Abstract
Quality of life (QOL) has been recognized as a crucial domain of outcome in schizophrenia treatment, and yet its determinants are not well understood. Recent meta-analyses suggest that symptoms have only a modest relationship to QOL (Eack SM, Newhill CE. Psychiatric symptoms and quality of life in schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:1225–1237). Individuals with schizophrenia show 1–2 SD deficits on measures of elementary neurocognition, and links between these deficits and objective measures of community functioning (eg, employment and independent living) are well established. While objective measures of community functioning and measures of QOL would appear to be closely related, studies investigating the ability of neurocognitive variables to predict QOL in individuals with schizophrenia have yielded conflicting results. One potential explanation for opposing findings in the schizophrenia literature is the interchangeable use of objective and subjective indices of QOL. This study used quantitative methods of meta-analysis to clarify the relationship between neurocognitive determinants of objective QOL (ie, observable, clinician-rated) and subjective QOL (ie, patient satisfaction) separately in individuals with schizophrenia. A total of 20 studies (10 objective and 10 subjective) consisting of 1615 clients were aggregated from relevant databases. Weighted effect size analysis revealed that there were small–moderate relationships (d ≤ 0.55) between crystallized verbal ability, working memory verbal list learning, processing speed, and executive function and objective indices of QOL. In contrast, results revealed either nonsignificant or inverse relationships for the vast majority of neurocognitive measures and measures of subjective QOL. Moderating variables and implications for future research and treatment development are discussed.
Keywords: outcome, cognition, psychotic illness
Introduction
With the emergence of more effective pharmacologic management of acute psychiatric symptoms in schizophrenia over the past 20 years, increasing attention has been paid to the development of interventions targeted at improving the long-term functional and subjective outcomes for people with the illness. One of the dominant approaches to measurement of outcome in the schizophrenia literature has been the use of scales designed to assess the construct of quality of life (QOL2). Although there is not a single definition of QOL, most agree that it is a multidimensional construct that includes a person’s subjective sense of well-being, functional status, and access to resources and opportunities.3 Thus, knowledge of the determinants of QOL in individuals with schizophrenia is of key importance in tailoring effective interventions to improve the lives of people with the disease. Despite this fact, an understanding of determinants of QOL in schizophrenia remains elusive. An obvious candidate would be persistent psychiatric symptoms. However, a recent meta-analysis by Eack and Newhill1 found only small negative relationships between levels of psychiatric symptoms and QOL, with general psychopathology (eg, anxiety and depression) showing the strongest association across all QOL domains. Therefore, although psychiatric symptoms clearly influence QOL in individuals with schizophrenia, they explain only a modest proportion of variance in QOL.
Neurocognitive deficits are a core aspect of schizophrenia.4 Individuals diagnosed with schizophrenia consistently show 1–2 SD deficits on measures of speed of processing, attention/vigilance, working memory, verbal learning and memory, visual learning and memory, reasoning, and problem solving.5 Particular significance has been attached to these deficits as many have been moderately associated with impaired community functioning (eg, living or vocational status) in individuals with schizophrenia, both cross-sectionally and longitudinally.6–8 Moreover, these deficits may actually better account for the diversity of community outcomes in schizophrenia than positive or negative symptoms.9
While neurocognitive deficits have been linked to impairment on measures of objective measures of community function, results of studies examining the relationship between neurocognition and QOL in patients with schizophrenia have been mixed. Some studies have demonstrated a positive relationship between neurocognitive domains and aspects of QOL,10,11 whereas others show an inverse relationship.12,13 In other cases, the data revealed no relationship between neurocognitive deficits and QOL.14,15 For example, the same measure of executive function, the Wisconsin Card Sorting Test (WCST) perseverative error (PE) score, has been linked to QOL when measured by the Heinrichs–Carpenter Quality of Life Scale (QLS),10 an objective measure of QOL, but was shown to be unrelated to QOL in a second study that selected the World Health Organization Quality of Life Scale-Brief (WHOQOL-BREF15), a subjective measure of QOL.
Given: (1) the importance of understanding determinants of QOL in schizophrenia for developing effective clinical treatment interventions that would improve patient functional and subjective well-being, (2) the growing literature on neurocognitive predictors of QOL in patients with schizophrenia over the past 10 years, and (3) the highly contradictory findings across studies, a quantitative meta-analysis of the literature was warranted. The present study was formulated with the hypothesis that the discordance in findings regarding neurocognition and QOL might be explained by the considerable variance in types of QOL measures used by different research teams. Indeed, a review of the general medical literature, Gill and Feinstein16 found 159 different “QOL” measures used in the 75 studies they evaluated.
One approach to classifying measures of QOL is to view global well-being as a composite of at least somewhat independent dimensions of objective QOL indicators and subjective QOL indicators as well as personal characteristics.17 By this view, objective QOL refers to observable life conditions of the client and may be assessed through clinician ratings or client self-report, but in either case, the patient’s current or recent functional status is under review. In this regard, the construct of objective QOL has considerable overlap with more general constructs and measures of community/social functioning. Subjective QOL, according to this model, specifically refers to client satisfaction across parallel life domains. For example, the domain of social relations might be measured by asking questions about the frequency of the patient’s social contacts, eg, “How often do you spend time with close friends?” In contrast, subjective QOL for social relations measures patient satisfaction, asking for a subjective assessment of quality of the patient’s interactions with others, eg, “How do you feel about the amount of time you spend with other people?” (eg, Quality of Life Interview [QOLI]17).
To our knowledge, there have been no systematic literature analyses examining the relationship between neurocognition and QOL in patients with schizophrenia. We sought to use quantitative meta-analytic methods to: (1) determine whether there was a differential relationship between neurocognition and objective and subjective QOL in individuals with schizophrenia, (2) estimate the overall magnitude of these relationships, and (3) examine variables that moderate the relationship between neurocognition and subjective and objective QOL (eg, age, illness duration, and symptoms). We predicted: (1) because objective QOL indicators overlap considerably with measures of community functioning, we would replicate previous findings6,7 and uncover small–medium effect sizes between neurocognitive measures and objective QOL; (2) in contrast, we predicted a negligible relationship between neurocognition and subjective QOL, as previous studies in both schizophrenia and nonpsychiatric populations have shown a nonsignificant relationship between general neurocognitive functioning and subjective QOL instruments.15,18–20
Methods
Literature Search
We conducted parallel literature searches in the PUBMED and PSYCINFO databases for all peer-reviewed, English-language articles published between January 01, 1980 and January 10, 2009 using the search terms (“cognition” AND “schizophrenia” AND “QOL”) and (“cognition” AND “schizophrenia” AND “social functioning”) and (“severe mental illness” AND “QOL”). The year 1980 was selected as the cutoff in light of the introduction of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition for more reliable diagnostic criteria for schizophrenia illness.21 The reference sections of articles located from both searches were studied for relevant citations.
Inclusion Criteria
General study inclusion criteria were as follows: (1) at least one-third participants with schizophrenia or schizoaffective disorder, (2) use of standard neuropsychological test battery, (3) cross-sectional relationship without treatment intervention, (4) use of either an objective and/or subjective QOL measure that relied on client self-report, measured multiple life domains, and that had been validated for use in schizophrenia, and (5) study statistics were convertible to effect size d (eg, Pearson r, beta regression coefficients).
These database searches yielded 518 potential studies. The majority of these studies were excluded because they did not use a standard neuropsychological test battery or a dedicated QOL instrument that met criteria for inclusion or did not study individuals with schizophrenia. Others measured neurocognitive functioning and QOL but did not present data relating the 2 variables. In addition, studies not using a cross-sectional paradigm were excluded. However, longitudinal studies that presented baseline correlations between neurocognitive measures and QOL were included. Upon review, 27 studies met our study inclusion criteria. Of these, 11 authors who did not publish correlation coefficients between individual neurocognitive measures and total QOL were solicited for additional data. In total, 20 studies (10 objective and 10 subjective) were included in our analysis. See table 1 for detailed study descriptions.
Table 1.
Neurocognition and QOL in Schizophrenia
| Study | Sample | Neurocognitive Measures | QOL Measure | Major Findings |
| Studies of objective QOL | ||||
| Addington and Addington10 | 50 FE participants (88% S), 53 ME participants (100% S), and 55 NPC | WAIS-digit symbol, letter–number sequencing; CPT; WMS-LMI, LMII; RAVLT-immediate, delayed; WCST-CAT, PE | QLS | Cognition predicted QLS scores at time 1 and time 2 for FE, ME, and NPC groups. |
| Addington and Addington22 | 80 outpatient participants (100% S) | WAIS-vocabulary subtest; CPT; WMS-LMI, LMII; WCST-CAT, PE | QLS | Poor executive function was significantly correlated with low scores on the QLS. |
| Dickinson and Coursey23 | 20 outpatient participants (92.5% S or SA) | WAIS-vocabulary, digit span, letter–number sequencing, symbol-digit subtests | LOF | Neurocognitive measures (except for digit span) were positively associated with LOF. |
| Fiszdon et al24 | 151 outpatient participants (100% S or SA) | WAIS-digit span and digit symbol subtests; WMS-LMI; HVLT-immediate | QLS | At intake, none of the neurocognitive variables were significantly associated with QLS total. |
| Heslegrave et al14 | 42 outpatient participants (100% S) | Computerized WCST-PE | SIP | Neurocognitive impairment generally unrelated to objective QOL. |
| Lipkovich et al25 | 414 outpatient participants (100% S or SA) | WAIS-letter–number sequencing, RAVLT (with 10 min Crawford alternative) | QLS | At baseline, multiple QLS domains significantly related to processing speed, working memory, and verbal memory. |
| Lysaker and Davis11 | 65 outpatient participants (100% S or SA) | WAIS-vocabulary subtest; HVLT-delayed, WCST-PE | QLS | All 3 neurocognitive measures were correlated with at least one domain of the QLS. |
| Matsui et al26 | 53 outpatient participants (100% S) and 31 NPC | JVLT-immediate | QLS | QLS total score was significantly predicted by the script and sentence memory tests. |
| Narvaez et al27 | 88 outpatient participants (100% S or SA) | WAIS-digit span, digit symbol, letter–number sequencing subtests; WMS-LMI, LMII; WCST-PE, CAT | Objective section of QOLI | List learning and WCST measures were positively associated with objective QOL. |
| Savilla et al28 | 57 outpatient participants (100% S) | BACS-list learning-immediate, digit sequencing task, symbol coding | QLS | Cognitive functioning was positively associated objective QOL. |
| Studies of subjective QOL | ||||
| Alptekin et al29 | 38 outpatient participants (100% S), 31 NPC | WAIS-digit span; COWAT-letter fluency | WHOQOL-BREF | The social domain scores of the WHOQOL were positively correlated with digit span and COWAT. |
| Brekke et al30 | 40 outpatient participants (100% S) | WCST-PE | SWL | Negative relationship between WCST and SWL. |
| Brissos et al18 | 30 euthymic bipolar I participants, 23 remitted schizophrenia participants (100% S), and 23 NPC | WAIS-digit span subtest, WMS-LMI, LMII; Symbol-Digit Modalities Test; Trail Making Test-A, B; COWAT-letter fluency | WHOQOL-BREF | No correlations between any of the domains of the WHOQOL-BREF and any neurocognitive variables. |
| Chino et al19 | 36 outpatient participants (100% S) | RAVLT-immediate; Letter Fluency Test | WHOQOL-BREF | Neurocognitive test results were not correlated with subjective QOL. |
| Corrigan and Buican12 | 49 participants in transition out of state hospital (80.8% S, SA or mood disorder) | WAIS-vocabulary subtest | Subjective section of QOLI | Verbal ability was inversely related to subjective QOLI. |
| Dickerson et al31 | 72 outpatient participants (100% S) | WAIS-vocabulary, digit span, digit symbol subtests; WMS-LMI, LMII; Trail Making Test-A, B; WCST-PE, CAT | Subjective section of QOLI | Inverse relationship between WMS-LMI and subjective QOLI. |
| Herman32 | 46 inpatients dually diagnosed with schizophrenia and substance abuse, 43 inpatients with schizophrenia | WAIS-vocabulary, digit span, digit symbol; subtests COWAT-letter fluency; WMS-LMI, LMII; Trail Making Test-A, B | WHOQOL-BREF | Subjective QOL was only positively correlated with COWAT. |
| Hofer et al15 | 60 outpatient participants (100% S) | CVLT-immediate (German version); WCST-PE, CAT | WHOQOL-BREF | No significant relationship found between neurocognitive variables and subjective QOL. |
| Narvaez et al27 | 88 outpatient participants (100% S or SA) | WAIS-digit span, digit symbol, letter–number sequencing subtests; Letter Fluency; WMS-LMI, LMII, Trail Making Test-A, B; WCST-PE, CAT | Subjective section of QOLI | Better neuropsychological functioning independently predicted worse subjective QOL. |
| Smith et al33 | 46 outpatient participants (100% S or SA) | CVLT-immediate; WCST-CAT | Subjective section of QOLI | Subjective QOL was not correlated with any neurocognitive variables. |
Note: FE, first episode; ME, multiepisode; NPC, nonpsychiatric control; S, schizophrenia; SA, schizoaffective disorder; COWAT, Controlled Word Association Test; WMS, Weschler Memory Scale; LMI, Logical Memory Immediate Recall; LMII, Logical Memory Delayed Recall; WCST, Wisconsin Card Sorting Test (CAT, categories; PEs, perseverative errors); SPAN, Span of Apprehension; CPT, Continuous Performance Test; CVLT, California Verbal Learning Test; JVLT, Japanese Verbal Learning Test; HVLT, Hopkins Verbal Learning Test; RAVLT, Rey Auditory Verbal Learning Test; QLS, Heinrichs–Carpenter Quality of Life Scale; SIP, Sickness Impact Profile; LOF, Strauss–Carpenter Level of Functioning Scale; QOLI, Lehman Quality of Life Interview; SWL, Satisfaction with Life Scale; WAIS, Wechsler Adult Intelligence Scale; WHOQOL-BREF, World Health Organization Quality of Life Scale (short form); QOL, Quality of life.
Measure Selection
Neurocognitive Measures.
To ensure stability of findings, neurocognitive measures were selected for inclusion in this meta-analysis based on their use in at least 3 different studies. A total of 14 different neurocognitive measures were selected (see table 2). The following neurocognitive domains were included for analysis: crystallized verbal ability, fluency, vigilance, working memory, prose recall, list learning, processing speed, and executive function. Effect sizes were calculated and aggregated from individual cognitive tests with consistent outcome measures to minimize the combination of effect sizes from different tests and different outcome measures from the same test that could be tapping different neurocognitive constructs. For example, performance on the WCST is measured with multiple scores, most often either categories achieved (CAT) or number of PEs. These 2 outcome measures, while clearly related and from the same test, measure different presumed underlying constructs, concept formation and flexibility on the one hand, and set-shifting on the other. Thus, we examined these scores separately in this analysis.
Table 2.
Neurocognitive Measures Included in the Meta-Analysis
| Neurocognitive Domain | Measures |
| Crystallized verbal ability | Weschler Adult Intelligence Scale-Vocabulary Subtest |
| Fluency | Letter Fluency, Controlled Oral Word Association Test (COWA-FAS) |
| Vigilance | Continuous Performance Test |
| Working memory | Digit Span Subtest of the WAIS (Digit Span) |
| Prose recall | Weschler Memory Scale-Logical Memory, Immediate (LMI) and Long Delay (LMII) |
| List learning | California Verbal Learning Test, Hopkins Verbal Learning Test, Rey Auditory Verbal Learning Test (CVLT/HVLT/RAVLT immediate and delayed) |
| Processing speed | Digit Symbol Substitution Test, Trail Making Test-A |
| Executive function | Wisconsin Card Sorting Test-categories achieved (CAT) and -perseverative errors; Trail Making Test B |
In light of their high degree of test similarity, outcome measures were combined across each of 3 verbal list-learning measures, the Hopkins Verbal Learning Test (HVLT), Rey Auditory Verbal Learning Test (RAVLT), and the California Verbal Learning Test (CVLT). Results from Logical Memory subtests from the Wechsler Memory Scale (WMS) and WMS-Revised were also combined, as were results from the paper-and-pencil and computerized versions of the WCST.
QOL Measures.
In order to be considered for inclusion in the meta-analysis both subjective and objective QOL measures had to be: (1) validated in samples of individuals with schizophrenia, (2) measure multiple life domains (eg, occupation, social interactions, and recreation/leisure etc.), and (3) rely on client self-report. Four objective QOL measures meeting these criteria were selected: (1) the Heinrichs–Carpenter QLS,34 (2) the Lehman QOLI17 objective subscale, (3) the Strauss–Carpenter Specific Levels of Function scale (SLOF35), and (4) the Sickness Impact Profile (SIP36). There are differences among the scales chosen in that SIP uses a written questionnaire completed by the patient, whereas the QLS, QOLI, and SLOF use a rated interview format. In addition, the QLS, QOLI, SLOF, and SIP all assess multiple patient life domains; they include questions specifically related to occupation, social interactions, recreation/leisure, and emotional status. Other scales that measured patient’s multidimensional life function but did not rely on patient self-report were excluded (eg, Global Assessment of Function). It should be noted that some researchers have categorized the SIP as a “subjective” measure of QOL because it utilizes patient self-report.14,37,38 For the current study, however, we classified the measure as an objective index in light of its focus on objective life conditions and not on subjective ratings of life satisfaction.
Three subjective QOL measures met our criteria: (1) WHOQOL-BREF Version39, (2) the Lehman QOLI17 subjective subscale, and (3) the Satisfaction with Life Scale (SWL40). All 3 of these scales also rely on self-report of the patient, 2 in the form of a written questionnaire (WHOQOL-BREF and SWL), and 1 in the form of a structured interview (QOLI). All included scales assess multiple life domains; however, they are distinct from the objective scales in that they specifically measure the patient’s subjective satisfaction with their life conditions, as opposed to assessing objective functional status. We note that scales that combined objective QOL and subjective QOL questions in the same overall measure were excluded due to the comparative nature of the present study (eg, Lancashire Quality of Life Profile41).
Data Analysis
The software program “DSTAT v. 1.11”42 was used to calculate effect sizes and to carry out subsequent homogeneity and moderator variable analyses. The unit of analysis in a meta-analysis is the effect size (d). For purposes of the present study, the d score was always defined as the strength of the relationship between each neurocognitive variable and objective or subjective QOL measure expressed in SD units. For 14 studies, we converted r into Cohen d values. One study reported beta coefficients from a multiple regression not correlation coefficients. In this study, we converted beta values into an approximate r for meta-analysis using the method outlined by Peterson and Brown.43 Nonsignificant results from 5 studies lacking supporting statistical information were coded as an effect size of zero.44 Four studies did not present correlations for total QOL score but instead reported correlations of specific neurocognitive domains with specific QOL domains. For these studies, as we predicted a positive relationship between neurocognition and objective QOL, we conservatively coded the lowest summary domain correlation for studies of objective QOL. In contrast, because we predicted a negligible relationship between neurocognition and subjective QOL, for subjective QOL studies, we coded the highest domain correlation. Effects were categorized as small (d < 0.5), medium large (d = 0.5–0.8), or large (d > 0.845). All effect sizes were expressed in a way such that positive values indicate better performance on neurocognitive tests.
Individual values of d were thereafter combined across studies and weighted according to their variance using a fixed-effects model. Potential differences in effect size between studies were analyzed using the method of Hedges and Olkin.46 This procedure computes mean weighted effect sizes and 95% CIs for each variable subset and allows for the testing of the influence of each individual factor on the overall results using the Q statistic. To assess stability of underlying effects, we used a test for heterogeneity QT, which is based on the sum of squares of the individual effect sizes around the mean when each square is weighted by the inverse of the estimated variance of the effect size. Q has an asymptotic χ2 distribution and is analogous to the ANOVA. Studies were evaluated for within-group differences (QW) and between-group differences (QB) following the same model.
To partially address the “file drawer” or publication bias problem in meta-analytic investigations, in which null results in a research area are collected but not reported in the literature, we calculated a fail-safe N (NFS) for each class of outcome variable by the method of Orwin.47 This measure provides an estimate of the number of studies with null results that would be needed to reduce the obtained mean effect size to a nonsignificant level. In the absence of a universally accepted significance level for effect sizes, we considered an effect size of 0.05 nonsignificant.
Moderator Analyses
Moderator analyses were conducted when the test for heterogeneity (Qw) for a specific neuropsychological measure was significant. Results are not reported for nonsignificant moderator analyses. Study characteristics hypothesized to moderate the relationship of neurocognition and QOL were: treatment setting (inpatient, outpatient, or mixed), symptomatology (Positive and Negative Syndrome Scale48), sample age, gender (% male), illness duration, age of onset, number of hospitalizations, average daily antipsychotic medication dose in chlorpromazine (CPZ) equivalents, and type of QOL measure. In addition, we included 2 study quality characteristics in our moderator analysis (1) confirmatory Structured Clinical Interview for DSM-IV diagnostic interviews49 and (2) QOL raters blind to neuropsychological test results. All study characteristics were coded independently by 2 raters (A.W.T) and (M.M.K.) in a subsample of 40% of studies to ensure reliability of extraction of study characteristics. Inter-rater concordance for coding was calculated to be 96%. Continuous moderator variables (eg, participant age and illness duration) were analyzed with a continuous model50 with a z test for significance of model fit. Mean weighted effect sizes were directly compared for relationships between neurocognition and objective QOL and neurocognition and subjective QOL. These effect size comparisons were made only between independent samples of clients administered neurocognitive measures and objective or subjective QOL measures. While alpha was set at .05 for the study as a whole, given the high number of moderator analyses and corresponding inflation in risk for alpha error, we used a reduced alpha level of .01 for these specific comparisons.
Results
Study Characteristics
A summary of sample characteristics of the 10 objective QOL studies the 10 subjective QOL studies that met inclusion criteria for the meta-analysis are presented in table 3.
Table 3.
Sample Characteristics
| Variable | Objective QOL Studies, N = 10 | Subjective QOL Studies, N = 10 |
| Mean sample size | 107.40 (106.39) | 54.1 (22.53) |
| % Reporting | 100 | 100 |
| % Schizophrenia spectrum | 98.05 (4.25) | 94.25 (18.18) |
| % Reporting | 100 | 100 |
| Age in years | 38.57 (5.77) | 37.31 (5.21) |
| % Reporting | 100 | 100 |
| % Male | 71.33 (11.66) | 63.47 (6.52) |
| % Reporting | 100 | 100 |
| Education in years | 12.50 (0.71) | 11.63 (1.15) |
| % Reporting | 60 | 60 |
| Illness duration in years | 13.03 (7.73) | 14.12 (5.73) |
| % Reporting | 30 | 60 |
| Age of onset | 23.75 (1.01) | 21.90 (3.56) |
| % Reporting | 60 | 30 |
| Number of hospitalizations | 7.19 (4.51) | 4.13 (3.16) |
| % Reporting | 30 | 30 |
| PANSS positive | 16.06 (1.70) | 13.27 (3.26) |
| % Reporting | 50 | 40 |
| PANSS negative | 18.09 (3.34) | 16.65 (2.74) |
| % Reporting | 50 | 40 |
| HAM-D | 10.90 | 6.97 (5.56) |
| % Reporting | 10 | 20 |
| CPZ equivalents | 636.20 (223.14) | 399.38 (157.32) |
| % Reporting | 50 | 50 |
| Study quality score | 0.90 (1.1) | 0.40 (0.52) |
| % Reporting | 100 | 100 |
Note: PANSS, Positive and Negative Syndrome Scale; HAM-D, Hamilton Depression Rating Scale.
Neurocognitive Deficits and Objective QOL
As can be seen in table 4, the majority of neurocognitive domains were positively correlated with objective QOL. Small effect sizes were found for the relationship between crystallized verbal ability (Wechsler Adult Intelligence Scale [WAIS]-Vocabulary, d = 0.34, 95% CI: 0.13/0.55), working memory (WAIS-Digit Span, d = 0.26, 95% CI: 0.11/0.41; WAIS-Letter–Number Sequencing, d = 0.17, 95% CI: 0.06/0.28), verbal list learning (CVLT/HVLT/RAVLT immediate, d = 0.37, 95% CI: 0.24/0.51; CVLT/HVLT/RAVLT delayed, d = 0.13, CI: 0.01/0.25), processing speed (WAIS-Digit Symbol, d = 0.23, 95% CI: 0.10/0.36), and objective QOL. Executive function was found to have a small-medium effect size relationship to objective QOL (WCST-PE, d = 0.28, 95% CI: 0.14/0.41; WCST-CAT, d = 0.55, 95% CI: 0.38/0.72). Attention and prose recall were the only neurocognitive domains that were not significantly correlated (Ps > .08) with objective QOL.
Table 4.
Estimated Effect Sizes of the Relationship between Neurocognition and Objective QOL
| Measure | k | N | d | 95% CI | z | P | Qw | NFS |
| Crystallized verbal ability | ||||||||
| WAIS-Vocabulary | 3 | 185 | 0.34 | 0.13/0.55 | 3.23 | .001 | 3.76 | 17 |
| Vigilance | ||||||||
| CPT | 3 | 271 | 0.15 | −0.02/0.32 | 1.70 | .089 | 1.50 | N/A |
| Working memory | ||||||||
| Digit Span | 4 | 336 | 0.26 | 0.11/0.41 | 3.35 | .001 | 5.77 | 17 |
| Letter–Number Sequencing | 4 | 626 | 0.17 | 0.06/0.28 | 2.96 | .003 | 13.23* | 10 |
| Prose recall | ||||||||
| LM-Immediate | 4 | 422 | 0.11 | −0.02/0.25 | 1.65 | .099 | 4.17 | N/A |
| LM-Long Delay | 3 | 271 | 0.12 | −0.05/0.29 | 1.35 | .176 | 1.22 | N/A |
| List learning | ||||||||
| CVLT/HVLT/RAVLT-immediate | 4 | 452 | 0.37 | 0.24/0.51 | 5.57 | .000 | 11.67* | 26 |
| CVLT/HVLT/RAVLT-delayed | 3 | 563 | 0.13 | 0.01/0.25 | 2.19 | .028 | 6.16* | 5 |
| Processing speed | ||||||||
| Digit Symbol | 5 | 439 | 0.23 | 0.10/0.36 | 3.40 | .000 | 19.27* | 18 |
| Executive function | ||||||||
| WCST-PE | 5 | 439 | 0.28 | 0.14/0.41 | 2.61 | .000 | 10.76* | 23 |
| WCST-CAT | 3 | 271 | 0.55 | 0.38/0.72 | 0.63 | .000 | 0.85 | 30 |
Note: LM, logical memory; K, number of studies; N, number of participants; Qw, within-group homogeneity statistic. Abbreviations are explained in the first footnote to table 1.
*P < .05.
Heterogeneity measures suggested that the overall weighted mean effect of the relationships between objective QOL and processing speed, verbal list learning, working memory (only the Letter–Number Sequencing test), and executive function (only the PE score from the WCST) were not stable. Moderator analyses of processing speed revealed that greater age (Z = −3.17, P = .002), more years of education (Z = −2.8, P = .005), and a greater number of hospitalizations (Z = −3.08, P = .002) attenuated the relationship between processing speed and objective QOL. In addition, moderator analyses revealed that greater years of education (z = −2.86, P = .004) attenuated the relationship between list learning (immediate recall) and objective QOL. Greater daily antipsychotic medication dose (Z = −2.70, P = .007) and more negative symptoms (Z = −2.82, P = .005) attenuated the relationship between executive function (WCST-PE) and objective QOL. Longer duration of illness correlated with larger effect sizes between both measures of list learning (Z = 2.63, P = .008) and executive function and objective QOL (Z = 0.284, P = .004). Greater percentage of males (Z = −3.57, P = .000) and greater mean sample age of onset (Z = −3.10, P = .002) attenuated the relationship between working memory (Letter–Number Sequencing) and objective QOL.
Neurocognitive Deficits and Subjective QOL
As can be seen in table 5, the majority of neurocognitive domains were not significantly correlated with subjective QOL, with the exception of crystallized verbal ability and processing speed, which were negatively correlated with subjective QOL and letter fluency, which was positively correlated with subjective QOL. Small effect sizes were revealed for verbal IQ (WAIS-vocabulary, d = −0.29, 95% CI: −0.49/−0.10), processing speed (WAIS-digit symbol, d = −0.19, 95% CI: −0.36/−0.02), and letter fluency (d = 0.26, 95% CI: 0.09/0.43). Measures of attention, working memory, verbal list learning, prose recall, and executive function were not significantly correlated with subjective QOL (all Ps > .06).
Table 5.
Estimated Effect Sizes of the Relationship between Neurocognition and Subjective QOL
| Measure | k | N | d | 95% CI | z | P | Qw | NFS |
| Crystallized verbal ability | ||||||||
| WAIS-Vocabulary | 3 | 210 | −0.29 | −0.49/−0.10 | −2.96 | .003 | 18.56* | 14 |
| Working memory | ||||||||
| Digit Span | 5 | 310 | 0.01 | −0.15/0.17 | 0.10 | .917 | 22.00 | N/A |
| Prose recall | ||||||||
| LM-Immediate | 4 | 272 | −0.16 | −0.33/0.01 | −1.89 | .059 | −2.25 | N/A |
| LM-Long Delay | 4 | 272 | −0.06 | −0.23/0.10 | −0.74 | .459 | 1.15 | N/A |
| List learning | ||||||||
| CVLT/HVLT/RAVLT-immediate | 4 | 230 | 0.03 | −0.15/0.21 | 0.30 | .762 | 0.63 | N/A |
| Fluency | ||||||||
| Letter Fluency | 5 | 274 | 0.26 | 0.09/0.43 | 3.00 | .002 | 32.12* | 21 |
| Processing speed | ||||||||
| Digit Symbol | 4 | 272 | −0.19 | −0.36/−0.02 | −2.20 | .027 | 10.58* | 11 |
| Trail Making Test-A | 4 | 272 | −0.06 | −0.23/0.11 | −0.73 | .464 | 1.13 | N/A |
| Executive function | ||||||||
| WCST-PE | 4 | 260 | 0.01 | −0.16/0.18 | 0.14 | .890 | 3.38 | N/A |
| WCST-CAT | 4 | 266 | 0.04 | −0.13/0.21 | 0.43 | .668 | 0.18 | N/A |
| Trail Making Test-B | 4 | 272 | −0.04 | −0.21/0.13 | −0.49 | .627 | 0.50 | N/A |
Note: Abbreviations are explained in the first footnote to table 1 and table 4.
*P < .05.
Heterogeneity measures suggested that the overall weighted mean effect of the relationships between subjective QOL and crystallized verbal ability, processing speed, and letter fluency were not stable. Moderator analyses of crystallized verbal ability revealed that greater age was related to smaller mean effect sizes (Z = −3.85, P = .001). Treatment setting (inpatient, outpatient, or mixed) also significantly moderated the relationship between crystallized verbal ability and subjective QOL (inpatient d = 0.00, outpatient d = −0.52, QB = 6.83, P = .009), as did QOL measure (WHOQOL d = 0.00, QOLI d = −0.52, QB = 6.83, P = .009). Moderator analyses also revealed that greater percentage of males was related to a stronger relationship between processing speed and subjective QOL (Z = 2.89, P = .003). The relationship of letter fluency to subjective QOL was moderated by treatment setting (inpatient d = 0.84, outpatient d = −0.19, QB = 26.97, P = .000) and QOL measure (WHOQOL d = 0.53, QOLI d = −0.28, QB = 19.35, P = .000).
Comparison of Relationship of Neurocognitive Measures to Objective vs Subjective Measures of QOL
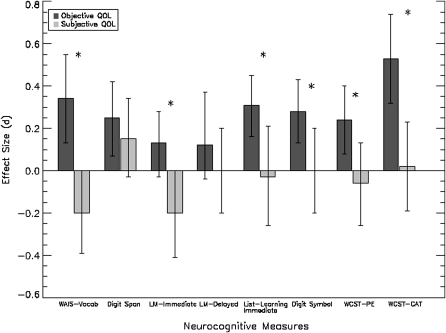
In addition, we made direct comparisons between relationships of the 2 QOL measure types (objective vs subjective) to each neurocognitive domain (see figure 1). One study that administered subjective and objective measures of QOL to the same participants was excluded as effect sizes across objective and subjective QOL indices from this study were not independent.27 Results revealed between-group differences in the relationship of neurocognition and subjective and objective QOL for crystallized verbal ability (WAIS-Vocabulary, QB = 13.86, P = .000), immediate prose recall (LM1, QB = 6.43, P = .011), list learning (CVLT/HVLT/RAVLT immediate QB = 5.66, P = .017), processing speed (WAIS-Digit Symbol, QB = 4.56, P = .033), and executive function (WCST-PE: QB = 5.42, P = .02; WCST-CAT: QB = 11.55, P = .020). The relationship between working memory (digit span) and delayed prose recall was not different for subjective and objective QOL.
Fig. 1.
Overall Effect Size Comparison (±95% CI) of the Relationship between Standardized Measures of Neurocognition and Subjective and Objective Quality of Life in Individuals with Schizophrenia. *P < .05.
File-Drawer Analyses
We sought to determine the extent to which our findings could be influenced by unpublished studies “the file-drawer problem” of nonsignificant effects. As shown in table 4, for objective QOL, there would need to be 17 unpublished studies of negative findings for crystallized verbal ability to be unrelated to QOL, 17 and 10 for attention (digit span and letter–number sequencing, respectively), 26 and 5 for list learning and memory (CVLT/HVLT/RAVLT-immediate and delayed, respectively), 18 for processing speed, and 23 and 30 for executive function (WCST-PE and -CAT, respectively). As shown in table 5, for subjective QOL, there would need to be 14 unpublished studies of negative findings for crystallized verbal ability to be unrelated to QOL, 21 for letter fluency, and 11 for processing speed.
Discussion
This is the first meta-analytic study to directly compare the pattern of relationships between elementary neurocognitive domains and subjective and objective measures of QOL. Our results revealed 3 major findings. First, consistent with our hypotheses, we found a disparity between the relationship of neurocognitive deficits to measures of subjective QOL and neurocognitive deficits and objective QOL in individuals with schizophrenia. With a few exceptions, neurocognitive measures were positively correlated with objective QOL but either unrelated or negatively correlated with subjective QOL. More specifically, direct comparisons between studies using subjective vs objective QOL measures showed that the neurocognitive domains of crystallized verbal ability, immediate prose recall, list learning, processing speed, and executive function were differentially related to subjective and objective QOL.
Second, we found positive relationships between measures of crystallized verbal ability, working memory, verbal list learning, and processing speed and objective QOL that were all in the small (d = 0.17–0.34) effect size range, and relationships between measures of executive function and objective QOL were in the small–medium (d = 0.28–0.55) effect size range. These results are consonant with our hypotheses and are consistent with several previous reviews and meta-analyses that have found measures of working memory, verbal memory, and executive function are related to a range of measures of functional outcome in people with schizophrenia in both cross-sectional and longitudinal designs.6–8 Attention was not related to objective QOL in our study. This finding is also generally consistent with previous studies of neurocognition and functional outcome, which suggest that measures of attention are more strongly associated with performance-based measures of skill acquisition and social problem solving than measures of objective community functioning that overlap with the measures of objective QOL selected for the current study.6
There is considerable face validity to the assertion that individuals with higher verbal IQ, verbal memory, executive function, and processing speed will be more likely to perform better vocationally, maintain larger social networks, and live independently. However, it is important to note that the effect sizes for the relationship between neurocognitive deficits and objective QOL were generally small, suggesting that there are likely many other individual and social determinants of objective QOL in addition to elementary neurocognition. Indeed, research over the past several years has suggested a variety of potential moderating variables between neurocognition and functional outcome, such as social cognition,51 learning potential,7 and negative symptoms.52 Already there is preliminary evidence that at least one measure of social cognition, facial affect recognition, moderates the relationship between neurocognitive deficits and objective QOL.53
Third, in contrast to the objective QOL findings, we found a largely nonsignificant relationship between neurocognitive variables and subjective QOL. Measures of attention, working memory, verbal memory, and executive function were not related to measures of subjective QOL. However, measures of crystallized verbal ability (d = −0.29) and processing speed (d = −0.19) were both negatively correlated with subjective QOL. Verbal fluency was the only measure found to be positively (d = 0.26) correlated with subjective QOL.
Moderator Analyses
Our moderator analyses for neurocognitive and objective QOL measures revealed that as the mean study sample age increased the relationship between processing speed (Digit Symbol) and objective QOL was attenuated. Similarly, as education increased, the relationships between list learning and processing speed and objective QOL weakened. A speculative explanation for these findings is that neurocognitive abilities have less of a direct effect on objective QOL in older and more educated samples as the clients in these samples have had more practice and acquired more compensatory skills to cope with persistent neurocognitive deficits than their younger, less-educated counterparts. In other words, the educational process may provide practice of processing speed and list learning skills serving to decrease heterogeneity between clients on these measures, making these findings relatively independent of the disease, whereas these cognitive skills may be more heterogeneous and reflect more about the illness for younger, less-educated people. In addition, there was little evidence that psychiatric symptoms influenced the relationship between neurocognition and objective or subjective QOL.
Differential Relationship between Neurocognitive Deficits and Objective and Subjective QOL
While the very different relationships between neurocognition and objective vs subjective QOL might appear paradoxical, they are consistent with a wealth of research that has revealed that objective QOL instruments that measure social and vocational status do not correlate with subjective QOL instruments that measures satisfaction with these same life domains.17,28 For example, Skantze et al54 in a sample of 66 schizophrenia outpatients found that objective standard of life scale scores, which included objective indicators of housing quality and current employment, did not significantly correlate with scores on their QLS, which measured participant satisfaction in the same life domains. Factor analyses of QOL assessment items have supported this view as well. Warner et al55 conducted a factor analysis of responses to the Lancashire Quality of Life Profile, a QOL instrument that measures both objective and subjective domains and found that objective QOL variables loaded separately from subjective satisfaction ratings. This dissociation in constructs supports the notion that objective QOL and subjective QOL could have different sets of predictors in individuals with schizophrenia.
One potential explanation for the inverse relationship between domains of neurocognition and subjective QOL is that those individuals with stronger cognitive abilities may have greater insight into their illness and functional disability, enabling negative social comparison and thus lower life satisfaction.27,30,56 Studies have shown that schizophrenia patients with better cognitive abilities had more severe depression57 and greater insight into their illness.58 Additional research that examines insight into illness as a potential moderator of the relationship between neurocognition and subjective QOL will prove helpful in elucidating this relationship.
Limitations
Several caveats to the current studies should be mentioned: (1) This is the first meta-analysis to date of a new and rapidly growing research area investigating the relationship between neurocognition and subjective and objective QOL, and thus, we had a relatively small number of studies (k = 20). Thus, these findings are preliminary and will need to be replicated with larger numbers of studies employing these same neurocognitive measures and QOL indices. (2) As our “fail-safe N” analyses revealed, for a few of our reported relationships, there would need to be a very low number of unpublished studies with nonsignificant effects required to negate our findings (eg, NFS = 5 for the relationship of verbal list learning to objective measures of QOL). (3) Some elementary neurocognitive domains in the current analysis were not well-represented in terms of numbers of measures (eg, attention) included in the current analysis, and current findings will be strengthened with the addition of other neurocognitive measures designed to measure similar constructs. (4) Many of our moderator analyses were underpowered with 50% or less of included studies reporting sample duration of illness, negative and positive symptom scores, depression ratings, or medication dosage (see table 3). (5) We note that some of our strongest findings were unstable as measured by our heterogeneity statistic (eg, Vocabulary and subjective QOL). This instability may represent the grouping of very different sample types into the same heterogeneity analysis. (6) Important domains of neurocognition, such as nonverbal memory, were not included in the analysis as an insufficient number of extant studies employed these measures and thus the relationship of these measures to subjective and objective QOL remains unknown. (7) Several moderator analyses revealed that the type of QOL measure within the subjective QOL domains influenced the relationship of elementary neurocognition and QOL. These findings suggest that there was considerable between-measure variability in the assessment of the construct of subjective QOL.
Implications
Taken together, the markedly different pattern of relation between neurocognition and objective and subjective QOL has implications for the potential effects of intervention on cognitive deficits of individuals with schizophrenia. In light of the MATRICS initiative work toward the development of cognition-enhancing pharmacological agents and the growth of a range of behavioral interventions targeted directly at deficits in neurcognition,59,60 the differential relationship between neurocognition and objective and subjective QOL revealed in this study may be particularly pertinent. Our results confirm the positive link between neurocognition and objective QOL, suggesting that improving neurocognition could have a positive impact on objective measures of client functioning. However, because our results indicated that neurocognition was largely unrelated or for some neurocognitive domains even negatively related to subjective QOL, the current study emphasizes the need for clinical researchers to craft new interventions alongside those targeting cognition in order to ensure that integrated treatment interventions attend to individuals’ subjective life satisfaction in addition to improving objective QOL.
Funding
Howard Hughes Medical Institute Summer Fellowship Program (to A.W.T.); National Institute of Mental Health (K08 MH-69888); National Alliance for Research in Schizophrenia and Depression (Young Investigator Award; to M.M.K.).
Acknowledgments
We thank Drs J. Addington, E. Twamley, and D. Dickinson for providing raw data used in these analyses. The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Eack SM, Newhill CE. Psychiatric symptoms and quality of life in schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:1225–1237. doi: 10.1093/schbul/sbl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awad AG, Voruganti LP. Intervention research in psychosis: issues related to the assessment of quality of life. Schizophr Bull. 2000;26:557–564. doi: 10.1093/oxfordjournals.schbul.a033477. [DOI] [PubMed] [Google Scholar]
- 3.Lehman AF. Measures of quality of life among persons with severe and persistent mental disorders. Soc Psychiatry Psychiatr Epidemiol. 1996;31(2):78–88. doi: 10.1007/BF00801903. [DOI] [PubMed] [Google Scholar]
- 4.Gold J, Harvey PD. Cognitive deficits in schizophrenia. The Psychiatr Clin North Am. 1993;16:295–312. [PubMed] [Google Scholar]
- 5.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 6.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 8.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14(1):1–21. [PubMed] [Google Scholar]
- 10.Addington J, Addington D. Social and cognitive functioning in psychosis. Schizophr Res. 2008;99(1–3):176–181. doi: 10.1016/j.schres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Lysaker P, Davis L. Social function in schizophrenia and schizoaffective disorder: associations with personality, symptoms and neurocognition. Health Qual Life Outcomes. 2004;2(1):15. doi: 10.1186/1477-7525-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan PW, Buican B. The construct validity of subjective quality of life for the severely mentally ill. J Nerv Ment Dis. 1995;183:281–285. doi: 10.1097/00005053-199505000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Proteau A, Verdoux H, Briand C, et al. Cognitive predictors of psychosocial functioning outcome in schizophrenia: a follow-up study of subjects participating in a rehabilitation program. Schizophr Res. 2005;77:343–353. doi: 10.1016/j.schres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Heslegrave RJ, Awad AG, Voruganti LN. The influence of neurocognitive deficits and symptoms on quality of life in schizophrenia. J Psychiatry Neurosci. 1997;22:235–243. [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer A, Baumgartner S, Bodner T, et al. Patient outcomes in schizophrenia II: the impact of cognition. Eur Psychiatry. 2005;20:395–402. doi: 10.1016/j.eurpsy.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272:619–626. [PubMed] [Google Scholar]
- 17.Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11:51–62. [Google Scholar]
- 18.Brissos S, Dias VV, Carita AI, Martinez-Arán A. Quality of life in bipolar type I disorder and schizophrenia in remission: clinical and neurocognitive correlates. Psychiatry Res. 2008;160(1):55–62. doi: 10.1016/j.psychres.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Chino B, Nemoto T, Fujii C, Mizuno M. Subjective assessments of the quality of life, well-being and self-efficacy in patients with schizophrenia. Psychiatry Clin Neurosci. 2009;63:521–528. doi: 10.1111/j.1440-1819.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 20.Bain GH, Lemmon H, Teunisse S, et al. Quality of Life in healthy old age: relationships with childhood IQ, minor psychological symptoms and optimism. Soc Psychiatry Psychiatr Epidemiol. 2003;38:632–636. doi: 10.1007/s00127-003-0685-5. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-III. Washington, DC: American Psychiatric Press. 1987. [Google Scholar]
- 22.Addington J, Addington D. Neurocognitive and social functioning in schizophrenia. Schizophr Bull. 1999;25:173–182. doi: 10.1093/oxfordjournals.schbul.a033363. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson D, Coursey RD. Independence and overlap among neurocognitive correlates of community functioning in schizophrenia. Schizophr Res. 2002;56(1–2):161–170. doi: 10.1016/s0920-9964(01)00229-8. [DOI] [PubMed] [Google Scholar]
- 24.Fiszdon JM, Choi J, Goulet J, Bell MD. Temporal relationship between change in cognition and change in functioning in schizophrenia. Schizophr Res. 2008;105(1–3):105–113. doi: 10.1016/j.schres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Lipkovich I, Deberdt W, Csernansky JG, et al. Relationships among neurocognition, symptoms and functioning in patients with schizophrenia: a path-analytic approach for associations at baseline and following 24-months of antipsychotic drug therapy. BMC Psychiatry. 2009;9:44. doi: 10.1186/1471-244X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui M, Sumiyoshi T, Arai H, Higuchi Y, Kurachi M. Cognitive functioning related to quality of life in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:280–287. doi: 10.1016/j.pnpbp.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Narvaez JM, Twamley EW, McKibbin CL, Heaton RK, Patterson TL. Subjective and objective quality of life in schizophrenia. Schizophr Res. 2008;98:201–208. doi: 10.1016/j.schres.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savilla K, Kettler L, Galletly C. Relationships between cognitive deficits, symptoms and quality of life in schizophrenia. Aust N Z J Psychiatry. 2008;42:496–504. doi: 10.1080/00048670802050512. [DOI] [PubMed] [Google Scholar]
- 29.Alptekin K, Akvardar Y, Akdede BB, et al. Is quality of life associated with cognitive impairment in schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:239–244. doi: 10.1016/j.pnpbp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Brekke JS, Kohrt B, Green MF. Neuropsychological functioning as a moderator of the relationship between psychosocial functioning and the subjective experience of self and life in schizophrenia. Schizophr Bull. 2001;27:697–708. doi: 10.1093/oxfordjournals.schbul.a006908. [DOI] [PubMed] [Google Scholar]
- 31.Dickerson F, Ringel NB, Parente F. Subjective quality of life in out-patients with schizophrenia: clinical and utilization correlates. Acta Psychiatr Scand. 1998;98(2):124–127. doi: 10.1111/j.1600-0447.1998.tb10053.x. [DOI] [PubMed] [Google Scholar]
- 32.Herman M. Neurocognitive functioning and quality of life among dually diagnosed and non-substance abusing schizophrenia inpatients. Int J Ment Health Nurs. 2004;13:282–291. doi: 10.1111/j.1440-0979.2004.00346.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith TE, Hull JW, Goodman M, et al. The relative influence of symptoms, insight, and neurocognition on social adjustment in schizophrenia and schizoaffective disorder. J Nerv Ment Dis. 1999;187(2):102–108. doi: 10.1097/00005053-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for assessing the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–396. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 35.Strauss J, Carpenter WT. Prediction of outcome in schizophrenia. III. Five-year outcome and its predictors. Arch Gen Psychiatry. 1977;34(2):159–163. doi: 10.1001/archpsyc.1977.01770140049005. [DOI] [PubMed] [Google Scholar]
- 36.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Voruganti LN, Heslegrave RJ, Awad AG. Neurocognitive correlates of positive and negative syndromes in schizophrenia. Can J Psychiatry. 1997;43:854–855. doi: 10.1177/070674379704201008. [DOI] [PubMed] [Google Scholar]
- 38.Sota TL, Heinrichs RW. Demographic, clinical, and neurocognitive predictors of quality of life in schizophrenia patients receiving conventional neuroleptics. Compr Psychiatry. 2004;45:415–421. doi: 10.1016/j.comppsych.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 39.WHO Group. The World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 40.Stein LI, Test MA. Alternatives to mental hospital treatment: I. Conceptual model treatment program and clinical evaluation. Arch Gen Psychiatry. 1980;37:392–397. doi: 10.1001/archpsyc.1980.01780170034003. [DOI] [PubMed] [Google Scholar]
- 41.Oliver JP, Huxley PJ, Priebe S, Kaiser W. Measuring the quality of life of severely mentally ill people using the Lancashire Quality of Life Profile. Soc Psychiatry Psychiatr Epidemiol. 1997;32(2):76–83. doi: 10.1007/BF00788924. [DOI] [PubMed] [Google Scholar]
- 42.Johnson BT. DSTAT: Software for Meta-Analytic Reviews of Research Literature [computer program] Hillsdale, NJ: Erlbaum; 1993. [Google Scholar]
- 43.Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. J Appl Psychol. 2005;90(1):175–181. doi: 10.1037/0021-9010.90.1.175. [DOI] [PubMed] [Google Scholar]
- 44.Lipsey MW, Wilson DB. The way in which intervention studies have “personality” and why it is important to meta-analysis. Eval Health Prof. 2001;24:236–254. doi: 10.1177/016327870102400302. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Academic Press; 1977. [Google Scholar]
- 46.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York, NY: Academic Press; 1985. [Google Scholar]
- 47.Orwin RG. A fail-safe N for meta-analysis. J Educ Stat. 1983;8(2):157–159. [Google Scholar]
- 48.Kay SR, Fizszbein A, Opler LA. The positive and negative syndrome scale for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 49.Spitzer R, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for the DSM-III-R. Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- 50.Rosenthal R. Meta-Analytic Procedures for Social Research. London, UK: Sage; 1986. [Google Scholar]
- 51.Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowie CR, Leung WW, Reichenberg AA, et al. Predicting schizophrenia patients’ real world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63:505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006;85(1–3):142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 54.Skantze K, Malm U, Dencker SJ, May PR, Corrigan P. Comparison of quality of life with standard of living in schizophrenic out-patients. Br J Psychiatry. 1992;161:797–801. doi: 10.1192/bjp.161.6.797. [DOI] [PubMed] [Google Scholar]
- 55.Warner R, de Girolamo G, Belelli G, Bologna C, Fioritti A, Rosini G. The quality of life of people with schizophrenia in Boulder, Colorado, and Bologna, Italy. Schizophr Bull. 1998;24:559–568. doi: 10.1093/oxfordjournals.schbul.a033349. [DOI] [PubMed] [Google Scholar]
- 56.Karow A, Pajonk FG. Insight and quality of life in schizophrenia: recent findings and treatment implications. Curr Opin Psychiatry. 2006;19:637–641. doi: 10.1097/01.yco.0000245754.21621.c9. [DOI] [PubMed] [Google Scholar]
- 57.Bowie CR, Twamley EW, Anderson H, Halpern B, Patterson TL, Harvey PD. Self-assessment of functional status in schizophrenia. J Psychiatr Res. 2007;41:1012–1018. doi: 10.1016/j.jpsychires.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subotnik KL, Nuechterlein KH, Irzhevsky V, Kitchen CM, Woo SM, Mintz J. Is unawareness of psychotic disorder a neurocognitive or psychological defensiveness problem? Schizophr Res. 2005;75(2–3):147–157. doi: 10.1016/j.schres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Marder S. Initiatives to promote the discovery of drugs to improve cognition in severe mental illness. J Clin Psychiatry. 2006;67(7):e03. doi: 10.4088/jcp.0706e03. [DOI] [PubMed] [Google Scholar]
- 60.McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:437–441. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]