Abstract
Background.
Patients’ perception of pain during hemodialysis (HD) and at times between HD treatment and its association with survival have not been well studied in end-stage renal disease (ESRD). We evaluated the experience of pain during HD and at times when the patient was not receiving HD, and assessed possible associations of the perception of pain and sleep disturbance with patient survival.
Methods.
A total of 128 ESRD patients treated with HD completed questionnaires on psychosocial status, quality of life and sleep disorders. A modified McGill Pain questionnaire was used to assess the nature, location, frequency, intensity and duration of pain both during and at times between HD sessions. The Pittsburgh Sleep Quality Index was used to screen for sleep disturbances over a 30-day period.
Results.
Controlling for age, diabetes mellitus, serum albumin concentration and human immunodeficiency virus infection, there was a significant association between mortality and both frequency and intensity of pain while patients were not on HD. There was no association between survival and duration of pain while off HD or any of the pain parameters while patients were on HD. There was no association between survival and the presence of a sleep disorder.
Conclusions.
Pain perception while off HD may be of more importance to patients than pain during HD. The mechanisms underlying the association are unknown but may involve linkage of pain with severity of medical illness or the generation of a maladaptive cytokine response. Multicenter prospective studies of pain interventions using well-validated pain perception tools are needed to establish causal relationships. Interventions directed toward treating pain on non-HD days may improve ESRD patient survival.
Keywords: chronic kidney disease, depression, satisfaction with life, satisfaction with care, psychosocial
Introduction
Pain and sleeplessness have been considered important symptoms for general medical patients for many years [1, 2]. Only recently, however, have pain and sleep been carefully evaluated in patients with end-stage renal disease (ESRD) and chronic kidney disease (CKD) [2–6]. There is increasing recognition that pain is one of the most common issues experienced by ESRD patients, associated with increased depression and diminished quality of life (QOL) [1, 2, 4, 6–11]. Increased depressive affect and perception of QOL by ESRD patients have been linked to patient survival in large populations [12, 13]. Perception of pain has been linked to mortality in several general medical populations [14, 15].
Pain, although often underappreciated by clinicians, has been recognized as an important concern for dialysis patients since the seminal work of Binik et al. [1]. More than one-fifth of a US hemodialysis (HD) population reported pain as a troublesome issue [8]. A Canadian study showed 50% of HD patients reported a problem with chronic pain, with 55% of these patients rating their pain as severe [6].
Pain, however, is not the only predictor of poor QOL for CKD patients [2, 16]. Sleep quality has been shown to be an important mediating factor in pain-related disabilities. Chronic pain and sleep disturbances each independently and synergistically have profound detrimental effects on QOL in general medical and ESRD populations [2, 17]. Poor sleep quality is independently associated with several QOL indices, medication use patterns and mortality in HD patients as shown in 308 dialysis units in seven countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS) between 1996 and 2001 [18].
Recent studies confirmed that sleep problems are frequent in patients with ESRD [3, 4]. In a 2004 study, 65% of patients reported symptoms of at least one specific sleep disorder [3]. Patients with sleep disorders reported that they had higher illness intrusiveness and worse self-perceived health than those without sleep problems. The experience of a sleep disorder was an independent predictor of illness intrusiveness, a health-related QOL parameter [3].
The perception of pain during dialysis and at times between dialysis treatment and their association with survival has not been well studied in ESRD patients. Little is known about the experience of pain by HD patients on non-dialysis days, in contrast to that perceived during dialysis treatment. The aims of this study were to evaluate the experience of pain during dialysis, and at times when the patient was not receiving dialysis and to assess possible associations of perception of pain and sleep disturbance with survival in HD patients. We hypothesized the subjective report of a sleep disorder and increased perception of pain would be associated with mortality in HD patients.
Materials and methods
Patient population and demographics
HD patients were recruited from two dialysis units located in Washington, DC, under the direction of George Washington University Medical Center full-time faculty nephrologists. The population was composed of primarily African-American patients and has been previously described [19, 20].
Recruitment
All patients enrolled in chronic HD programs at the two dialysis units were eligible to participate. The recruitment period ranged between 3 October 2001 and 26 November 2003. Each patient was initially approached by a trained research assistant, who explained the study in detail and invited patients to complete a series of questionnaires regarding pain, psychological status and QOL. Questionnaires were administered by research assistants, who recorded verbal responses. At the time of data collection, patients gave consent to use the data in mortality analyses. Data collection was approved by the George Washington University Medical Center Committee on Human Research. Follow-up for vital status continued through July 2005.
Data collection
Research assistants tracked the status of all patients by interviews with nephrologists and staff at both dialysis units, who verified information in the facilities’ computer databases. Data obtained included current vital status (alive or dead), date of death, date of transplantation and last day of treatment (if no follow-up data were available).
Measures
Participants were evaluated using the Beck Depression Inventory (BDI), Illness Effects Questionnaire (IEQ), Single Question QOL Scale (SQQOL), Multidimensional Scale of Perceived Social Support (MSP) and Satisfaction with Life Scale (SWLS), Karnofsky scores, Pittsburgh Sleep Questionnaire (PSQ) scores and the modified McGill Pain Questionnaire.
Beck Depression Inventory.
The BDI is a 21-item questionnaire that examines the somatic and cognitive effects of depression. The BDI quantifies the severity of depression as none, mild, moderate or severe. This depression-screening tool has previously been validated in ESRD patients [21–25]. A score of ≥11 indicates depression in a general medical population, while scores >14 have been suggested as cutoff values in ESRD patients [24–26]. Higher BDI scores have been associated with mortality in ESRD patient populations [21, 27].
Illness Effects Questionnaire.
The IEQ is used to quantify the patients' perception of burden of illness. It contains 20 items scored on a 7-point Likert scale. The IEQ has been validated in ESRD patient populations and has also been associated with mortality [7, 16, 28, 29].
Single Question QOL scale.
The SQQOL has been used in several recent studies of dialysis patients [8, 16, 19, 30]. We showed this single question global QOL measure correlated with depression, number of symptoms, life satisfaction scores, perception of burden of illness, social support and satisfaction with nephrologist scores but not with age, level of albumin, hemoglobin, Kt/V or Karnofsky scores, demonstrating its validity as a QOL measure [8, 16, 19].
Social support was assessed using the MSP [31, 32]. The scale, frequently used in CKD patients [7, 28, 33–35], includes 12 questions measuring perceived support from family, friends and significant others. Patients’ scores were reported on a 7-point Likert scale with total scores ranging from 0 to 84, indicating low to high-perceived social support. We previously reported higher ESRD HD patient social support scores were associated with improved survival [20, 28].
The SWLS contains five items rated on a scale from 1 to 7, designed to measure overall perception of QOL [36, 37]. The SWLS correlates with a number of subjective QOL measures [36]. The SWLS has been validated in ESRD populations [16, 28, 29].
The PSQ [38, 39] measured patients’ satisfaction levels both with their nephrologist and with their dialysis center staff. Topics covered by the 10-question survey included the patients’ perception of how much the nephrologist and dialysis staff cared for, respected, and supported them as individuals. The Satisfaction with Doctor score is the sum of all questions relating to perceptions of the nephrologist, while the Satisfaction with Staff score is the sum of all questions relating to perceptions of dialysis center staff.
The Karnofsky Performance Status Scale [40] measures a patient’s functional status using a scale ranging from 0 to 100. A score of 100 signifies full capacity to carry out normal activities of daily living, while a score of 0 indicates death. Scores <70 indicate that some level of assistance is needed to carry out daily activities. The Karnofsky scale has been used in prior QOL studies in ESRD patients [16, 28, 29, 33, 35].
The Pittsburgh Sleep Quality Index (PSQI) screens for sleep disturbances over a 1-month period [41]. There are 19 questions with seven component scores including ‘subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction [41]’. The seven component scores are summed to yield one global score [41]. A global PSQI score >5 has an 89.6% sensitivity and 86.5% specificity for determining disturbance in sleep [41]. The PSQI has previously been used in ESRD patients [9, 42].
The modified McGill Pain questionnaire was used to measure perception of pain. This survey includes questions regarding the nature, location, frequency, intensity and duration of pain [43]. The McGill Pain questionnaire and modifications of the instrument have previously been used in patients with CKD and ESRD [2, 4–6]. We evaluated two variables: pain during HD treatment (excluding needle insertion) and at times between dialysis treatment (‘non-dialysis days’). Pain frequency was measured by asking patients how often they experienced pain on a 1–10 Likert scale, with ‘1’ defining rarely experiencing pain and ‘10’ defining always experiencing pain. Pain duration was measured by asking patients how long their pain lasted in minutes. The perceived intensity of pain was rated on a 1–10 scale [4, 43]. The percentage of patients with pain was calculated based on how many patients reported experiencing pain over the past month. In addition, we asked whether the patient had spoken to his or her nephrologist regarding pain.
Statistical considerations
All statistical analyses were performed using either SAS version 9.1 (SAS Institute, Cary, NC) or Excel 2007. Mantel-Haenszel chi-square tests were used for comparisons between nominal variables and were performed using categories defined by the mean scores. Correlation analyses with Pearson or Spearman coefficients were used to assess relationships between the demographic, pain and sleep variables as appropriate. Cox regression analyses were used to assess survival. A P-value of 0.05 was considered significant. Results are expressed as mean ± SD.
Results
Demographics and patient population
The sample was composed of 77 men (60.2%) and 51 women (39.8%). The study patients were 91.4% African-American, 7.0% were Caucasian and 1.6% identified themselves as Asian or Pacific Islander. The study patients' demographics are outlined in Table 1. The mean age of the population was 57.3 ± 13.8 years (range 24–86 years). A total of 48.4% had diabetes mellitus and 10.2% were human immunodeficiency virus (HIV) infected. The mean serum albumin concentration was 3.8 ± 0.4 g/dL. Patients’ mean Kt/V was 1.5 ± 1.0. The mean duration of treatment on dialysis was 39.9 ± 40.9 months. The mean Karnofsky score was 74.6 ± 14.6 (Table 1).
Table 1.
Patient demographic and clinical characteristics
| N = 128 | |
| African-American | 91.4% |
| White | 7.0% |
| Asian or Pacific Islander | 1.6% |
| Male | 60.2% |
| Mean age, years | 57.3 ± 13.8 |
| Diabetes mellitus | 48.4% |
| HIV infected | 10.2% |
| Mean Karnofsky score | 74.6 ± 14.6 |
| Mean duration of dialysis (months) | 39.9 ± 40.9 |
| Mean serum albumin concentration (g/dL) | 3.8 ± 0.4 |
| Mean hemoglobin concentration (g/dL) | 11.6 + 1.6 |
| Mean Kt/V | 1.5 ± 1.0 |
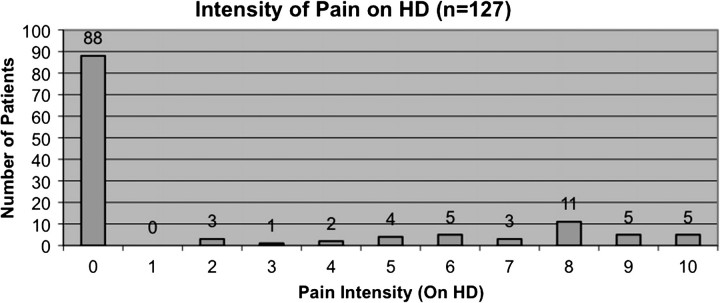
There was incomplete information regarding the experience of pain and sleep disturbances for one patient. A total of 30.7% of patients experienced pain during dialysis (excluding needle insertion). A total of 44.1% of patients experienced pain on non-dialysis days (P < 0.05 versus during dialysis). The mean intensity score of pain experienced by patients during dialysis was 2.1 ± 3.5, the mean score for frequency of pain during dialysis was 1.8 ± 3.1, and the mean duration of pain score during dialysis was 9.8 ± 31.7 min (Figure 1, Table 2). A total of 3.9% of patients experienced the highest level of pain (Figure 1) and the highest frequency of pain during HD (data not shown). A total of 28.4% of patients scored above the mean of intensity of pain during dialysis, 29.1% scored above the mean frequency of pain during dialysis and 21.3% scored above the mean duration of pain during dialysis.
Fig. 1.
Pain intensity on HD.
Table 2.
Patient pain, sleep and psychosocial characteristics
| Mean | Median | Mode | |
| Intensity of pain, during dialysis | 2.1 ± 3.5 | 0 | 0 |
| Frequency of pain, during dialysis | 1.8 ± 3.1 | 0 | 0 |
| Duration of pain, during dialysis, minutes | 9.8 ± 31.7 | 0 | 0 |
| Intensity of pain, on non-dialysis days | 3.1 ± 3.8 | 0 | 0 |
| Frequency of pain, on non-dialysis days | 2.9 ± 3.6 | 0 | 0 |
| Duration of pain, on non-dialysis days, minutes | 49.8 ± 220.8 | 0 | 0 |
| PSQI total | 6.2 ± 4.6 | 5 | 4 |
| MSP | 69.2 ± 12.9 | 72 | 84 |
| BDI | 11.0 ± 8.2 | 8 | 4 |
| IEQ | 50.7 ± 25.5 | 47 | 38 |
| SWLS | 21.3 ± 8.1 | 22 | 25 |
| Patient satisfaction with nephrologist | 18.4 ± 2.8 | 19 | 19 |
| Patient satisfaction with dialysis staff | 18.5 ± 2.8 | 19 | 19 |
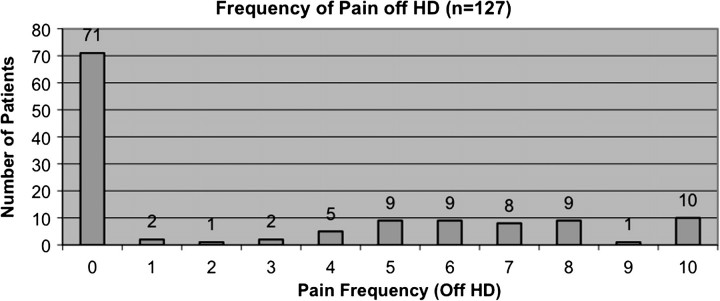
The mean intensity of pain score on non-dialysis days was 3.1 ± 3.8, the mean frequency of pain score on non-dialysis days was 2.9 ± 3.6, and the mean duration of pain on non-dialysis days was 49.8 ± 220.8 min (Figure 2, Table 2). The median and modal score for all of the pain parameters was zero. A total of 41.0% of patients scored above the mean intensity of pain on non-dialysis days, 42.1% scored above the mean of frequency of pain on non-dialysis days and 12.0% scored above the mean of duration of pain on non-dialysis days. A total of 7.9% of patients experienced the highest level of pain (data not shown) and 9.5% of patients experienced the highest frequency of pain during HD.
Fig. 2.
Pain frequency on non-dialysis days.
The mean global PSQI score was 6.2 ± 4.6. A total of 45.6% of patients had a global PSQI score >5. The mean BDI score was 11.0 ± 8.2, the mean MSP score was 69.2 ± 12.9, the mean IEQ score was 50.7 ± 25.5 and the mean SWLS was 21.3 ± 8.1.
Chi-square analyses
There was no significant association of gender, Kt/V, hemoglobin and albumin level, presence of diabetes mellitus, MSP or SWLS and any of the pain parameters when assessed in categorical relationships. There was no significant association of any of the pain variables and the patient satisfaction with nephrologist or dialysis staff.
Married patients experienced less intensity (P = 0.026) and frequency (P = 0.013) of perceived pain on non-dialysis days than unmarried subjects. Younger patients experienced greater intensity (P = 0.037) and frequency (P = 0.024) of perceived pain during dialysis than older subjects. The magnitude of all pain parameters on dialysis days was significantly increased in patients with high IEQ scores. Patients with higher BDI scores experienced more intensity (P = 0.005 and P = 0.008) and frequency (P = 0.003 and P = 0.012) of perceived pain both during dialysis and on non-dialysis days. Patients with lower Karnofsky scores experienced more intensity (P = 0.028) and frequency (P = 0.017) of pain during dialysis than those with higher functional status. The magnitude of all pain parameters during dialysis and on non-dialysis days was significantly decreased if the patient had spoken to his or her nephrologist about pain.
There was no significant association between gender, marital status or whether the patient had spoken to his or her nephrologist about pain and the presence of a sleep disorder. There was no significant association between Kt/V, hemoglobin and albumin levels, Karnofsky score and the presence of a sleep disorder. There was no significant association between the presence of a sleep disorder and patient satisfaction with nephrologist or dialysis staff. Diabetic patients were less likely to perceive a sleep disorder (p = 0.046). Patients with higher IEQ scores (P < 0.001), higher BDI scores (P < 0.001), lower MSP scores (P = 0.005) and lower SWLS scores (P = 0.001) were more likely to perceive a sleep disorder (P < 0.001). Younger patients were more likely to experience a sleep disorder (P = 0.023).
Correlational analyses
There was no correlation of age, Kt/V, Karnofsky score, hemoglobin or albumin concentration and any pain or sleep variable. There was no correlation of any pain or sleep variable and patient satisfaction with nephrologist or dialysis staff.
SWLS scores correlated with intensity of perceived pain during dialysis and on non-dialysis days as well as perception of globally disturbed sleep (Table 3). IEQ scores correlated with all pain parameters on dialysis and non-dialysis days, with the exception of duration of pain on non-dialysis days. Extent of depression correlated with frequency and intensity of pain on dialysis and non-dialysis days and perception of globally disturbed sleep. Perception of lower social support correlated with magnitude of perception of disturbed sleep.
Table 3.
Correlation of pain and sleep indicators with QOL, medical and treatment parametersa
| On dialysis |
Non-dialysis |
PSQI | |||||
| Frequency | Duration | Intensity | Frequency | Duration | Intensity | ||
| Age | −0.113 | −0.114 | −0.122 | −0.081 | 0.047 | −0.118 | −0.120 |
| 0.206 | 0.202 | 0.172 | 0.367 | 0.603 | 0.186 | 0.183 | |
| Kt/V | −0.050 | −0.019 | −0.052 | −0.061 | −0.016 | −0.063 | −0.073 |
| 0.577 | 0.832 | 0.562 | 0.497 | 0.859 | 0.482 | 0.419 | |
| Hb | 0.095 | 0.032 | 0.115 | −0.032 | 0.033 | −0.051 | −0.024 |
| 0.288 | 0.721 | 0.198 | 0.722 | 0.715 | 0.569 | 0.790 | |
| Alb | −0.126 | 0.001 | −0.056 | −0.084 | 0.012 | −0.059 | −0.149 |
| 0.158 | 0.991 | 0.532 | 0.350 | 0.894 | 0.509 | 0.097 | |
| Karnofsky | −0.103 | 0.005 | −0.061 | 0.050 | 0.134 | 0.090 | −0.131 |
| 0.249 | 0.956 | 0.496 | 0.578 | 0.136 | 0.314 | 0.145 | |
| SWLS | −0.135 | −0.158 | −0.174 | −0.120 | 0.011 | −0.174 | −0.299 |
| 0.130 | 0.076 | 0.050 | 0.181 | 0.903 | 0.050 | 0.0007 | |
| IEQ | 0.395 | 0.230 | 0.324 | 0.228 | −0.014 | 0.299 | 0.548 |
| 0.00004 | 0.009 | 0.0002 | 0.010 | 0.877 | 0.0006 | <0.001 | |
| BDI | 0.284 | 0.143 | 0.294 | 0.207 | 0.070 | 0.279 | 0.576 |
| 0.001 | 0.109 | 0.0008 | 0.020 | 0.438 | 0.001 | <0.001 | |
| MSP | −0.019 | −0.151 | −0.047 | −0.011 | 0.063 | −0.034 | −0.342 |
| 0.832 | 0.090 | 0.600 | 0.903 | 0.485 | 0.704 | 0.0001 | |
| Satisfaction with doctor | 0.038 | −0.068 | 0.030 | 0.112 | 0.064 | 0.086 | −0.057 |
| 0.672 | 0.448 | 0.738 | 0.211 | 0.478 | 0.336 | 0.528 | |
| Satisfaction with staff | −0.116 | 0.003 | −0.087 | 0.040 | −0.001 | 0.049 | −0.011 |
| 0.194 | 0.973 | 0.331 | 0.657 | 0.991 | 0.584 | 0.903 | |
Values are given as r above and P below. Key, Karnofsky (Karnofsky Performance Status Scale). Hb, hemoglobin; Alb, albumin. See text for details. Statistically significant relationships are presented as bold values.
Survival
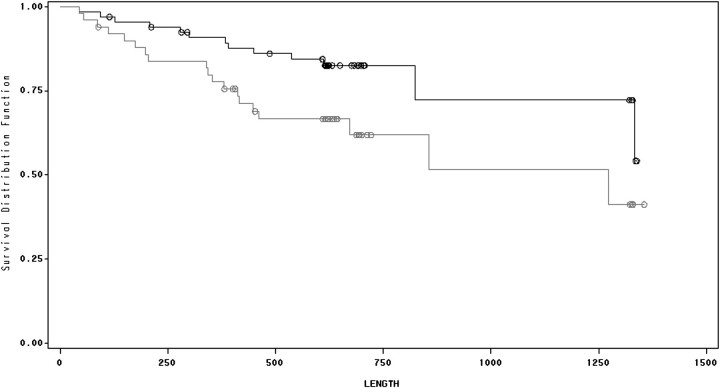
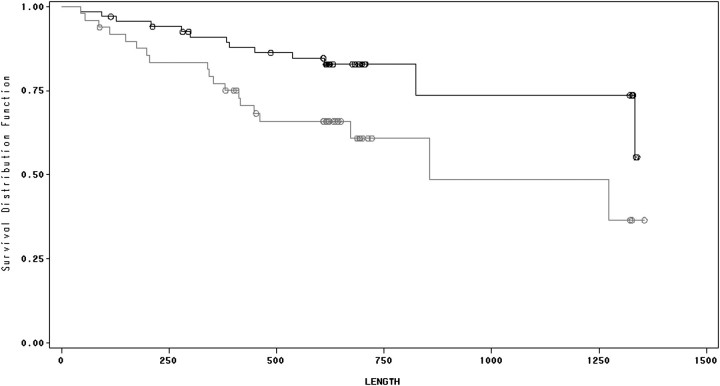
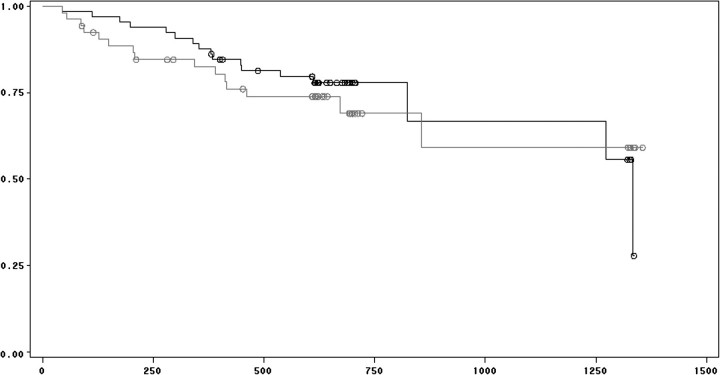
Ninety-six of the 128 patients survived the study and observation periods. There was a significant association between survival and perception of both frequency and intensity of pain while patients were not on dialysis (Figures 3 and 4). There was no significant association between survival and duration of pain while patients were off dialysis, and there was no association between survival and any of the perceived pain parameters while patients were on dialysis. Controlling for age, diabetes mellitus, serum albumin concentration and HIV infection, mortality was associated with frequency of pain on non-dialysis days [hazard ratio (HR) = 1.097, confidence interval (CI) = 1.001–1.201, P = 0.047; Table 4] as well as perceived intensity of pain on non-dialysis days (HR = 1.09, CI = 1.001–1.187, P = 0.047; Table 5). There was no association between survival and the presence of a sleep disorder (Figure 5).
Fig. 3.
Survival and pain frequency on non-dialysis days. Kaplan–Meier analysis of HD patients with high versus low frequency of pain on non-dialysis days. Lower line represents high frequency (above the mean) and upper line represents low frequency (below the mean). See text for details.
Fig. 4.
Survival and pain intensity on non-dialysis days. Kaplan–Meier analysis of HD patients with high versus low intensity of pain on non-dialysis days. Lower line represents high intensity (above the mean) and upper line represents low intensity (below the mean). See text for details.
Table 4.
Cox regression analysis showing association between survival and frequency of pain on non-dialysis daysa
| Variable | HR | 95% CI | P-value |
| Age | 1.044 | 1.014–1.074 | 0.004 |
| Albumin | 0.282 | 0.098–0.808 | 0.018 |
| Frequency of pain on non-dialysis days | 1.097 | 1.001–1.201 | 0.047 |
All analyses are controlled for diabetes mellitus and HIV infection.
Table 5.
Cox regression analysis showing association between survival and intensity of pain on non-dialysis daysa
| Variable | HR | 95% CI | P-value |
| Age | 1.042 | 1.013–1.071 | 0.0039 |
| Albumin | 0.288 | 0.1–0.823 | 0.02 |
| Intensity of pain on non-dialysis days | 1.09 | 1.001–1.187 | 0.047 |
All analyses are controlled for diabetes mellitus and HIV infection.
Fig. 5.
Survival and sleep. Kaplan–Meier analysis of HD patients with presence and absence of sleep disorders. Heavy line represents presence of sleep disorders and light line represents absence of sleep disorders. See text for details.
Discussion
Pain has been noted as a distressing issue for HD patients for more than a quarter of a century [1], associated with increased depression and decreased QOL [2]. Both depression and QOL have been linked with differential survival in ESRD patients [12, 13, 21, 28]. Recent studies have confirmed that pain is a common issue for patients treated with maintenance HD for ESRD [7–9, 11]. The experience of pain between dialysis treatment has not been studied in HD patients.
We found perception of pain was common in HD patients, although a substantial proportion of patients did not report pain during dialysis or on non-dialysis days. An important subpopulation of patients did, however, perceive a high level of pain, both during dialysis and on non-dialysis days. Interestingly, the number of participants who perceived pain, and the intensity, frequency and duration of pain perceived was higher for the portion of the week between dialysis treatment compared with perceptions of pain during HD. There was no association of pain parameters and gender, presence of diabetes or patient satisfaction with care or treatment indices evaluated using several analytic approaches in this population. Age, marital status, satisfaction with life, perception of burden of illness and increased depression, in contrast, appear to be important factors associated with differential experience of pain. Associations of several parameters of perception of pain with QOL indicators such as level of depression, satisfaction with life and burden of illness on non-dialysis days suggest pain is intertwined with the patients’ general perception of QOL exclusive of the dialysis experience. The findings suggest that patients may compartmentalize dialysis treatment as a separate portion of their lives, less associated with general perceptions of QOL than those pertaining to non-dialysis days, the overwhelming majority of time spent by the non-hospitalized HD patient during the week.
The causes of pain for dialysis patients relate to complications of uremia, their underlying comorbid illnesses and factors related to the procedure, and have been well outlined [6, 11], as are effective treatment approaches [5, 11, 44].
The finding that perception of pain is associated with mortality outcomes in ESRD patients is novel. Macfarlane et al. [15] showed that pain was associated with mortality in a general population and acknowledged that pain could be a marker of poor health, a symptom of an undiagnosed illness or associated with psychological distress. Pain perception could be associated with increased stress or diminished perception of QOL [22] or high levels of pro-inflammmatory cytokines in ESRD patients [22], which have been linked to mortality. In addition, perception of pain could be associated with increased disability or depression, both of which are associated with mortality. It is interesting that in this study, there was not sufficient power to link sleep disorders with mortality, although the association has been documented in the larger DOPPS study [18].
Our findings emphasize the potentially robust nature of the relationship between perception of pain and mortality, and suggest treatment of pain might modify both perception of QOL and mortality. The study of Barakovy and Moss [5], a randomized controlled trial of aggressive treatment of pain in ESRD patients, addresses the possibility that such interventions are practical. The interrelationship between pain perception and various psychosocial factors suggests interventions directed against depression, anxiety and sleep disturbances or other psychosocial parameters might be effective in reducing pain, which should be evaluated in any such studies in HD patients [45].
A limitation of the study pertains to the patient population, which is primarily comprised of African-Americans. There would seem to be little evidence that relationships between symptoms and outcomes would differ in different ethnic groups, however, black patients enjoy superior survival in the US ESRD program. The study population includes prevalent patients and thus may be subject to survivor bias. Unfortunately, there was not sufficient power to address survival solely in incident patients. Future studies should both assess perceptions of pain during and exclusive of dialysis in incident patients, and follow these parameters longitudinally, to strengthen causal inferences [21].
Assessment of symptoms is underappreciated in dialysis practices, and pain treatment is inadequate [44, 46]. Inadequate treatment of pain may relate to concerns about iatrogenic addiction or inadequate or insufficient training of health personnel [45], and these pathways should be addressed in HD units. Nephrologists should inquire regarding the symptom of pain, experienced on dialysis and away from the dialysis unit. The experience of pain and perception of QOL exclusive of dialysis treatment may, however, be more important as patient-centered outcomes and as loci for intervention. Pain treatment should be a routine part of therapy by nephrologists for dialysis patients, inclusive and exclusive of treatment. Randomized controlled studies of pain interventions with assessment of hard outcomes are appropriate.
Acknowledgments
Funding. Funded in part by Gill Fellowships, George Washington University School of Medicine, Washington, D.C., USA.
Conflict of interest statement. None declared.
References
- 1.Binik YM, Baker AG, Kalogeropoulos D, et al. Pain, control over treatment, and compliance in dialysis and transplant patients. Kidney Int. 1982;21:840–848. doi: 10.1038/ki.1982.108. [DOI] [PubMed] [Google Scholar]
- 2.Shayamsunder AK, Patel SS, Jain V, et al. Sleepiness, sleeplessness, and pain in end-stage renal disease: distressing symptoms for patients. Semin Dial. 2005;18:109–118. doi: 10.1111/j.1525-139X.2005.18218.x. [DOI] [PubMed] [Google Scholar]
- 3.Mucsi I, Molnar MZ, Rethelyi J, et al. Sleep disorders and illness intrusiveness in patients on chronic dialysis. Nephrol Dial Transplant. 2004;19:1815–1822. doi: 10.1093/ndt/gfh130. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SD, Patel SS, Khetpal P, et al. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:919–925. doi: 10.2215/CJN.00820207. [DOI] [PubMed] [Google Scholar]
- 5.Barakzoy AS, Moss AH. Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17:3198–3203. doi: 10.1681/ASN.2006050477. [DOI] [PubMed] [Google Scholar]
- 6.Davison SN. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42:1239–1247. doi: 10.1053/j.ajkd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Weisbord SD, Fried LF, Arnold RM, et al. Prevalence, severity, importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16:2487–2494. doi: 10.1681/ASN.2005020157. [DOI] [PubMed] [Google Scholar]
- 8.Kimmel PL, Emont SL, Newmann JM, et al. ESRD patient quality of life: symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am J Kidney Dis. 2003;42:713–721. doi: 10.1016/s0272-6386(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 9.Davison SN, Jhangri GS. The impact of chronic pain on depression, sleep, and the desire to withdraw from dialysis in hemodialysis patients. J Pain Symptom Manage. 2005;30:465–473. doi: 10.1016/j.jpainsymman.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Cohen LM, Germain MJ, Woods AL, et al. The family perspective of ESRD deaths. Am J Kidney Dis. 2005;45:154–161. doi: 10.1053/j.ajkd.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Davison SN. The prevalence and management of chronic pain in end-stage renal disease. J Palliat Med. 2007;10:1277–1287. doi: 10.1089/jpm.2007.0142. [DOI] [PubMed] [Google Scholar]
- 12.Lopes AA, Albert JM, Young EW, et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66:2047–2053. doi: 10.1111/j.1523-1755.2004.00977.x. [DOI] [PubMed] [Google Scholar]
- 13.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 14.McBeth J, Symmons DP, Silman AJ, et al. Musculoskeletal pain is associated with a long-term increased risk of cancer and cardiovascular-related mortality. Rheumatology. 2009;48:74–77. doi: 10.1093/rheumatology/ken424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macfarlane GJ, McBeth J, Silman AJ. Widespread body pain and mortality: prospective population based study. BMJ. 2001;323:662–665. doi: 10.1136/bmj.323.7314.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimmel PL, Patel SS. Quality of life in patients with chronic kidney disease: focus on end-stage renal disease treated with hemodialysis. Semin Nephrol. 2006;26:68–79. doi: 10.1016/j.semnephrol.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Veldhuijzen DS, Greenspan JD, Smith MT. Sleep and Quality of Life in Clinical Medicine. Totowa, NJ: Human Press; 2008. Sleep and quality of life in chronic pain. In; pp. , pp. 187–197. [Google Scholar]
- 18.Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23:998–1004. doi: 10.1093/ndt/gfm630. [DOI] [PubMed] [Google Scholar]
- 19.Patel SS, Shah VS, Peterson RA, et al. Psychosocial variables, quality of life, and religious beliefs in ESRD patients treated with hemodialysis. Am J Kidney Dis. 2002;40:1013–1022. doi: 10.1053/ajkd.2002.36336. [DOI] [PubMed] [Google Scholar]
- 20.Spinale J, Cohen SD, Khetpal P, et al. Spirituality, social support, and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1620–1627. doi: 10.2215/CJN.01790408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimmel PL, Peterson RA, Weihs KL, et al. Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int. 2000;57:2093–2098. doi: 10.1046/j.1523-1755.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 22.Cukor D, Cohen SD, Peterson RA, et al. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18:3042–3055. doi: 10.1681/ASN.2007030345. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 24.Craven JL, Rodin GM, Littlefield C. The Beck Depression Inventory as a screen device for major depression in renal dialysis patients. Int J Psychiatry Med. 1988;18:365–374. doi: 10.2190/m1tx-v1ej-e43l-rklf. [DOI] [PubMed] [Google Scholar]
- 25.Hedayati SS, Bosworth HB, Kuchibhatla M, et al. The predictive value of self-reported questionnaires compared to physician diagnosis of depression in end-stage renal disease patients receiving chronic hemodialysis. Kidney Int. 2006;69:1662–1669. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 26.Cukor D, Peterson RA, Cohen SD, et al. Depression in end-stage renal disease hemodialysis patients. Nat Clin Pract Nephrol. 2006;2:678–687. doi: 10.1038/ncpneph0359. [DOI] [PubMed] [Google Scholar]
- 27.Boulware LE, Liu Y, Fink NE, et al. The temporal relation between depression symptoms, cardiovascular disease events and mortality in end-stage renal disease: contribution of reverser causality. Clin J Am Soc Nephrol. 2006;1:496–504. doi: 10.2215/CJN.00030505. [DOI] [PubMed] [Google Scholar]
- 28.Kimmel PL, Peterson RA, Weihs KL, et al. Psychosocial factors, behavioral compliance and survival in urban hemodialysis patients. Kidney Int. 1998;54:245–254. doi: 10.1046/j.1523-1755.1998.00989.x. [DOI] [PubMed] [Google Scholar]
- 29.Unruh ML, Weisbord SD, Kimmel PL. Health-related quality of life in nephrology research and clinical practice. Semin Dial. 2005;18:82–90. doi: 10.1111/j.1525-139X.2005.18206.x. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds JT, Homel P, Cantey L, et al. A one-year trial of in-center daily hemodialysis with an emphasis on quality of life. Blood Purif. 2004;22:320–328. doi: 10.1159/000079186. [DOI] [PubMed] [Google Scholar]
- 31.Zimet GD, Dahlem NW, Zimet SG. The multidimensional scale of perceived social support. J Pers Assess. 1988;52:30–41. [Google Scholar]
- 32.Dahlem NW, Zimet G, Walker R. The multidimensional scale of perceived social support: a confirmation study. J Clin Psychol. 1991;47:756–761. doi: 10.1002/1097-4679(199111)47:6<756::aid-jclp2270470605>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Shidler NR, Peterson RA, Kimmel PL. Quality of life and psychosocial relationships in patients with chronic renal insufficiency. Am J Kidney Dis. 1998;32:557–566. doi: 10.1016/s0272-6386(98)70017-4. [DOI] [PubMed] [Google Scholar]
- 34.Sacks CR, Peterson RA, Kimmel PL. Perception of illness and depression in chronic renal disease. Am J Kidney Dis. 1990;15:31–39. doi: 10.1016/s0272-6386(12)80589-0. [DOI] [PubMed] [Google Scholar]
- 35.Kimmel PL. Psychosocial factors in dialysis patients. Kidney Int. 2001;59:1599–1613. doi: 10.1046/j.1523-1755.2001.0590041599.x. [DOI] [PubMed] [Google Scholar]
- 36.Diener E, Eammons RA, Larsen RJ, et al. The satisfaction with life scale. J Pers Asses. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 37.Pavot W, Diener E. Review of the satisfaction with life scale. Psychol Asses. 1993;5:164–172. [Google Scholar]
- 38.Kirchgessner J, Perera-Chang M, Klinkner G, et al. Satisfaction with care in peritoneal dialysis patients. Kidney Int. 2006;70:1325–1331. doi: 10.1038/sj.ki.5001755. [DOI] [PubMed] [Google Scholar]
- 39.Kovac JA, Patel SS, Peterson RA, et al. Patient satisfaction with care and behavioral compliance in end-stage renal disease patients treated with hemodialysis. Am J Kidney Dis. 2002;39:1236–1244. doi: 10.1053/ajkd.2002.33397. [DOI] [PubMed] [Google Scholar]
- 40.Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. [Google Scholar]
- 41.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 42.Iliescu EA, Yeates KE, Holland DC. Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant. 2004;19:95–99. doi: 10.1093/ndt/gfg423. [DOI] [PubMed] [Google Scholar]
- 43.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–279. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 44.Davison SN, Chambers EJ, Ferro CJ. Management of pain in renal failure. In: Chambers EJ, Brown EA, Germain MJ, editors. Supportive Care for the Renal Patient. 2nd edn, Oxford, UK: Oxford University Press; 2010, pp.. pp. 139–188. [Google Scholar]
- 45.Ghodse H. Pain, anxiety and insomnia—a global perspective on the relief of suffering. Br J Psychiatry. 2003;183:15–21. doi: 10.1192/bjp.183.1.15. [DOI] [PubMed] [Google Scholar]
- 46.Claxton RN, Blachall L, Weisbord SD, et al. Undertreatment of symptoms in patients on maintenance hemodialysis. J Pain Symptom Manage. 2010;39:211–218. doi: 10.1016/j.jpainsymman.2009.07.003. [DOI] [PubMed] [Google Scholar]