Abstract
Cannabis use is highly prevalent among people with schizophrenia, and coupled with impaired cognition, is thought to heighten the risk of illness onset. However, while heavy cannabis use has been associated with cognitive deficits in long-term users, studies among patients with schizophrenia have been contradictory. This article consists of 2 studies. In Study I, a meta-analysis of 10 studies comprising 572 patients with established schizophrenia (with and without comorbid cannabis use) was conducted. Patients with a history of cannabis use were found to have superior neuropsychological functioning. This finding was largely driven by studies that included patients with a lifetime history of cannabis use rather than current or recent use. In Study II, we examined the neuropsychological performance of 85 patients with first-episode psychosis (FEP) and 43 healthy nonusing controls. Relative to controls, FEP patients with a history of cannabis use (FEP + CANN; n = 59) displayed only selective neuropsychological impairments while those without a history (FEP − CANN; n = 26) displayed generalized deficits. When directly compared, FEP + CANN patients performed better on tests of visual memory, working memory, and executive functioning. Patients with early onset cannabis use had less neuropsychological impairment than patients with later onset use. Together, these findings suggest that patients with schizophrenia or FEP with a history of cannabis use have superior neuropsychological functioning compared with nonusing patients. This association between better cognitive performance and cannabis use in schizophrenia may be driven by a subgroup of “neurocognitively less impaired” patients, who only developed psychosis after a relatively early initiation into cannabis use.
Keywords: schizophrenia, psychosis, marijuana, drug, neuropsychology, comorbidity
Introduction
Rates of substance use disorders among individuals with schizophrenia are higher than the general population,1–3 with cannabis being the most commonly abused illicit drug.4 While cannabis use has been associated with poorer treatment outcomes, including symptom exacerbation,5–7 whether it is also associated with cognitive deficits remains contentious. Cannabis use among healthy users has been associated with cognitive impairments,8–12 including residual deficits in memory and attention, even several days following abstinence.13–16 Our studies in individuals with no medical or psychiatric history have shown that long-term cannabis use is associated with structural brain abnormalities and subthreshold psychotic symptoms in a dose-dependent manner.8 However, among patients with schizophrenia, the association is less clear. While acute administration of tetrahydrocannabinol (THC) to patients with schizophrenia exacerbates symptoms and cognitive impairments and may have enduring effects,17 cannabis has also been found to have some beneficial effects on cognition, at least in certain subgroups of patients.18–20 Thus, while cannabis use is traditionally associated with cognitive impairment, the relationship is more complex in the case of schizophrenia.
Although a recent meta-analysis conducted by Potvin and colleagues21 supports this notion, their analysis does not include studies conducted after 2006 and is not focused on cannabis specifically but includes patients with polysubstance abuse. Here, we present 2 studies that aim to clarify the nature of the association between cannabis use and cognitive impairments observed in schizophrenia. The first study comprises a meta-analysis of empirical investigations of cannabis effects on cognition in patients with established schizophrenia. In the second study, we compared the neuropsychological functioning of patients with first-episode psychosis (FEP) with and without a history of cannabis use as well as healthy controls with no substance use history.
Study I: Meta-analysis of Findings To Date
Methods
We performed a literature search using PUBMED, PsycINFO, and Scopus covering the period between 1987 and March 2010. Combinations of the following keywords were used: schizophrenia, psychosis, cannabis, substance, cognit*, and neuropsycholog*. The reference lists of the published articles were also reviewed. Inclusion criteria included: (1) diagnosis of schizophrenia, (2) cognitive abilities of patients with and without cannabis use were directly compared, (3) cannabis was the predominant substance used by patients, and (4) studies reported sufficient data to calculate effect sizes in d metrics.
The initial search yielded 145 studies but only 15 studies considered the effect of cannabis on cognition in schizophrenia (table 1). Five of these studies were excluded because: (1) they lacked data for calculating effect size,22,23, (2) the study sample included a mixture of patients with schizophrenia and affective disorders,24 and (3) they were primarily FEP studies (too few studies in this group to conduct appropriate meta-analyses).25,26 In 6 of the 10 studies included in the meta-analysis, cannabis use was defined as lifetime use and in the other 4 studies it was defined as recent use (current use or use in last 6 months). In 2 of the included studies,27,28 not all patients in the substance-using groups were abusing cannabis, but they were still included in the meta-analysis since cannabis was the most commonly used substance within the samples. In one study,29 we used data from the 3-month follow-up assessment only since some patients had very recently used substances at baseline or were acutely intoxicated at the time of testing. Overall, our meta-analysis included 10 studies involving 572 patients with schizophrenia (with and without comorbid cannabis use).
Table 1.
Studies that Investigated Cannabis Use and Cognition Association in Schizophrenia
| Studies | Sample | Cannabis Criteria | Cannabis Use | Other Drugs | Cognitive Tests | Results |
| Lifetime history | ||||||
| Joyal et al27 | 16 SCZ + SUD14 SCZ −SUD | Cannabis abuse or dependency Not all the patients use cannabis but cannabis was the preferred substance for the sample | No information regarding abstinence and duration/onset of cannabis use | TMT-A, TMT-B, WCST, COWAT, RCFT, WAIS PA, Porteus Maze, Go/No Go | Impaired WCST, COWAT in nonusers Fewer neurological soft signs | |
| Stirling et al33 | 24 SCZ + CANN39 SCZ − CANN | Cannabis use prior to onset of psychosis | No information regarding abstinence and duration/onset of cannabis use | Design memory, verbal fluency, WAIS OA, BD, PC, PA, face recognition memory | SCZ + CANN better in 7/9 measures SCZ − CANN more deficit schizophrenia and more neurological soft signs | |
| Jockers-Scherubl et al19 | 19 SCZ + CANN20 SCZ − CANN39 HC | At least 0.5 g daily For 2 y prior to onset | Abstinence, at least 4 wk (weekly urine screenings) | Alcohol excluded, SCZ − no other substance abuse | CPT-IP, TMT-A, TMT-B, WCST, WMS-R verbal and visual memory, WAIS-R (BD, PA, DSST, Comprehension) | SCZ + CANN performs better than SCZ − CANN in some cognitive domains. Regular use before 17 associated with even better performance |
| Sevy et al32 | 14 SCZ + CANN13 SCZ − CANN | Cannabis abuse or dependency 0.5 g/day, >4 d of the week. Only 1 subject used in last 2 mo | No information regarding abstinence and duration/onset of cannabis use 78% started prior to onset of psychosis | Cannabis main substance, 7 alcohol abuse, 3 cocaine abuse Nicotine use is more in SCZ + CANN | WRAT, CPT-IP, CVLT, DS (backward and forward), TMT-A, TMT-B, COWAT (semantic and phonemic), IGT | No difference No impact of alcohol |
| Løberg et al29 (Study I) | 13 SCZ + CANN18 SCZ − CANN | History of cannabis abuse or dependency | No information regarding abstinence and duration/onset of cannabis use | All abuse cannabis 8 additional substance abuse mainly amphetamine | Memory, attention, psychomotor speed, general abilities, and executive function domains based on various test | SCZ + CANN performs better except memory |
| Schnell et al20 | 35 SCZ + CANN34 SCZ −CANN | Cannabis abuse or dependency All started prior to onset of illness (average 6 y) | Minimum 3 wk abstinence (self and medical reports and urine screening) Age of onset = 17 Duration = 8.5 y Frequency/month = 53 joints | No other substances except nicotine | AVLT, LNS, DSST, TMT-B, MWT-B, CPT, Verbal fluency, Dual processing task | SCZ + CANN better in WM, executive functions, and verbal memory. High frequency use was associated with even better performance |
| Current/recent use | ||||||
| Potvin et al28 | 44 SCZ + CANN32 SCZ − CANN | Abuse or dependence Not all the patients use cannabis but cannabis was the preferred substance for the sample Last 6 mo | No information regarding abstinence and duration/onset of cannabis use | 12 cocaine, 17 polysubstance | PAL, MOT | SCZ + CANN better in MOT No difference for PAL. However, SCZ + CANN patients who use cocaine are more impaired in PAL. Non cocaine user but SCZ + CANN patients are better than SCZ − CANN in PAL |
| Løberg et al29 (Study II) | 13 SCZ + CANN13 SCZ − CANN | Current cannabis abuse or dependency Acute admission with schizophrenia or schizophrenia like psychoses | No information regarding abstinence and duration/onset of cannabis use | All abuse cannabis 10 also abuse other substances (4 amphetamine, 3 cocaine, 4 alcohol) | Global cognition score based on various cognitive tests longitudinal study, 3 mo follow-up | In acute admission, SCZ + CANN performs worse, in follow-up they significantly improved SCZ − CANN no change in follow-up |
| Ringen et al78 | 117 SCZ + CANN23 SCZ − CANN | Cannabis use in last 6 mo | DSST, Digit span, 2-Back, Verbal fluency, Logical memory, word interference, CVLT | SCZ + CANN performs worse in verbal memory and attention | ||
| Scholes et al79 | 22 SCZ + CANN | Current cannabis use | Stroop, WCST, Spatial span, LNS | No difference | ||
| 49 SCZ − CANN | ||||||
Note: TMT-A, Trial Making Test—Part A; TMT-B, Trial Making Test—Part B; TMT B/A, Trial Making Test—Part B minus Part A; WCST, Wisconsin Card Sorting Task; COWAT, Controlled Oral Word Association Task; RCFT, Rey Complex Figure Test; PAL, Paired Associate Learning; MOT, Motor Screening; OA, Object Assembly; BD, Block Design; PC, Picture Completion; PA, Picture Arrangement; DS, Digit Span; WAIS, Wechsler Adult Intelligence Scale, WRAT, Wide Range Achievement Test; CPT-IP, Continuous Performance Task—Indentical Pairs; CVLT, California Verbal Learning Task; IGT, Iowa Gambling Task; RAVLT, Rey Auditory Verbal Learning Task; LNS, Letter Number Sequencing; DMST, Delayed Matching to Sample Test; MWT-B, Mehrfachwahl-Wortschatztest—Form B; HC, Healthy Control; SUD, Substance Use Disorder; FEP, First-Episode Psychosis; SCZ, Schizophrenia; DSST, digit symbol substitution task. Superscript numbers refer to the relevant reference number in the citations list.
Neuropsychological Variables.
Neuropsychological variables were grouped according to the 6 cognitive domains of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery30 (table 2). A “Global cognition index” was calculated by averaging effect sizes from each domain for each study. We used global scores of cognition from the Study I of Løberg et al29 because their classification of cognitive domains differed from the MATRICS.
Table 2.
NIMH-MATRICS Cognitive Domains
| Cognitive Domain | Cognitive Test |
| Processing Speed | Phonetic fluency, semantic fluency, Trail-Making Test, Symbol Coding, Motor Screening Task, Stroop |
| Attention | Continuous Performance Test, Dual Processing Task |
| Working Memory | Digit span, Letter Number Sequencing, Wechsler Memory Scale—Revised Verbal Memory |
| Reasoning and Problem Solving | WAIS Block Design, Object Assembly, Picture Arrangement, Porteus Maze, Wisconsin Card Sorting Test |
| Verbal Learning and Memory | California Verbal Learning Test, Rey Auditory Verbal Learning Test |
Note: Abbreviations are explained in the footnote to table 1.
Meta-analytical Procedure.
For each cognitive test, effect size (Cohen’s d) and SE were calculated. Effect sizes were defined as the mean difference between the schizophrenia patients’ and healthy controls’ performance divided by the pooled SD. Effect sizes were weighted using the inverse variance method. A random effects model was used. Homogeneity of the mean-weighted effect sizes was tested using the Q-test, while publication bias was assessed using Egger’s test. Only instances where there were heterogeneity or publication biases are described in the Results. Meta-analyses were performed using MIX software.31 Meta-regression analyses examined the effect of moderator variables on between-group differences. This was performed with random effects modeling using the restricted-information maximum likelihood method with a significance level set at P < .05. These analyses were conducted in SPSS version 16.0 using the macros written by DB Wilson (http://mason.gmu.edu/∼dwilsonb/ma.html). Separate meta-analyses were also performed for studies defining cannabis use as “lifetime” or “recent” (current/last 6 months) use.
Results
There were significant differences between the SCZ + CANN and SCZ − CANN groups for gender, age, education, age of onset of illness, and positive symptoms. The SCZ + CANN group comprised more males (RR = 1.10, CI = 0.99–1.22, Z = 1.75, P = .08), had less education (d = 0.40, CI = 0.21–0.60, Z = 4.01, P < .001), a younger age (d = 0.57, CI = 0.36–0.79, Z = 5.29, P < .001), more severe positive symptoms (d = 0.65, CI = 0.41–0.90, Z = 5.15, P < .001), and earlier illness onset (d = 0.42, CI = 0.19–0.65, Z = 3.59, P = .003). There were no significant between-group differences for duration of illness, negative symptoms, and premorbid IQ.
Global Cognition Index.
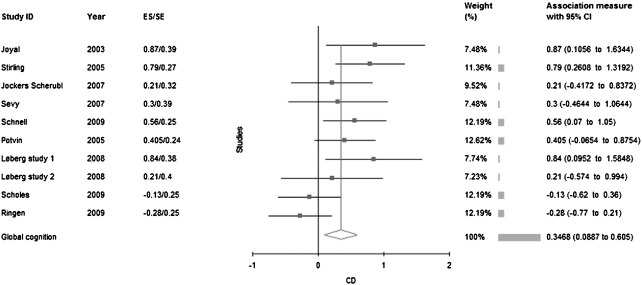
Eight studies reported superior performance for SCZ + CANN patients on global cognition (see table 3; figure 1). The studies that reported better performance in SCZ + CANN tended to define cannabis use as lifetime use. Overall, SCZ + CANN patients performed significantly better than SCZ − CANN patients (d = 0.35). The distribution of effect sizes observed in these studies was not homogeneous. In separate analyses for studies using lifetime or recent cannabis use criteria, no more heterogeneity was observed. SCZ + CANN patients performed significantly better only in the studies defining use by lifetime exposure (d = 0.55) but not in the studies using recent use criteria (d = 0.03). Meta-regression analyses did not reveal any effect of demographic (effect size of age difference and gender ratio) or clinical variables (effect size of differences for onset of illness, duration of illness, positive, and negative symptoms) on the magnitude of the between-group differences.
Table 3.
Mean-Weighted Effect Sizes for Cognitive Domains for Cannabis Using and Nonusing Schizophrenia Patients Defined by Lifetime Use Vs Current or Recent Use Criteria
| Test | Number of Studies | SCZ + CANN | SCZ − CANN | Cohen’s d | 95 % CI | z | P | Q-test, P | Bias Test |
| Global cognition | 10 | 223 | 349 | 0.35 | 0.09 to 0.61 | 2.63 | .009 | 17.13* | 0.34 |
| Lifetime | 6 | 121 | 138 | 0.55 | 0.30 to 0.80 | 4.29 | <.001 | 3.95 | |
| Current/recent | 4 | 102 | 211 | 0.03 | −0.30 to 0.37 | 0.20 | .84 | 4.59 | |
| Attention | 3 | 68 | 67 | 0.26 | −0.08 to 0.61 | 1.51 | .13 | 0.50 | 0.26 |
| Processing speed | 8 | 192 | 322 | 0.40 | 0.11 to 0.69 | 2.75 | .006 | 14.62* | 0.39 |
| Lifetime | 5 | 103 | 124 | 0.65 | 0.38 to 0.92 | 4.70 | <.001 | 1.72 | |
| Current/recent | 3 | 89 | 198 | 0.09 | −0.34 to 0.52 | 0.42 | .67 | 4.87 | |
| Visual memory | 3 | 87 | 91 | 0.45 | 0.13 to 0.77 | 2.76 | .006 | 2.24 | 0.98 |
| Verbal memory | 4 | 101 | 184 | 0.0 | −0.51 to 0.51 | 0 | .99 | 10.1* | 0.75 |
| Lifetime | 3 | 78 | 67 | 0.18 | −0.32 to 0.77 | 0.63 | .53 | 4.78 | |
| Planning | 4 | 81 | 122 | 0.47 | 0.05 to 0.90 | 2.20 | .03 | 5.92 | 0.39 |
| Lifetime | 3 | 59 | 73 | 0.67 | 0.31 to 1.03 | 3.65 | <.001 | 1.20 | |
| Working memory | 4 | 94 | 213 | 0.13 | −0.40 to 0.55 | 0.48 | .63 | 11.58* | 0.61 |
| Lifetime | 2 | 49 | 47 | 0.64 | 0.22 to 1.05 | 3.00 | .003 | 0.16 | |
| Current/recent | 2 | 45 | 166 | −0.28 | −0.61 to 0.06 | 1.62 | .10 | 0.12 |
Note: Bold values indicate comparisons that were significantly different between the SCZ + CANN and the SCZ − CANN groups.
*P < .05.
Fig. 1.
Effect Size Analysis of All Studies Conducted To Date. A summary score global cognition index was calculated by averaging effect sizes from each domain for each study.
Attention.
Three studies examined attention. There were minimal between-group differences on a sustained attention task (d = 0.26).
Processing Speed.
Eight studies examined processing speed. The SCZ + CANN patients had significantly faster processing speeds than SCZ − CANN patients (d = 0.40). Effect sizes were not homogeneously distributed, however, when recent use vs lifetime defining studies was examined separately, there was no evidence for heterogeneous distribution of effect sizes. SCZ + CANN patients performed significantly better than SCZ − CANN only within the lifetime defining studies (d = 0.65) but not in those studies with recent use criteria (d = 0.09).
Visual Memory.
Three studies assessed visual memory. Again, SCZ + CANN patients performed better than SCZ − CANN patients (d = 0.45).
Verbal Memory.
Four studies reported verbal memory measures. There was no significant between-group difference for verbal memory (d = 0.0). The Q-test showed evidence for heterogeneity for distribution of effect sizes. When the 3 lifetime defining studies were examined separately, between-group comparisons remained nonsignificant (d = 0.18).
Working Memory.
Four studies examined working memory. There was no significant between-group difference (d = 0.13). While distribution of effect sizes was heterogeneous, the between-group difference for 2 lifetime defining studies was d = 0.64, with the SCZ + CANN group demonstrating significantly superior performance. The between-group difference for recent use criteria studies was not significant (d = −0.28). There was no evidence of heterogeneity for these separate analyses.
Executive Functioning.
In the 4 studies included in this domain, cannabis use was associated with better planning and reasoning abilities (d = 0.47). When the lifetime defining studies were examined separately, the between-group difference was larger (d = 0.67).
Recency of Cannabis Use.
For this analysis, we excluded the study by Jockers et al19 as they did not exclude patients using cannabis in the week prior to testing. Patients with SCZ + CANN without recent use demonstrated better verbal memory performance than the SCZ − CANN group (d = 0.46, CI = 0.05–0.88, Z = 2.20, P = .03). Distribution of effect sizes was more homogenous with this analysis (P = .52).
Frequency of Cannabis Use.
One study reported an association between higher frequency of cannabis use and better cognitive performance (working memory and attentional tasks; Schnell et al).20
Age of Onset of Cannabis Use.
Only one study examined the relationship between age of onset of cannabis use and cognitive deficits.19 In this study, earlier starting age of cannabis use (ie, before 17 years of age) was associated with superior cognitive performance.
Effect of Other Substances.
A separate meta-analysis for the 2 studies19,20 consisting of patients without comorbid substance use (other than nicotine) was conducted. We found a similar mean effect size for global cognition (d = 0.43) to that observed in the overall meta-analysis. One study also examined the effect of comorbid alcohol use in cannabis-using patients and failed to find any significant difference between alcohol-using and nonusing groups.32 One study found that cocaine-using SCZ + CANN patients were cognitively more impaired.28
Summary of Study I
These meta-analyses demonstrate that patients with established schizophrenia with a cannabis use history display superior cognitive abilities compared with noncannabis-using patients. These results may appear counterintuitive because healthy individuals who are heavy cannabis users have cognitive deficits and brain abnormalities.8,9 However, our findings are consistent with previous studies conducted in schizophrenia patients with comorbid polysubstance use,21 while showing specificity to a cannabis-using sample. Furthermore, we showed that better cognitive performance is seen only in lifetime users but not in recent users.
Importantly, the majority of studies to date have been conducted in patients with chronic illness without assessing whether cannabis use preceded the onset of psychosis. It is possible that the cognitive profile of patients whose cannabis use may have contributed to the onset of their psychosis might be different from those who started using cannabis after the onset of their psychosis. Studies examining cannabis use prior to the onset of FEP are especially useful in this regard because cannabis use may contribute to the onset of psychosis only in these patients. Five studies have examined cognitive functioning in a sample who used cannabis before the onset of psychosis.25,26,33 However, the studies of Stirling et al,33 Jockers-Scherubl et al,19 and Schnell et al20 included a chronic sample, and it was not possible to exclude the effects of cannabis use after the onset of psychosis or illness-related factors. While the studies of de la Serna et al25 and Mata et al26 were also FEP samples, they only assessed cannabis use in the previous one month or in the previous 12 months, meaning that their cannabis nonuser samples were likely to have also included patients who had a past history of cannabis use.
Thus, there are no FEP studies that have examined cognitive functioning in FEP patients with a lifetime history of cannabis use. In our second study, we report on such a sample. Additionally, given that the age of cannabis use onset has been found to be an important moderator of the association between cannabis use and psychotic illness,34–37 we investigated the impact of age of cannabis use onset on cognitive functioning.
Study II: New Data in FEP Patients
Methods
Sample and Setting.
Participants included 85 FEP patients and 43 healthy nonusing controls. Participants were selected from a larger neuropsychological research database (described previously38–40) only if they were assessed for substance use. While all participants agreed to a cognitive test battery, some participants had an incomplete cognitive assessment. A detailed substance use history, including information concerning the amount, frequency, duration, and type of substance use, diagnosis, time since last use, and age of onset of regular use, was obtained using a combination of medical history notes and structured clinical and research interviews (ie, substance use module of the Structured Clinical Interview for DSM-IV [Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition], Patient version [SCID-I/P],41 Clinical Global Impressions-Severity of Illness Scale42). In addition, substance use data were gathered from the Early Psychosis File Questionnaire,43 which was used to systematically extract data on premorbid, entry, treatment, and discharge characteristics from consecutive medical files. Cannabis was the predominant substance used in FEP patients. FEP patients with a history of regular use (defined as >2 years of use and >2 g per week) were classified as cannabis users (n = 59; FEP + CANN), and a large majority of these patients met criteria for Diagnostic and Statistical Manual of Mental Disorders, Third Revision (DSM-III-R) cannabis abuse or dependence (see below). FEP patients without a history of regular cannabis use were classified as nonusers (n = 26; FEP − CANN). Cannabis use was assessed over each subject’s lifetime, and none of the FEP − CANN group had ever used cannabis regularly (ie, had not used on a weekly basis for >12 months), while all FEP + CANN patients used cannabis before the onset of the illness. Of the 26 FEP − CANN patients, 3 had a past history of alcohol abuse and 4 others had experimented with stimulants and lysergic acid diethylamide (LSD).
All patients with FEP were recruited from the Early Psychosis Prevention and Intervention Centre at Orygen Youth Health and were diagnosed with schizophrenia-spectrum disorders according to DSM-III-R criteria based on a medical record review and structured clinical interview using the Positive and Negative Syndrome Scale,44 SCID-I/NP41 and the Royal Park Multi-diagnostic Instrument for Psychosis (McGorry et al45). For controls, we used the SCID-I (nonpatient edition). Of the 59 patients classified as cannabis users (FEP + CANN), 55 met criteria for past or current cannabis abuse or dependence. Thirty-two of the FEP + CANN had started to use cannabis at 16 years of age or earlier (FEP + CANN[early]) and 27 had started at age 17 or thereafter (FEP + CANN[later]). We chose to compare those who had commenced using cannabis before or after ages 16–17 years on the basis of previous literature suggesting that exposure to cannabis prior to this period of adolescence has relatively greater adverse consequences compared with later initiation,12,46 and this cutoff enabled a roughly equal division of the sample. All were abstinent at the time of assessment (according to self-report, medical records/case notes, and researcher observations), 34 for >1 month, while 10 had consumed cannabis within the previous week, and a further 10 for between 8 and 30 days prior to the assessment (data were missing for 5 cases). Fifteen of the FEP + CANN patients also had a history of stimulant and hallucinogen use (mainly amphetamine), and 16 others had a history of alcohol abuse. Most patients (n = 51) were prescribed antipsychotic medication (35 risperidone, 14 olanzapine, and 2 typical antipsychotics), while 10 were prescribed mood stabilizers (5 lithium and 5 sodium valproate) and 7 were prescribed antidepressants (5 SSRI, 1 imipramine, and 1 moclobemide). Chlorpromazine equivalents (cpz) of antipsychotics were calculated.47
Healthy controls comprised 43 individuals with no current or past history of illicit substance abuse or dependence. Healthy volunteers were recruited by approaching ancillary hospital staff (eg, nonprofessional staff and students) and through advertisements in local newspapers in the same catchment area as the patients (ie, north-western regions of Melbourne) as well as through “word of mouth” (ie, friends of patients).
All participants were screened for comorbid medical conditions. Exclusion criteria were a history of head injury causing loss of consciousness for greater than 1 minute and/or hospitalization, neurological disorders, thyroid disorders, documented poor eyesight or hearing, and history of corticosteroid use. Control participants with family or personal histories of psychiatric illness were also excluded. Local research and ethics committees approved the study, and written informed consent was obtained from all individuals prior to study participation.
Cognition.
All participants were administered a cognitive test battery by experienced psychologists. Neuropsychological tasks targeted general intelligence, processing speed, verbal memory, visual memory, working memory, and executive functioning (planning and reasoning). These domains reflect 5 of 6 dimensions in the MATRICS battery.30 In order to facilitate comparison with the meta-analysis in Study I, tasks that were, and those that were not reflected in the MATRICS battery were allocated to a relevant domain following a consensus agreement between 2 authors (E.B. and M.Y.).
General Intellectual Ability.
Performance on the National Adult Reading Test was used to estimate premorbid IQ.48 The Wechsler Adult Intelligence Scale—Revised (WAIS-R) was used to estimate current IQ.49
Processing Speed.
The Trail-Making Test (Part A) and Digit Symbol Coding were used as measures of processing speed.49,50
Visual Memory.
Visual Paired Associates (part I) and Visual Reproduction (part I) from the Wechsler Memory Scale—Revised (WMS-R) provided measures of visual learning and memory.51 Spatial and Pattern Recognition tests from the Cambridge Automated Neuropsychological Test Automated Battery (CANTAB) were used to index recognition memory.52,53
Verbal Memory.
A modified version (3 trials instead of 5) of the Rey Auditory Verbal Learning Test was used as a measure of verbal learning and delayed recall.54 The Logical Memory (parts I and II) component of the WMS-R was also used.51
Working Memory.
Spatial Span and Spatial Working Memory (SWM) from the CANTAB were used to index working memory.53,55 Regarding SWM, 2 scores were included in the analysis: (1) total between-search errors (SWM-errors), which is the number of times that a subject returns to a box in which a token had already been found during a previous search sequence and (2) strategy score (SWM-strategy), a measure of the ability to adopt a systematic search approach (ie, initiating a search from the same token across trials shows better strategy use). While SWM-errors is a more pure measure of short-term working memory, strategy score also measures central executive functioning.
Executive Functioning.
Block Design from the WAIS-R and the Tower of London (ToL) tests were used as measures of planning and reasoning ability.49,56
Statistical Analyses.
Statistical analyses were conducted using the Statistical Package for Social Sciences Version 16.0 (SPSS 16.0). Between-group differences on categorical variables were tested using chi-square analysis. Patients’ scores on cognitive tests were converted to z scores based on the controls’ performance. ANCOVA was used to test group differences between FEP − CANN and FEP + CANN on continuous measures with gender and age as covariates. ANCOVA analyses were also used to compare FEP − CANN, FEP + CANN[early], and FEP + CANN[later]. Significant F-statistics were followed up with post hoc analyses using the Tukey test. Cohen’s d also characterized between-group differences.57 Pearson product moment correlation was used to examine relationships between cognition, parameters of cannabis use, and clinical measures.
Results
Demographic.
There was no significant between-group difference for age and gender (see table 4). FEP patients who use cannabis were less educated than controls but did not differ from FEP − CANN, while the latter did not differ from controls.
Table 4.
Demographic and Clinical Characteristics of Patient and Control Groups
| Control | FEP + CANN | FEP − CANN | P | |
| Number (male/female) | 43 (33/10) | 59 (43/16) | 26 (15/11) | .44 |
| Age | 21.6 (5.8) | 20.7 (2.8) | 20.6 (3.5) | .49 |
| Education | 12.3 (1.4) | 10.9 (1.8) | 11.6 (2.0) | .001a |
| PANSS positive | 24.7 (6.3) | 21.8 (6.5) | .06 | |
| PANSS negative | 20.7 (7.4) | 20.9 (8.2) | .93 | |
| GAF-premorbid | 70.1 (13.5) | 76.2 (12.0) | .20 | |
| GAF-entry | 29.9 (8.5) | 31.7 (7.5) | .32 |
Note: Bold values indicate comparisons that were significantly different between the FEP + CANN and the FEP − CANN groups. GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale. Additional abbreviations are explained in the footnote to table 1.
FEP + CANN < Controls.
FEP − CANN and FEP + CANN vs Controls.
FEP patients (with and without comorbid cannabis) were significantly impaired on all 16 tasks compared with controls. Effect sizes of these impairments in all 6 cognitive domains were moderate to large, ranging from 0.52 to 2.11. The most pronounced impairments were observed for Current IQ (d = 2.11), Logical Memory (d = 1.70), SWM-errors (d = 1.60), and Digit Symbol Coding (d = 1.48).
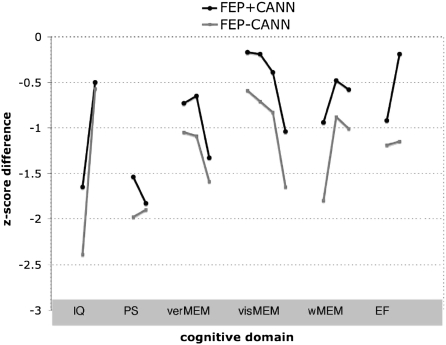
When we compared each of the FEP + CANN and FEP − CANN groups separately to controls, the FEP − CANN patients were significantly impaired on 15 of the 16 tasks administered across the 6 cognitive domains, while the FEP + CANN group showed only selective impairments (9 of the 16 tasks) (table 5; figure 2). Both FEP + CANN and FEP − CANN patients were significantly impaired for all tasks of general intelligence, verbal memory, and processing speed. Unlike the FEP − CANN group, the FEP + CANN group was not significantly different from controls on most tasks of visual memory and planning/reasoning. In the working memory domain, FEP + CANN patients were impaired in SWM-errors but not in SWM-strategy or Spatial Span. FEP + CANN patients did not differ from controls on the ToL task involving planning and reasoning.
Table 5.
Mean Z Scores and Between-Group Differences for Cannabis Using and Nonusing FEP Groups
| N | FEP + CANN | N | FEP − CANN | p | ES | |
| General Intelligence | ||||||
| Current IQ | 54 | −1.65 (1.40)* | 20 | −2.39 (0.96)* | .07 | 0.40 |
| Premorbid IQ | 41 | −0.50 (1.14) | 16 | −0.57 (1.24) | .83 | 0.06 |
| Verbal Memory | ||||||
| RAVLT—total | 40 | −0.73 (0.88)* | 19 | −1.05 (1.17)* | .21 | 0.33 |
| RAVLT—recall | 40 | −0.65 (0.96)* | 19 | −1.09 (1.24)* | .15 | 0.44 |
| Logical Memory | 41 | −1.33 (0.85)* | 16 | −1.59 (0.80)* | .21 | 0.30 |
| Visual Memory | ||||||
| Visual PA—total errors | 47 | −0.17 (0.99) | 23 | −0.59 (1.08)* | .10 | 0.50 |
| Visual Reproduction | 35 | −0.19 (0.70) | 19 | −0.71 (1.17)* | .05 | 0.60 |
| Spatial Recognition | 49 | −0.39 (1.01) | 23 | −0.83 (1.39)* | .18 | 0.39 |
| Pattern Recognition | 49 | −1.04 (1.20)* | 23 | −1.65 (1.50)* | .09 | 0.47 |
| Processing Speed | ||||||
| Trails A | 39 | −1.54 (1.58)* | 19 | −1.98 (3.24)* | .48 | 0.19 |
| Digit Symbol Coding | 44 | −1.83 (1.61)* | 17 | −1.90 (1.60)* | .67 | 0.04 |
| Working Memory | ||||||
| SWM-errors | 49 | −0.94 (1.13)* | 22 | −1.80 (1.30)* | .01 | 0.74 |
| SWM-strategy | 49 | −0.48 (0.91) | 22 | −0.88 (0.86)* | 0.12 | 0.44 |
| Spatial Span | 49 | −0.58 (0.91) | 22 | −1.01 (1.02)* | 0.09 | 0.44 |
| Executive Functioning | ||||||
| Block Design | 36 | −0.92 (1.23)* | 18 | −1.19 (1.65)* | 0.62 | 0.22 |
| ToL minimum solution | 24 | −0.19 (0.93) | 12 | −1.15 (1.37)* | 0.03 | 0.90 |
Note: Corrected for age and gender differences; ES, effect size (Cohen’s d; unadjusted values); Asterisks reflect that these measures are significantly different to healthy controls. “p” represents the P values for comparisons between the FEP + CANN and FEP − CANN groups. Values in parentheses represent the SD of z scores. SWM, Spatial Working Memory; ToL, Tower of London. Additional abbreviations are explained in the footnote to table 1. Bold values indicate comparisons that were significantly different between the FEP + CANN and the FEP − CANN groups.
Fig. 2.
Mean Z Score Differences for Cannabis Using and Nonusing FEP Groups. Note: IQ, intelligence; PS, processing speed; verMEM, verbal memory; visMEM, visual memory; wMEM, working memory; EF, executive functioning. Note that each point reflects a specific task within the 6 domains of interest (refer to the Methods section and table 5 for the specific tasks).
FEP − CANN vs FEP + CANN.
FEP + CANN patients tended to perform better than FEP − CANN patients but this difference was significant only for 3 variables (table 5; figure 2). On verbal and nonverbal memory, between-group differences were small to moderate (d ranged from 0.3 to 0.6) and FEP + CANN performed significantly better on visual reproduction. For the working memory domain, small to medium effect sizes were observed and a between-group difference for SWM-errors reached significance (ie, with FEP + CANN performing better). For executive functioning, a significant between-group difference with a large effect size (d = 0.90) was observed for the ToL test (again with FEP + CANN performing better).
Comorbid Substance Use.
ANCOVA analyses were conducted to examine the effect of additional illicit substance use (over lifetime) on cognition in the FEP + CANN patients. FEP + CANN patients who also used amphetamines (n = 12) did not perform significantly differently to the remaining (nonamphetamine using) FEP + CANN patients on any cognitive measure (d ranged from −0.50 to −0.39). Similarly, no significant differences were found between the alcohol-using and nonusing FEP + CANN patients (d ranged from −0.10 to +0.54).
Age of Onset of Cannabis Use.
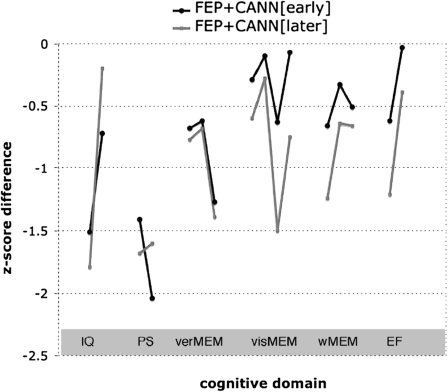
When comparing the noncannabis-using patients (FEP − CANN) with early onset (≤16 years; FEP + CANN[early]) and later onset (≥17 years; FEP + CANN[later]) cannabis-using patients, we found group differences for 4 cognitive tests (SWM-errors, ToL, Pattern Recognition, and Spatial Recognition). The FEP − CANN patients did not differ from FEP + CANN[later] on any cognitive tests (figure 3). FEP + CANN[early] patients performed significantly better than FEP − CANN on all 4 of the above cognitive tests and better than FEP + CANN[later] on 2 tests (Pattern Recognition and Spatial Recognition). The FEP + CANN[early] group were younger than the FEP + CANN[later] group (table 6) but did not differ in duration or monthly quantity of cannabis use, diagnosis, treatment, or antipsychotic levels (based on chlorpromazine equivalents; see table 6).
Fig. 3.
Mean Z Score Differences for Early and Late Cannabis Using FEP Groups.
Note: IQ, intelligence; PS, processing speed; verMEM, verbal memory; visMEM, visual memory; wMEM, working memory; EF, executive functioning. Note that each point reflects a specific task within the 6 domains of interest (refer to the Methods section and table 5 for the specific tasks).
Table 6.
Cannabis Use and Clinical Characteristics of Patient Groups Based on Age of Onset of Cannabis Use
| FEP − CANN (n = 26) | FEP + CANN[later] (n = 27) | FEP + CAN[early] (n = 32) | P Value | |
| Demographic | ||||
| Age (y) | 20.6 (3.5) | 22.2 (2.6) | 19.5 (2.5) | .003a |
| Gender (male/female) | 15/11 | 20/7 | 23/9 | .39 |
| Education | 11.1 (1.6) | 11.0 (1.5) | 11.5 (1.5) | .52 |
| GAF-premorbid | 74.8 (11.7) | 64.9 (14.3) | 75.8 (10.4) | .06 |
| GAF-entry | 32.4 (7.5) | 31.2 (6.3) | 27.9 (8.9) | .34 |
| Cannabis Use | ||||
| Age of Onset (y) | 17.9 (1.0) | 14.4 (1.6) | <.001 | |
| Duration (y) | 3.9 (2.4) | 4.8 (2.3) | .13 | |
| Quantity (g/month) | 11.5 (15.8) | 20.8 (21.5) | .10 | |
| Diagnosis | .68 | |||
| Schizophreniform | 8 (31%) | 8 (30%) | 11 (34%) | |
| Schizophrenia | 7 (27%) | 11 (41%) | 12 (38%) | |
| Mood disorder | 8 (31%) | 5 (18%) | 6 (19%) | |
| Other Psychoses | 3 (11%) | 3 (11%) | 3 (9%) | |
| Symptom | ||||
| Positive | 21.9 (6.7) | 25.8 (6.3) | 24.3 (5.8) | .08 |
| Negative | 20.4 (8.1) | 20.8 (7.4) | 21.4 (7.3) | .89 |
| Antipsychotic use | .77 | |||
| Risperidone | 12 (46%) | 18 (67%) | 18 (56%) | |
| Olanzapine | 7 (27%) | 5 (19%) | 7 (22%) | |
| Typicals | 2 (8%) | 0 (0%) | 1 (3%) | |
| No antipsychotics | 4 (16%) | 4 (15%) | 4 (13%) | |
| Missing | 1 (4%) | 2 (6%) | ||
| Chlorpromazine equivalent | 204 (259) | 136 (102) | 169 (141) | 0.40 |
Note: GAF, Global Assessment of Functioning. Additional abbreviations are explained in the footnote to table 1.
Post hoc tests; FEP + CANN[later] vs FEP + CANN[early] P = .002; FEP + CANN[later] vs FEP − CANN P = .12; FEP + CANN[early] vs FEP − CANN P = .36.
Recency of Cannabis Use.
FEP patients who had used cannabis in the previous week were more impaired on Logical Memory compared with patients who were abstinent for greater than 1 month prior to testing (P = .04). There were no significant correlations between cognition and other cannabis use parameters (frequency, quantity, and duration of use).
Associations between Clinical Variables and Cognition.
In both FEP groups, the Positive and Negative Syndrome Scale negative symptoms score was negatively correlated with Digit Symbol Coding (FEP + CANN: r = −.41, P = .006, FEP − CANN: r = −.58, P = .02) and Current IQ (FEP + CANN: r = −.43, P = .002, FEP − CANN: r = −.55, P = .01). The positive symptoms score was not significantly correlated with any cognitive measure.
Discussion
In Study I, we demonstrated that regular cannabis use was associated with better cognitive performance in schizophrenia. Cannabis-using patients performed moderately better than nonusing patients on measures of global cognition, visual memory, processing speed, working memory, planning, and reasoning. This difference was largely driven by studies that included patients with a lifetime history of cannabis use rather than just those with current or recent use. Consistent with previous studies, cannabis use was associated with a younger age of psychosis onset, male sex, and more positive symptoms.7,36,58–60 Study II examined the effect of regular cannabis use on cognition in FEP patients. Cognitive performance of FEP patients with a history of cannabis use was compared with FEP patients without a history of substance use and healthy controls on 6 cognitive domains. Unlike most previous studies, we also examined the effect of variables such as recency and frequency of use and age of onset of cannabis use. The findings were consistent with Study I and demonstrated that FEP patients who used cannabis (especially those who used prior to age 17) also performed better than nonusing patients in some domains and were less impaired relative to healthy controls on cognitive domains, including visual memory, working memory, planning, and reasoning. When age of onset of cannabis use was considered, superior cognitive performance in cannabis-using patients relative to nonusing patients was only observed among those who started using cannabis at an early age (≤16 years). Together, the findings of our meta-analysis and empirical study suggest that comorbid cannabis use is associated with a superior cognitive profile in schizophrenia-spectrum disorders.
Several explanations regarding the nature of the association between pre-illness onset cannabis use and subsequent development of schizophrenia have been suggested. One explanation is that cannabis is a risk factor that precipitates (but does not cause) the onset of psychosis in genetically predisposed individuals.61 However, there is also evidence that cannabis use prior to illness onset is “causally” related to the development of subsequent psychosis in vulnerable individuals.62–64 Similarly, several recent systematic reviews have indicated that cannabis may double the risk of later developing a psychotic illness and that this association is dose dependent.34,63,65 However, few previous studies have considered the role of cognition. Our findings raise a number of further questions concerning the relationship between cannabis, cognition, and schizophrenia.
Some authors have suggested a neuroprotective role of cannabis66 to explain the association between cannabis use and enhanced cognition in schizophrenia patients.19,67 Other authors suggest that, in the short-term, cannabinoids could stimulate prefrontal neurotransmission to enhance cognitive functions,22,34,67,68 but in the long-term, repeated administration is detrimental.12 However, these postulates remain speculative and further work is necessary. It is well recognized that acute administration of THC impairs rather than enhances cognitive performance in both healthy controls12,17 and patients with psychosis17,69, suggesting that acute neuroprotective effects of THC are unlikely. Findings of our meta-analysis and FEP study also provide evidence regarding adverse effects of recent cannabis use, which may partly mask the positive association between lifetime cannabis use and better cognition. Another possibility is that cannabis-using patients had superior social skills in order to be able to acquire and sustain a drug habit,12,21 which is also reflected in their cognition. Few studies have directly examined this possibility, and this notion is not always supported by the extant data70; however, we did not find any current or premorbid Global Assessment of Functioning (GAF) differences between FEP users and nonusers in our study.
While the FEP patients with a cannabis use history were generally superior in their cognitive profile to those without a history of cannabis use, the former group was not homogeneous. Indeed, the cognitive profile was markedly different depending on the age of onset of cannabis use, with the early onset group having superior cognition, which cannot be explained by differences in the duration or dosage of cannabis used. This finding is somewhat against expectation and is counterintuitive given the associations between cannabis use and cognition in healthy controls (ie, worse cognition in those with early onset use12). It is possible that in the FEP group with comorbid cannabis use (characterized by only relatively mild cognitive impairments), early onset cannabis use increased the risk for developing psychosis, thereby facilitating the transition to frank psychosis that might otherwise not have occurred. That is, early cannabis use may induce psychosis onset in less cognitively vulnerable individuals. Schnell and colleagues20 as well as Løberg and colleagues29 have proposed a similar hypothesis. Support for this comes from findings that early onset of cannabis use in adolescence, particularly before the age of 15, is associated with greater risk for the subsequent development of psychotic disorders, even after controlling for preexisting psychotic symptoms.46 It is possible that these individuals would have remained asymptomatic or would have been symptomatic only in subsequent years, if they did not abuse cannabis. This notion is consistent with Jockers-Scherubl et al19 who found an association between earlier onset of cannabis use and better cognition in schizophrenia. Such a notion could also help explain why cessation of substance use after the first onset of psychosis significantly increases the probability of remission and improves long-term outcomes in FEP patients.71 Interestingly, a recent study also found fewer neurological soft signs in heavy cannabis-using FEP patients, supporting the notion that these patients have less neurological impairment.72
Other factors might also influence the association between cannabis use and cognitive deficits in schizophrenia, including dosage and frequency of use and cumulative exposure. Interestingly, while higher frequency of cannabis use could be expected to be associated with poorer cognition, the only 2 studies that have examined frequency of use in schizophrenia patients found the opposite effect (ie, better performance).20,67 While some authors propose that such results relate to the neuroprotective effects of cannabis,19,66,67 the findings also support our hypothesis of less severe cognitive impairment in a subgroup of neurocognitively less vulnerable patients, with more frequent and heavy use of cannabis necessary to induce psychosis.
Our findings should be interpreted in the context of several limitations. First, while the preceding discussion raises several alternative explanations for the association between cannabis use and cognition in schizophrenia, the cross-sectional nature of the studies considered prevents firm conclusions being drawn. Longitudinal studies are necessary to better understand causal interactions between relevant factors.
Second, our findings of poorer cognitive performance in patients without comorbid cannabis use could relate to a sampling bias, whereby the FEP − CANN group may include a greater proportion of “deficit syndrome” patients or patients with poorer premorbid functioning, worse cognition,73 and lower substance use.74 However, the fact that the FEP + CANN group in our Study II did not differ from the FEP − CANN group in terms of positive or negative symptoms, suggests that the latter group cannot be readily identified as deficit syndrome type. Furthermore, prepsychotic GAF scores and education did not differ between the 2 FEP groups. Consequently, our findings do not support this contention, but more detailed measures of premorbid functioning are required to conclusively address this issue.
Third, given the high rates of cannabis use in FEP and the relationship between cannabis use and the development of psychotic symptoms, it is important to consider what proportion of the FEP + CANN group had a cannabis-induced psychosis. The diagnostic criteria for substance-induced psychosis have recently been criticized,75 as a diagnosis of cannabis-induced psychosis is unlikely to be made if psychotic symptoms are still apparent 1 month following intoxication. Therefore, better cognitive performance, early onset use, and improvement with abstinence may be partially explained by inclusion of FEP + CANN patients with a cannabis-induced psychosis rather than an actual schizophrenia-spectrum disorder. Better diagnostic instruments and longitudinal assessments are required to examine this issue further.
Fourth, comorbid abuse of other substances could also affect the results because both cocaine and alcohol use have been associated with worse cognitive functioning in schizophrenia. However, the 2 studies (including our own) that examined the effect of comorbid alcohol use on cognition did not find an association.21 Examination of larger samples is necessary to properly examine the effect of comorbid alcohol abuse on cognition. The study of Potvin et al21 suggested that comorbid cocaine use could have a detrimental effect on cognition in cannabis-abusing patients with schizophrenia,28 but this finding is unlikely to influence the current results because there was minimal cocaine abuse in the studies included in our meta-analysis or among patients in our FEP study.
Fifth, it is possible that some components of cannabis, such as cannabidiol (a nonintoxicating constituent of cannabis sativa), might relieve psychotic symptoms and thereby improve cognition.76,77 There is also some evidence to suggest that, in certain subgroups of schizophrenia patients, THC administration shows beneficial effects.18 However, more research is required to understand these complex actions in the context of schizophrenia.
Sixth, the number of studies included was small and restricted our analysis to investigate broad rather than specific aspects of cognition (or individual cognitive tasks). Finally, studies included in the meta-analysis had small samples and low quality of data on several important parameters, such as duration of illness; age of onset of either cannabis use or psychosis; and frequency, quantity, or duration of cannabis use.
In conclusion, our meta-analysis and experimental data converge to indicate that cannabis use in both FEP and established schizophrenia is associated with better cognitive performance than nonuse, and fewer cognitive impairments relative to healthy controls. The association between better cognitive performance and cannabis use is driven by a subgroup of neurocognitively less impaired patients, who only developed psychosis after an early initiation of cannabis use (ie, during early adolescence). However, longitudinal studies in high-risk populations are needed to test this notion more decisively. Together, these findings suggest that a subgroup of psychotic patients may show improved outcomes (ie, partial recovery of cognitive functioning and less disability) if their cannabis use can be controlled. Moreover, our findings support the notion that, in some vulnerable individuals, abstinence from cannabis abuse could potentially prevent the development of psychosis.
Funding
National Health and Medical Research Council (NHMRC) of Australia (Grant 236175, 459111, 514604) and the University of Melbourne; NHMRC Clinical Career Development Awards (Grant 509345/454792 to M.Y. and W.J.B.); Colonial Foundation (to D.I.L.); NHMRC Clinical Career Developmental Award and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to S.J.W.); NHMRC CJ Martin Training Fellowship (454797 to A.F.); Ronald Phillip Griffith Fellowship, Faculty of Medicine, Dentistry, and Health Sciences, the University of Melbourne (to S.C.); NHMRC Senior Principal Research Fellowship (628386) and NHMRC Program Grants (350241, 566529 to C.P.); the Leenaards Foundation Switzerland (to P.C.).
Acknowledgments
All authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Gregg L, Barrowclough C, Haddock G. Reasons for increased substance use in psychosis. Clin Psychol Rev. 2007;27:494–510. doi: 10.1016/j.cpr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Mueser KT, Yarnold PR, Levinson DF, et al. Prevalence of substance abuse in schizophrenia: demographic and clinical correlates. Schizophr Bull. 1990;16(1):31–56. doi: 10.1093/schbul/16.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND. Substance use disorders in schizophrenia—clinical implications of comorbidity. Schizophr Bull. 2009;35:469–472. doi: 10.1093/schbul/sbp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. doi: 10.1093/schbul/sbp031. 2010;36:1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coldham EL, Addington J, Addington D. Medication adherence of individuals with a first episode of psychosis. Acta Psychiatr Scand. 2002;106:286–290. doi: 10.1034/j.1600-0447.2002.02437.x. [DOI] [PubMed] [Google Scholar]
- 6.Grech A, Van Os J, Jones PB, Lewis SW, Murray RM. Cannabis use and outcome of recent onset psychosis. Eur Psychiatry. 2005;20:349–353. doi: 10.1016/j.eurpsy.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry. 1994;51:273–279. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- 8.Yücel M, Solowij N, Respondek C, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 9.Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 10.Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- 11.Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26:309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- 12.Solowij N, Michie PT. Cannabis and cognitive dysfunction: parallels with endophenotypes of schizophrenia? J Psychiatry Neurosci. 2007;32(1):30–52. [PMC free article] [PubMed] [Google Scholar]
- 13.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 14.Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 15.Pope HG, Jr., Gruber AJ, Yurgelun-Todd D. Residual neuropsychologic effects of cannabis. Curr Psychiatry Rep. 2001;3:507–512. doi: 10.1007/s11920-001-0045-7. [DOI] [PubMed] [Google Scholar]
- 16.Solowij N. Do cognitive impairments recover following cessation of cannabis use? Life Sci. 1995;56:2119–2126. doi: 10.1016/0024-3205(95)00197-e. [DOI] [PubMed] [Google Scholar]
- 17.D'Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Schwarcz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29:255–258. doi: 10.1097/JCP.0b013e3181a6bc3b. [DOI] [PubMed] [Google Scholar]
- 19.Jockers-Scherubl MC, Wolf T, Radzei N, et al. Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1054–1063. doi: 10.1016/j.pnpbp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Schnell T, Koethe D, Daumann J, Gouzoulis-Mayfrank E. The role of cannabis in cognitive functioning of patients with schizophrenia. Psychopharmacology (Berl) 2009;205:45–52. doi: 10.1007/s00213-009-1512-9. [DOI] [PubMed] [Google Scholar]
- 21.Potvin S, Joyal CC, Pelletier J, Stip E. Contradictory cognitive capacities among substance-abusing patients with schizophrenia: a meta-analysis. Schizophr Res. 2008;100:242–251. doi: 10.1016/j.schres.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Coulston CM, Perdices M, Tennant CC. The neuropsychology of cannabis and other substance use in schizophrenia: review of the literature and critical evaluation of methodological issues. Aust N Z J Psychiatry. 2007;41:869–884. doi: 10.1080/00048670701634952. [DOI] [PubMed] [Google Scholar]
- 23.Kumra S, Thaden E, DeThomas C, Kranzler H. Correlates of substance abuse in adolescents with treatment-refractory schizophrenia and schizoaffective disorder. Schizophr Res. 2005;73:369–371. doi: 10.1016/j.schres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Liraud F, Verdoux H. [Effect of comorbid substance use on neuropsychological performance in subjects with psychotic or mood disorders] Encephale. 2002;28(2):160–168. [PubMed] [Google Scholar]
- 25.de la Serna E, Mayoral M, Baeza I, et al. Cognitive functioning in children and adolescents in their first episode of psychosis: differences between previous cannabis users and nonusers. J Nerv Ment Dis. 2010;198(2):159–162. doi: 10.1097/NMD.0b013e3181cc0d41. [DOI] [PubMed] [Google Scholar]
- 26.Mata I, Rodriguez-Sanchez JM, Pelayo-Teran JM, et al. Cannabis abuse is associated with decision-making impairment among first-episode patients with schizophrenia-spectrum psychosis. Psychol Med. 2008;38:1257–1266. doi: 10.1017/S0033291707002218. [DOI] [PubMed] [Google Scholar]
- 27.Joyal CC, Halle P, Lapierre D, Hodgins S. Drug abuse and/or dependence and better neuropsychological performance in patients with schizophrenia. Schizophr Res. 2003;63:297–299. doi: 10.1016/s0920-9964(02)00387-0. [DOI] [PubMed] [Google Scholar]
- 28.Potvin S, Briand C, Prouteau A, et al. CANTAB explicit memory is less impaired in addicted schizophrenia patients. Brain Cogn. 2005;59(1):38–42. doi: 10.1016/j.bandc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Løberg EM, Hugdahl K. Cannabis use and cognition in schizophrenia. Front Hum Neurosci. 2009;3:53. doi: 10.3389/neuro.09.053.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 31.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sevy S, Burdick KE, Visweswaraiah H, et al. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr Res. 2007;92(1–3):74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr Res. 2005;75(1):135–137. doi: 10.1016/j.schres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Cohen M, Solowij N, Carr V. Cannabis, cannabinoids and schizophrenia: integration of the evidence. Aust N Z J Psychiatry. 2008;42:357–368. doi: 10.1080/00048670801961156. [DOI] [PubMed] [Google Scholar]
- 35.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161:501–506. doi: 10.1176/appi.ajp.161.3.501. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Pinto A, Vega P, Ibanez B, et al. Impact of cannabis and other drugs on age at onset of psychosis. J Clin Psychiatry. 2008;69:1210–1216. doi: 10.4088/jcp.v69n0802. [DOI] [PubMed] [Google Scholar]
- 38.Velakoulis D, Pantelis C, McGorry PD, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56(2):133–141. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- 39.Velakoulis D, Wood SJ, Smith DJ, et al. Increased duration of illness is associated with reduced volume in right medial temporal/anterior cingulate grey matter in patients with chronic schizophrenia. Schizophr Res. 2002;57(1):43–49. doi: 10.1016/s0920-9964(01)00307-3. [DOI] [PubMed] [Google Scholar]
- 40.Wood SJ, Pantelis C, Proffitt T, et al. Spatial working memory ability is a marker of risk-for-psychosis. Psychol Med. 2003;33:1239–1247. doi: 10.1017/s0033291703008067. [DOI] [PubMed] [Google Scholar]
- 41.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (Clinician Version) 1st ed. Washington, DC: APA; 1997. [Google Scholar]
- 42.Haro JM, Kamath SA, Ochoa S, et al. The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand Suppl. 2003;107(416):16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- 43.Morley KI, Cotton SM, Conus P, et al. Familial psychopathology in the First Episode Psychosis Outcome Study. Aust N Z J Psychiatry. 2008;42:617–626. doi: 10.1080/00048670802119754. [DOI] [PubMed] [Google Scholar]
- 44.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 45.McGorry PD, Kaplan I, Dossetor C, Herrman H, Copolov D, Singh B. Royal Park Multidiagnostic Instrument for Psychosis. Melbourne, Australia: National Health and Medical Research Council; 1989. [Google Scholar]
- 46.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 48.Nelson HE, Willison JR. Restandardisation of the NART against the WAIS-R. In: Nelson HE, editor. National Adult Reading Test (NART): Test Manual. Windsor, ON: NFER-Nelson; 1991. pp. 13–23. [Google Scholar]
- 49.Wechsler D. Wechsler Abbreviated Intelligence Scale—Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 50.Reitan RM, Wolfson D. The Trail Making Test as an initial screening procedure for neuropsychological impairment in older children. Arch Clin Neuropsychol. 2004;19:281–288. doi: 10.1016/S0887-6177(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 51.Wechsler D. Wechsler Memory Scale—Revised Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 52.Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33(1):1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- 53.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 54.Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 55.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 56.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298(1089):199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 57.Cohen JD. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 58.DeLisi LE. The effect of cannabis on the brain: can it cause brain anomalies that lead to increased risk for schizophrenia? Curr Opin Psychiatry. 2008;21(2):140–150. doi: 10.1097/YCO.0b013e3282f51266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dubertret C, Bidard I, Ades J, Gorwood P. Lifetime positive symptoms in patients with schizophrenia and cannabis abuse are partially explained by co-morbid addiction. Schizophr Res. 2006;86:284–290. doi: 10.1016/j.schres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Talamo A, Centorrino F, Tondo L, Dimitri A, Hennen J, Baldessarini RJ. Comorbid substance-use in schizophrenia: relation to positive and negative symptoms. Schizophr Res. 2006;86:251–255. doi: 10.1016/j.schres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Hambrecht M, Hafner H. Cannabis, vulnerability, and the onset of schizophrenia: an epidemiological perspective. Aust N Z J Psychiatry. 2000;34:468–475. doi: 10.1080/j.1440-1614.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- 62.Smit F, Bolier L, Cuijpers P. Cannabis use and the risk of later schizophrenia: a review. Addiction. 2004;99:425–430. doi: 10.1111/j.1360-0443.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 63.Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- 64.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 65.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 66.Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coulston CM, Perdices M, Tennant CC. The neuropsychological correlates of cannabis use in schizophrenia: lifetime abuse/dependence, frequency of use, and recency of use. Schizophr Res. 2007;96(1–3):169–184. doi: 10.1016/j.schres.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Verrico CD, Jentsch JD, Roth RH. Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse. 2003;49(1):61–66. doi: 10.1002/syn.10215. [DOI] [PubMed] [Google Scholar]
- 69.Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39:1607–1616. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- 70.Ringen PA, Melle I, Birkenaes AB, et al. The level of illicit drug use is related to symptoms and premorbid functioning in severe mental illness. Acta Psychiatr Scand. 2008;118:297–304. doi: 10.1111/j.1600-0447.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- 71.Lambert M, Conus P, Lubman DI, et al. The impact of substance use disorders on clinical outcome in 643 patients with first-episode psychosis. Acta Psychiatr Scand. 2005;112(2):141–148. doi: 10.1111/j.1600-0447.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz-Veguilla M, Gurpegui M, Barrigon ML, et al. Fewer neurological soft signs among first episode psychosis patients with heavy cannabis use. Schizophr Res. 2009;107(2–3):158–164. doi: 10.1016/j.schres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Cohen AS, Saperstein AM, Gold JM, Kirkpatrick B, Carpenter WT, Jr., Buchanan RW. Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophr Bull. 2007;33:1201–1212. doi: 10.1093/schbul/sbl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirkpatrick B, Ram R, Bromet E. The deficit syndrome in the Suffolk County Mental Health Project. Schizophr Res. 1996;22(2):119–126. doi: 10.1016/s0920-9964(96)00057-6. [DOI] [PubMed] [Google Scholar]
- 75.Mathias S, Lubman DI, Hides L. Substance-induced psychosis: a diagnostic conundrum. J Clin Psychiatry. 2008;69:358–367. doi: 10.4088/jcp.v69n0304. [DOI] [PubMed] [Google Scholar]
- 76.Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM. Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol. 2009;51:528–534. doi: 10.1016/j.jhep.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 77.Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192:306–307. doi: 10.1192/bjp.bp.107.046649. [DOI] [PubMed] [Google Scholar]
- 78.Ringen PA, Vaskinn A, Sundet K, et al. Opposite relationships between cannabis use and neurocognitive functioning in bipolar disorder and schizophrenia. Psychol Med. 2009;6:1–11. doi: 10.1017/S0033291709991620. [DOI] [PubMed] [Google Scholar]
- 79.Scholes KE, Martin-Iverson MT. Cannabis use and neuropsychological performance in healthy individuals and patients with schizophrenia. Psychol Med. 2009;17:1–12. doi: 10.1017/S0033291709992078. [DOI] [PubMed] [Google Scholar]