2 regions are important nodes in the TPN and TNN, respectively, and have been observed to play vital roles in resting-state brain function.10,35 We have previously used the functional connectivities of these 2 regions to identify the intrinsic networks.5 In this study, we averaged the BOLD time series of the voxels within each seed region to obtain the reference time series for the seed region.
Correlation Maps of the Initial Seed Regions.
For each participant and each initial seed region, we computed the correlation coefficients between the seed region's time series and the time series of each voxel in the brain. The correlation coefficients were converted to z values using Fisher's r-to-z transform in order to improve the normality. One-sample t test was performed in a voxel-wise manner on the individual z values data to determine which brain regions were significantly correlated with the seed region. The false discovery rate (FDR)33 procedure was used to control the expected proportion of false positives at q = 0.05. This correction method has been introduced in detail in SPM2 (http://www.fil.ion.ucl.ac.uk/spm) and has been widely used in resting-state functional connectivity analysis based on individual seed region.5,11,36 A minimum cluster size of 30 voxels was set for the identified brain regions, and the positive and negative correlation maps of each initial seed region were obtained for each group.
Construction of the Intrinsic Networks.
The ImCalc toolbox of the SPM2 was used to construct the intrinsic networks for the patient, sibling, and control groups. For each group, the composition of the TNN was obtained by intersecting the brain regions significantly positively correlated to the PCC/PCu with those significantly negatively correlated to the right DLPFC. Similarly, the composition of the TPN was obtained by intersecting the regions significantly positively correlated to the right DLPFC with those significantly negatively correlated to the PCC/PCu. By creating a binary mask for each subject group in which each voxel value was set to 1 if the voxel belonged to the intrinsic networks and set to 0 if it did not, a patient mask, a sibling mask, and a control mask were generated separately. These 3 masks were then intersected to obtain the overlapped mask. The combination of the overlapped method and the conservative threshold (P < 0.05, FDR corrected) used in generating the initial correlation maps (see “Correlation Maps of the Initial Seed Regions” section) makes it possible to avoid clusters that might represent large or heterogeneous areas, a method we have used in a previous study.36
Interregional Functional Connectivity Analyses in the Intrinsic Networks.
The separate clusters of the overlapped network were used as seed regions for the interregional functional connectivity analysis. For each group, the mean time series of each seed region was obtained by averaging the BOLD time series over all the voxels in the seed region (table 2). Pearson's correlation coefficients were computed between each pair of these seed regions. After Fisher's r-to-z transform, individual z values were entered into the following analysis.
Table 2.
The Regions of the Overlapped Intrinsic Networks Across the 3 Groups
| Index | Region | Broadmann Area | Cluster Size (Number of Voxels) |
| Task-positive network | |||
| 1 | L.DLPFC | 9/10/46 | 299 |
| 2 | R.DLPFC | 9/10/46 | 249 |
| 3 | L.IFG/L.Ins | 44/45/13/47 | 259 |
| 4 | R.IFG/R.Ins | 44/45/13/47 | 392 |
| 5 | L.IPL | 40/2 | 523 |
| 6 | R.IPL | 40/2 | 531 |
| 7 | R.dPM | 6 | 16 |
| 8 | SMA | 8/6 | 52 |
| 9 | R.OFC | 11 | 17 |
| 10 | R.MT | 21 | 6 |
| Task-negative network | |||
| 11 | PCC/PCu | 23/31/7 | 363 |
| 12 | vMPFC | 10/11 | 178 |
| 13 | L.dMPFC | 8/9/10 | 211 |
| 14 | R.dMPFC | 9/10 | 33 |
| 15 | R.LPC | 39/40 | 18 |
| 16 | L.ITG | 20/21 | 82 |
| 17 | R.ITG | 20/21 | 76 |
Note: L, left; R, right; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; Ins, insula; IPL, inferior parietal lobule; dPM, dorsal premotor area; SMA, supplementary motor area; OFC, orbital frontal cortex; MT, middle temporal region; PCC, posterior cingulated cortex; PCu, precuneus; d/vMPFC, dorsal/ventral medial prefrontal cortex; LPC, lateral parietal cortex; ITG, inferior temporal gyrus.
The connectivity among the 3 groups was considered to be significantly different if: (1) the z values of this connectivity were significantly different from zero in at least 1 group by 1-sample t test (P < 0.05, FDR corrected) and (2) the 3 groups showed significantly different z values of this connectivity by ANOVA (P < 0.05, corrected). We used a permutation-based correction for multiple comparisons using Ptest software by 10 000 permutations.37 Similar to the traditional multiple-testing corrections, such as Bonferroni corrections, the permutation test resamples n times the total number of observations, in a population sample, to build an empirical estimate of the null distribution from which the test statistic has been drawn.38 To date, permutation tests have become widely accepted and recommended in studies that involved multiple statistical testing.37,39,40 This method was used to avoid false positives when examining differences in the strength of interregional connectivity, which is particularly important in resting-state studies.41 Post hoc (least significant difference) tests (P < 0.05, 2 tailed, corrected by permutation tests implemented by Ptest software) were used to examine the differences in the connectivities identified by ANOVA. Additionally, we compared each connectivity within the intrinsic networks between groups by a 2-sample t test (healthy controls vs unaffected siblings or schizophrenic patients) and a paired t test (unaffected siblings vs schizophrenic patients).
Results
Identification of the Intrinsic Networks
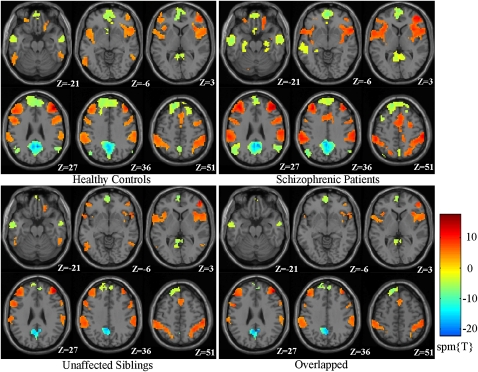
By using functional connectivity analysis and an overlap strategy, we identified the anticorrelated intrinsic networks in the 3-subject groups (table 2; Figure 1). The regions of the TNN included the PCC/PCu, bilateral medial prefrontal cortex (MPFC), bilateral inferior temporal gyri (ITG), and right lateral parietal cortex (LPC). The regions of the TPN included the DLPFC, inferior frontal gyrus/insula (IFG), inferior parietal lobule (IPL), and supplementary motor area (SMA) bilaterally and the dorsal premotor cortex (dPM), orbital frontal cortex (OFC), and middle temporal (MT) region on the right side of the brain only.
Fig. 1.
Intrinsic Networks for Each Subject Group and the Overlapped Intrinsic Network Among the 3 Groups. The red colors represent the regions of the task-positive network, while the light blue-yellow colors represent the regions of the task-negative network.
Connectivity Differences of Intrinsic Networks Among the 3 Groups
Among the 3 groups, connectivity differences were found between the PCC/PCu and the left ITG (F = 7.077, Pcorrected = 0.002), the right LPC and the ventral MPFC (vMPFC) (F = 3.508, Pcorrected = 0.034), the left and right ITG (F = 5.031, Pcorrected = 0.008), and the left DLPFC and right IFG (F = 3.264, Pcorrected = 0.044) by ANOVA (Pcorrected < 0.05). Post hoc tests for these connectivities showed significantly higher connectivity between the PCC/PCu and left ITG and between the vMPFC and the right LPC in the schizophrenic patients compared with unaffected siblings and the healthy controls. We also found significantly higher connectivity between the bilateral ITG in both the schizophrenic patients and the unaffected siblings compared with the healthy controls. Finally, significantly higher connectivity between the left DLPFC and IFG was found in the schizophrenic patients compared with the unaffected siblings (P < 0.05, 2 tailed; table 3), while this difference only approached statistical significance in the comparison between schizophrenic patients and healthy controls (P = 0.115, 2 tailed; table 3). Further comparison by 2-sample t tests and paired t tests of all the connectivities in the intrinsic networks validated the above findings because the ANOVA results constituted a subset of the t test results (see online supplementary material for tables S1–S3).
Table 3.
Differences Between Subject Groups in Connectivity Strength Within the Intrinsic Networks
| Connectivity | HC: z Value | SIB: z Value | SCZ: z Value | P Value (Post Hoc Tests, Corrected) | |||
| Region 1 | Region 2 | Mean ± SD | Mean ± SD | Mean ± SD | HC vs SIB | HC vs SCZ | SIB vs SCZ |
| Difference of correlation strength in the task-positive network | |||||||
| L.DLPFC | R.IFG/R.Ins | 0.36 ± 0.21 | 0.31 ± 0.22 | 0.47 ± 0.24 | 0.355 | 0.115 | 0.027 |
| Difference of correlation strength in the task-negative network | |||||||
| L.ITG | PCC/PCu | 0.31 ± 0.32 | 0.34 ± 0.24 | 0.57 ± 0.24 | 0.701 | 0.003 | <0.001 |
| R.LPC | vMPFC | 0.30 ± 0.25 | 0.31 ± 0.30 | 0.48 ± 0.24 | 0.948 | 0.012 | 0.032 |
| L.ITG | R.ITG | 0.46 ± 0.29 | 0.68 ± 0.32 | 0.69 ± 0.27 | 0.007 | 0.007 | 0.890 |
| Difference of anticorrelation strength between the 2 networks | |||||||
| None | |||||||
Note: Abbreviations are explained in the footnote to table 2. HC, healthy controls; SIB, unaffected siblings; SCZ, schizophrenic patients.
Discussion
Abnormal activity and connectivity in the intrinsic networks, the TNN and TPN, is increasingly hypothesized to play a role in schizophrenia pathophysiology.3–5,42–48 To our knowledge, there are few studies comparing the connectivity of the TNN and TPN among schizophrenic patients, their unaffected siblings, and healthy controls. In contrast to the only other published study of similar subject groups, which examined activity and connectivity differences of the default mode network (the TNN) among schizophrenia patients, their first-degree relatives, and healthy controls,45 our study comprehensively investigated the functional connectivity within the TNN and the TPN, as well as the anticorrelation between these 2 networks. In the TNN, schizophrenic patients demonstrated hyperconnectivity between the PCC/PCu and left ITG and between the vMPFC and the right LPC, while both schizophrenic patients and their unaffected siblings exhibited hyperconnectivity between the bilateral ITG. In the TPN, schizophrenic patients showed hyperconnectivity between the left DLPFC and right IFG in comparison to unaffected siblings, although this abnormality only trended toward significance relative to healthy controls. An advantage of our methodology is that we examined connectivity throughout the intrinsic networks rather than focusing on predefined regions of interest. Because we reconstructed the intrinsic networks in their entirety, we were able to examine the individual connectivities between each pair of regions in the networks. In addition, the overlap method enabled us to hone our connectivity analysis to precisely compare the same regions across the different subject groups and thereby avoid conflating differences in network composition and connectivity.
To situate the present study within the context of previous research on the intrinsic networks in schizophrenia, this study furthers the concept of distorted network connectivity in schizophrenia. Research to date, however, has produced conflicting results as to whether connectivity of the intrinsic networks is increased or decreased in schizophrenia. Our previous connectivity study of paranoid schizophrenic patients identified hyperconnectivity of the TNN in the resting state.5 Other studies, however, have demonstrated complex patterns of both increased and decreased connectivity in the TNN in schizophrenia,42,43,45,46 while still others have primarily demonstrated decreased connectivity of the TNN in schizophrenia.47 In the TPN, we identified resting-state hyperconnectivity associated with schizophrenia in the current study, which is consistent with our previous work.5 Studies of TPN connectivity in schizophrenia during task performance, by contrast, have demonstrated both increased connectivity45 and decreased connectivity46,47 of the TPN. Finally, analysis of the anticorrelation between the TNN and the TPN has also produced uneven results. In this study, we did not identify any differences in anticorrelation among the schizophrenic patients, their unaffected siblings, and controls by ANOVA. In comparing the subject groups by t tests, however, we found increased anticorrelation in schizophrenia relative to both the unaffected siblings and the healthy controls. Previous studies have identified both increased5,47 and decreased45,46 anticorrelations associated with schizophrenia, and thus a consensus has yet to develop. The inconsistency in intrinsic network connectivity results in schizophrenia thus spans the TNN, TPN, and the anticorrelation between these networks. It is possible that this variance is attributable to confounders associated with schizophrenia research, such as the biological changes that may arise secondary to the illness itself. For example, antipsychotic medications, substance abuse, and institutionalization may directly affect neural physiology, brain activity, and regional connectivity.27,49
Given the inconsistency to date of intrinsic network connectivity studies and the confounders associated with research in schizophrenia patients, it is hoped that the inclusion of the relatives of patients will further elucidate the connectivity changes that are primary to schizophrenia and those associated with illness risk. In the only other study of the intrinsic networks to include both schizophrenic patients and their first-degree relatives, Whitfield-Gabrieli et al.45 also demonstrated shared TNN hyperconnectivity. Furthermore, the increased connectivity was present both during task performance and in the resting state and correlated with psychopathology and working memory deficits in the schizophrenic patients as well as their first-degree relatives.45 Our current results of shared TNN hyperconnectivity are thus broadly consistent with those of Whitfield-Gabrieli et al. In this study, the specific TNN hyperconnectivity identified was between the bilateral ITG, which are involved in working memory as well as visual and language processing.50 Multiple studies of the relatives of schizophrenia patients have identified deficits in the functions supported by the ITG and suggested that such cognitive impairments may be a phenotypic marker of the genetic loading for schizophrenia that these individuals carry.28–31 Furthermore, gray matter losses in the bilateral ITG have been reported in both schizophrenic patients51,52 and their non-psychotic siblings.53 It is possible, therefore, that hyperconnectivity of the TNN underlies the cognitive deficits and increased risk for schizophrenia observed in the first-degree relatives of schizophrenic patients. The presence of TNN hyperconnectivity in the unaffected siblings suggests that this abnormality is a primary process associated with increased susceptibility to schizophrenia rather than a secondary effect of the disease. Because few studies have examined the connectivity of the intrinsic networks in the siblings of schizophrenic patients, future research is needed to elucidate the precise regions and connectivities that are abnormal. Such work may ultimately contribute to developing schizophrenia endophenotypes that will both aid in identifying individuals with the greatest illness risk and contribute to determining schizophrenia risk genes.24
Proper coordination and competition of cortical areas is crucial for optimal cognitive operations.54 The TNN is associated with cognitive processes that focus primarily on the internal world, including self-monitoring and stimulus-independent thoughts, while maintaining baseline monitoring for unpredicted external stimuli.7–9 Conversely, the TPN is involved in externally oriented stimulus-driven attention and goal-directed cognitive processes.10,11 The coordination between the TNN and TPN can be understood as integration between internal information processing and engagement with the external world. Abnormal connectivity of the intrinsic networks in schizophrenic patients may compromise network function and aversely affect the transitions between these 2 networks such that the normally strong boundary between internal and external information processing may be blurred. For example, schizophrenic patients may have difficulty distinguishing self-generated speech from external voices, suggesting a mechanism for the generation of auditory hallucinations. Hyperconnectivity of the TNN may represent increased introspective thinking and heightened salience monitoring such that external events are imbued with an inappropriate amount of self-relevance. Indeed, the correlation between psychopathology and aberrant activity and connectivity of the TNN has been repeatedly demonstrated in schizophrenic patients.42,43,45 Furthermore, the importance of proper coordination between the intrinsic networks was highlighted by a study in which working memory performance (TPN activity) was inversely correlated with the frequency of intrusion of unrelated stimulus-independent thoughts (TNN activity).55 Our current findings of TNN and TPN hyperconnectivity are broadly consistent with our previous work5 and further implicate disrupted network communication and competition in the symptoms of schizophrenia.
Interestingly, several of the regions identified as hyperconnected in this study underlie network coordination, the regulation of TNN and the TPN activity. Based on their widespread connectivity, high metabolic activity, and studies linking these regions to core functions of the TNN, the PCC7,8,56 and MPFC6–8,10,57 are postulated to modulate activity throughout the TNN. Similarly, in the TPN, the IFG 58–60 and the DLPFC8,10,61–63 are hypothesized to control activity throughout the network and underlie the core functions of the TPN. Moreover, studies have linked the MPFC with downward modulation of activity in the TPN64 and the IFG with decreasing activity in the TNN,60 thereby providing a mechanism for the anticorrelation observed between these networks. Previous schizophrenia research has identified abnormalities in connectivity, activity, gray matter volume, and metabolism in each of these 4 regions (PCC,42,43,45,65 MPFC,5,43,66–67 IFG,68–71 and DLPFC72–76). These diverse findings can be unified under the concept of intrinsic network abnormalities in schizophrenia and furthermore provide support for the changes in connectivity observed in the present study. Given the central and regulatory roles that the PCC, MPFC, IFG, and DLPFC play in the intrinsic networks, the regional hyperconnectivity identified in this study may imply that communication and coordination throughout the networks are disrupted in schizophrenia.
There are some methodological issues in this study that should be considered. First, a relatively low sampling rate (TR = 2 s) for multislice acquisition may reduce the specificity of the connectivity effects33 because low frequency cycles in respiration and cardiac activity may interfere with the low frequency fluctuations of the BOLD signal. Future studies may estimate, segregate, or remove these physiological effects by using multivariate connectivity analysis methods, such as independent component analysis,77 and by simultaneously recording the respiratory and cardiac cycles during data acquisition.78 Second, the anticorrelations were obtained by removing the global signal through linear regression, a popular preprocessing step in resting-state fMRI studies.10,45 Although the precise physiological interpretations of anticorrelations require further study, numerous investigators suggest that these anticorrelations reflect a competition for limited brain resources rather than an artifact of preprocessing.3,5,10,11 Moreover, analyses using noise reduction methods that do not require removal of the global signal, such as the CompCor approach,79 may clarify this issue. Third, although analyzing the overlapped intrinsic networks among the 3 groups allowed us to directly compare the strength of connectivity, this strategy excluded some regions from the comparison and did not address the compositional differences of the intrinsic networks between the groups. Finally, most of the schizophrenic patients in this study were receiving atypical antipsychotic medications at the time of scanning. The effect of antipsychotic medications on the intrinsic networks is still unclear, although some studies suggest these medications tend to normalize aberrant connectivity.49,80 Nevertheless, future studies may benefit from analyzing drug-naive first-episode schizophrenic patients.
Investigation of the resting-state connectivity of the anticorrelated intrinsic networks in schizophrenic patients and their unaffected siblings provides a unique opportunity to explore the pathophysiology of, and susceptibility to, schizophrenia. Hyperconnectivity of the TNN and TPN in schizophrenic patients may contribute to the pathophysiology of schizophrenia and may serve as a marker of the development of the illness. Similar, but milder, TNN hyperconnectivity observed in the unaffected siblings of schizophrenic patients may contribute to the identification of schizophrenia endophenotypes and ultimately to the determination of schizophrenia risk genes. Future studies based on a concept of distorted coordination of the intrinsic networks may improve our understanding of schizophrenia pathophysiology and susceptibility.
Funding
National Natural Science Foundation of China (30670752 to Z.L. and 30900487 to Y.Z.); National Basic Research Program of China (2006CB500808, 2007CB512300); 11th Five Year Key Program for Science and Technology Development of China (2007BAI17B05).
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The authors gratefully acknowledge Lin Xu from the Key Laboratory of Animal Models and Human Disease Mechanisms, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, People's Republic of China, and Zhong He from the Department of Radiology of Second Xiangya Hospital, Central South University, for their assistance in image acquisition and analysis.
All authors reported no potential conflicts of interest.
References
- 1.Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 2.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson P. Are anticorrelated networks in the brain relevant to schizophrenia? Schizophr Bull. 2007;33:994–1003. doi: 10.1093/schbul/sbm043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Liang M, Tian L, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 8.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 9.Mason MF, Norton MI, Van Horn JD, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frith C. Functional imaging and cognitive abnormalities. Lancet. 1995;346:615–620. doi: 10.1016/s0140-6736(95)91441-2. [DOI] [PubMed] [Google Scholar]
- 13.Frith C. The role of the prefrontal cortex in self-consciousness: the case of auditory hallucinations. Philos Trans R Soc Lond B Biol Sci. 1996;351:1505–1512. doi: 10.1098/rstb.1996.0136. [DOI] [PubMed] [Google Scholar]
- 14.Silbersweig DA, Stern E, Frith C, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- 15.Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- 16.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 17.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 18.Liang M, Zhou Y, Jiang T, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. NeuroReport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- 19.Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31(2-3):251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 20.Andreasen NC, Paradiso S, O'Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Liu Z, Liang M, et al. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. NeuroReport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Liang M, Zhou Y, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131(4):945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 23.Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to Star Wars Mx and functional genomics. Am J Med Genet. 2000;97:12–17. [PubMed] [Google Scholar]
- 24.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 25.Sadock BJ, Sadock VA. Schizophrenia. In: Sadock BJ, Sadock VA, editors. Kaplan and Sadock's Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 10th ed. New York, NY: Wolgters Kluwer and Lippincott Williams & Wilkins; 2007. pp. 467–497. [Google Scholar]
- 26.Lawrie SM, McIntosh AM, Hall J, et al. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull. 2008;34:330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald AW, III, Thermenos HW, Barch DM, et al. Imaging genetic liability to schizophrenia: systematic review of fMRI studies of patients' nonpsychotic relatives. Schizophr Bull. 2009;35:1142–1162. doi: 10.1093/schbul/sbn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snitz BE, Macdonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trandafir A, Meary A, Schurhoff F, et al. Memory tests in first-degree adult relatives of schizophrenic patients: a meta-analysis. Schizophr Res. 2006;81:217–226. doi: 10.1016/j.schres.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitskoorn MM, Aleman A, Ebisch SJH, et al. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Toulopoulou T, Rabe-Hesketh S, King H, et al. Episodic memory in schizophrenic patients and their relatives. Schizophr Res. 2003;63:261–271. doi: 10.1016/s0920-9964(02)00324-9. [DOI] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorder-Patient Edition (SCID-I/P) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 33.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7(2):119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 34.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlasbased interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 35.Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Yu C, Zheng H, et al. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010;121(3):220–230. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 37.Yu C, Liu Y, Li J, et al. Altered functional connectivity of primary visual cortex in early blindness. Hum Brain Mapp. 2008;29:533–543. doi: 10.1002/hbm.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belmonte M, Yurgelun-Todd D. Permutation testing made practical for functional magnetic resonance image analysis. IEEE Trans Med Imaging. 2001;20(3):243–248. doi: 10.1109/42.918475. [DOI] [PubMed] [Google Scholar]
- 39.Camargo A, Azuaje F, Wang H, et al. Permutation-based statistical tests for multiple hypotheses. Source Code Biol Med. 2008;3:15. doi: 10.1186/1751-0473-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sowell ER, Kan E, Yoshii J, et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci. 2008;11(6):637–639. doi: 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conneely KN, Boehnke M. So many correlated tests, so little time! Rapid adjustment of P values for multiple correlated tests. Am J Hum Genet. 2007;81:1158–1168. doi: 10.1086/522036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrity AG, Pearlson GD, McKiernan KA, et al. Aberrant ‘default mode’ functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 44.Jafri MJ, Pearlson GD, Stevens M, et al. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf RC, Vasic N, Sambataro F, et al. Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1464–1473. doi: 10.1016/j.pnpbp.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 47.Mannell MV, Franco AR, Calhoun VD, et al. Resting state and task-induced deactivation: a methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31:424–437. doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pomarol-Clotet E, Salvador R, Sarró S, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38(8):1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 49.Stephan KE, Magnotta VA, White T, et al. Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychol Med. 2001;31:1065–1078. doi: 10.1017/s0033291701004330. [DOI] [PubMed] [Google Scholar]
- 50.Ranganath C. Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139(1):277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 51.Onitsuka T, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. 2004;161:1603–1611. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuroki N, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. Am J Psychiatry. 2006;163:2103–2110. doi: 10.1176/appi.ajp.163.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gogtay N, Greenstein D, Lenane M, et al. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- 54.Bressler SL, Kelso JA. Cortical coordination dynamics and cognition. Trends Cogn Sci. 2001;5(1):26–36. doi: 10.1016/s1364-6613(00)01564-3. [DOI] [PubMed] [Google Scholar]
- 55.Teasdale JD, Dritschel BH, Taylor MJ, et al. Stimulus-independent thought depends on central executive resources. Mem Cognit. 1995;23(5):551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- 56.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 57.McGuire PK, Paulesu E, Frackowiak RS, et al. Brain activity during stimulus independent thought. Neuroreport. 1996;7(13):2095–2099. [PubMed] [Google Scholar]
- 58.Brass M, Derrfuss J, Forstmann B, et al. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9(7):314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Derrfuss J, Brass M, Neumann J, et al. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and stroop studies. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D'Esposito M, Detre JA, Alsop DC, et al. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 62.Procyk E, Goldman-Rakic PS. Modulation of dorsolateral prefrontal delay activity during self-organized behavior. J Neurosci. 2006;26:11313–11323. doi: 10.1523/JNEUROSCI.2157-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrides M. Mapping prefrontal cortex systems for the control of cognition. In: Toga AW, Mazziota JC, editors. Brain Mapping: The Systems. Academic Press; 2000. pp. 159–176. [Google Scholar]
- 64.Uddin LQ, Kelly AM, Biswal BB, et al. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu E, Hashimoto K, Ochi S, et al. Posterior cingulate gyrus metabolic changes in chronic schizophrenia with generalized cognitive deficits. J Psychiatr Res. 2007;41(1-2):49–56. doi: 10.1016/j.jpsychires.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 66.Haznedar MM, Buchsbaum MS, Luu C, et al. Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry. 1997;154:682–684. doi: 10.1176/ajp.154.5.682. [DOI] [PubMed] [Google Scholar]
- 67.Pomarol-Clotet E, Canales-Rodriguez EJ, Salvador R, et al. Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging [published online ahead of print January 12, 2010] Mol Psychiatry. doi: 10.1038/mp.2009.146. doi: 10.1038/mp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Honea R, Crow TJ, Passingham D, et al. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 69.Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shergill SS, Brammer MJ, Williams SCR, et al. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- 71.Sommer IE, Diederen KM, Blom JD, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131:3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- 72.Callicott JH, Mattay VS, Verchinski BA, et al. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 73.Potkin SG, Turner JA, Brown GG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreasen NC, O'Leary DS, Cizadlo T, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 76.Zhou Y, Liang M, Jiang TZ, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 77.Kiviniemi V, Kantola JH, Jauhiainen J, et al. Independent component analysis of nondeterministic fMRI signal sources. Neuroimage. 2003;19:253–260. doi: 10.1016/s1053-8119(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 78.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 79.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schlosser R, Gesierich T, Kaufmann B, et al. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. NeuroImage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.