Abstract
The wide clinical spectrum of the ABCB4 gene (ATP-binding cassette subfamily B member 4) deficiency syndromes in humans includes low phospholipid-associated cholelithiasis (LPAC), intrahepatic cholestasis of pregnancy (ICP), oral contraceptives-induced cholestasis (CIC), and progressive familial intrahepatic cholestasis type 3 (PFIC3). No ABCB4 mutations are found in a significant proportion of patients with these syndromes. In the present study, 102 unrelated adult patients with LPAC (43 patients) or CIC/ICP (59 patients) were screened for ABCB4 mutations using DNA sequencing. Heterozygous ABCB4 point or short insertion/deletion mutations were found in 37% (16/43) of the LPAC patients and in 27% (16/59) of the ICP/CIC patients. High-resolution gene dosage methodologies were used in the 70 negative patients. Here, we describe for the first time ABCB4 partial or complete heterozygous deletions in 7% (3/43) of the LPAC patients, and in 2% (1/59) of the ICP/CIC patients. Our observations urge to systematically test patients with LPAC, ICP/CIC, and also children with PFIC3 for the presence of ABCB4 deletions using molecular tools allowing detection of gross rearrangements. In clinical practice, a comprehensive ABCB4 alteration-screening algorithm will permit the use of ABCB4 genotyping to confirm the diagnosis of LPAC or ICP/CIC, and allow familial testing. An early diagnosis of these biliary diseases may be beneficial because of the preventive effect of ursodeoxycholic acid on biliary complications. Further comparative studies of patients with well-characterized genotypes (including deletions) and phenotypes will help determine whether ABCB4 mutation types influence clinical outcomes.
Keywords: ABCB4, deletion, biliary disease, LPAC, MDR3
Introduction
ABCB4 (ATP-binding cassette subfamily B member 4) membrane transporter translocates phosphatidylcholine from the inner to the outer leaflet of the canalicular membrane of the hepatocyte. This floppase activity makes phosphatidylcholine available for extraction into the canalicular lumen by bile salts. The ABCB4 gene (MIM 171060) has a crucial role as evidenced by cholestatic liver diseases caused by its deficiency.1 ABCB4 is also known as multidrug resistance 3 gene (MDR3), a member of the MDR/TAP subfamily involved in multidrug resistance as well as antigen presentation. The human ABCB4 gene is located on chromosome 7q21.1, contains 27 coding exons, and spans approximately 74 kb.2 The pathophysiology of the ABCB4 alterations resides in the lack of phospholipid protection in the bile against the detergent effect of the bile salts, resulting in damage to the biliary epithelium, bile ductular proliferation, and potential progressive portal fibrosis. As biliary cholesterol solubilization depends not only on the concentration of the sterol itself but also on the bile salt and phospholipid concentration, a decreased rate of phospholipid excretion can also be a cause of gallstone formation.
The wide clinical spectrum of the ABCB4 deficiency syndromes in humans covers cholestatic disorders presenting from the neonatal period of life to late adulthood.3, 4, 5 At least three distinct syndromes with variable severity have been clearly identified: progressive familial intrahepatic cholestasis type 3 (PFIC3; MIM 602347), low phospholipid-associated cholelithiasis (LPAC alias gallbladder disease 1, GBD1; MIM 60080), and familial intrahepatic cholestasis of pregnancy (ICP; MIM 147480). Evidences of ABCB4 mutations have also been found in transient neonatal cholestasis,6 or adult idiopathic biliary fibrosis or cirrhosis.4, 7, 8, 9, 10, 11
A recessive inheritance pattern of PFIC3 has been observed.7, 12 Most ABCB4 mutations in the patients with PFIC3 have been reported to be homozygous or compound heterozygous.3, 7, 9, 12, 13, 14 These mutations include missense and non-sense mutations, and short frameshift deletions or insertions. ABCB4 mutations are associated with an absence or a weak level of the canalicular ABCB4 protein, and with a low level of biliary phospholipids.7, 12, 15 Patients with PFIC3 usually present at a few years of age and suffer from severe chronic and progressive cholestasis. Liver histology often reveals fibrosis with portal inflammation and strong bile duct proliferation in an early stage.16 A characteristic high-serum gamma-glutamyltransferase activity is found in PFIC3. As a consequence of the cirrhosis, the PFIC3 patients are prone to gastrointestinal bleeding. About 50% of the patients need a liver transplantation. Interestingly, the other half may benefit from treatment with ursodeoxycholic acid (UDCA).7
Mutations in the ABCB4 gene that may reduce but not eliminate or drastically decrease the protein (leaving residual activity of the transporter), have been shown to cause a variety of milder cholestatic phenotypes, including LPAC and ICP. LPAC is a peculiar form of biliary gallstone disease characterized by intrahepatic sludge and/or symptomatic cholesterol cholelithiasis in young adults (usually before 40 years). LPAC is typically associated with mild chronic cholestasis, recurrence of symptoms after cholecystectomy, and prevention of recurrence by UDCA. About one third to half of patients have missense, frameshift, or non-sense mutations – mostly heterozygous – in the ABCB4 gene.17, 18, 19 One of the hallmarks of LPAC is the response and remission induced by the UDCA therapy. Heterozygous ABCB4 mutations were also identified in up to 15% of women suffering from ICP.12, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 ICP is a reversible form of cholestasis that may develop in the third trimester of pregnancy, usually rapidly resolves after delivery and recurs in 45–70% of subsequent pregnancies.27 The main symptoms are pruritus and, to a lesser extent, jaundice. Serum bile salt and aminotransferases levels are elevated. Increased incidence of fetal complications (including placental insufficiency, premature labor, and sudden fetal death) was reported in association with ICP. UDCA is the treatment of choice for ICP and produces relief from pruritus with improvement in liver tests, with no adverse maternal or fetal effects.27, 32, 33 It is generally accepted that women who have suffered from ICP are also susceptible to the development of cholestasis on the use of oral contraceptives (oral contraceptives-induced cholestasis (CIC)).34, 35 Evidences of ABCB4 heterozygous mutations have also been found in patients with drug-induced cholestasis or liver injury.17, 18, 35, 36
Loss of function ABCB4 mutations can present a large spectrum of cholestasis phenotypes, and genetic analysis of ABCB4 can now be performed to confirm the diagnosis.37 However, no ABCB4 mutations are found in a significant proportion of patients. The lack of ABCB4 mutation detection in remaining cases might be attributed to phenotyping errors, genetic heterogeneity, and inadequacy of genetic screening methods. In the present study, we have tested the last hypothesis by using recent high-resolution gene dosage methodologies in a large series of 102 adult patients with symptomatic intrahepatic cholelithiasis/cholestasis. Here, we describe for the first time ABCB4 partial or complete deletions in patients with LPAC and CIC.
Subjects and methods
Patients
A panel of 102 clinically diagnosed index cases was phenotypically selected, by routine genetic diagnosis. All patients were examined by reference hepatologists or gastroenterologists. The full clinical and radiological available information were recorded. The written informed consent from each patient included in the study was obtained. In total, 59 unrelated adult women with ICP and/or CIC and 43 unrelated adult patients with a clinical presentation compatible with LPAC syndrome were included in this present study. For the latter group, we have considered patients presenting with intrahepatic cholelithiasis despite cholecystectomy before 40 years associated with at least one of the following criteria: presence of complications (cholecystitis, cholangitis, or acute pancreatitis), intrahepatic hyperechoic foci with or without sludge or microlithiasis, response to UDCA therapy, and family history.
ABCB4 mutation screening
Total DNA was extracted from whole-blood leukocytes using the Nucleon BACC2 genomic DNA extraction kit (GE Health Care Europe, Amersham, UK). Genomic DNA was amplified with primers specific for the 27 ABCB4 (NM_018849.2)-coding exons and their intron boundaries. The primer sequences and PCR conditions are available upon request. Mutation was identified using bidirectional DNA sequencing of purified PCR products. Sequences were aligned with Seqscape v5.1 analysis software (Applied Biosystems, Foster City, CA, USA). The ABCB4 molecular analysis was performed in the Biochemistry and Molecular Genetics Laboratory, Beaujon Hospital, Clichy, France.
ABCB4 multiplex ligation-dependent probe amplification (MLPA) analysis
The ABCB4 deletion screening was performed by MLPA analysis using the SALSA MLPA kit P109 ABCB4, as recommended in the manufacturer's protocol (MRC-Holland, Amsterdam, The Netherlands). This SALSA MLPA kit is designed to detect deletions/duplications of one or more exons of the ABCB4 gene. It contains 22 probes covering ABCB4-coding exons, three probes for ABCB4 promoter, and three probes for the centromeric adjacent ABCB1 gene. For reference, several probes for other human genes located on different chromosomes are also included. Briefly, four control samples and patient samples (each containing 100 ng of genomic DNA) were used for overnight hybridization with the probe mixes. After ligation and amplification performed with FAM-labeled primers, PCR products were analyzed on an ABI Prism 3130 automatic DNA sequencer (Applied Biosystems). Peak areas for each separated fragment were measured by using Genemapper software v4.0 (Applied Biosystems). Normalized ratios of <0.6 and >1.3 were considered as deletions and duplications, respectively. Ratio profiles between 0.6 and 0.85 were considered as doubtful. DNA samples with ABCB4 copy number variation were further analyzed by real-time PCR-based gene dosage for confirmation.
Real-time PCR-based gene dosage
We quantified ABCB4 exons 10 and 11 by determining the threshold cycle (Ct) number at which the increase in the signal associated with exponential growth of PCR products begins. We also quantified the ALB gene (encoding albumin) as an endogenous DNA control, and each sample was normalized on the basis of its ALB content, as previously described.38 ALB was selected as an endogenous control, because it maps to chromosome 4q11-q13, while ABCB4 is at chromosome 7q21.1. The relative copy number of the ABCB4 exon targets was also normalized to a calibrator, consisting of genomic DNA from a normal subject. Final results, expressed as N-fold differences in the ABCB4 exonic targets copy number relative to the ALB gene and the calibrator were determined as follows: N-fold=2(ΔCtsample−ΔCtcalibrator), where ΔCt values of the sample and calibrator are determined by subtracting the average Ct value of the ABCB4 exon target from the average Ct value of the ALB gene. An N-fold value of <0.6 was considered deleted. All PCRs were performed on a LightCycler 480 with the LightCycler 480 SYBR Green I Master kit (Roche Applied Science, Basel, Switzerland). PCR conditions and primer sequences are available on request. Experiments were done with triplicates for each data point.
Microarray characterization of ABCB4 complete deletions
The practical aspects of array-comparative genomic hybridization (array-CGH) are described in detail elsewhere.39 Briefly, array-CGH labeling and hybridization were performed on Agilent whole human genome 400K microarrays as recommended in the Agilent manual (Protocol v6.3, October 2010, Agilent Technologies, Palo Alto, CA, USA). Patients' genomic DNA and six pooled normal control DNAs (reference) were labeled with Cy5-dUTP and Cy3-dUTP, respectively. Arrays were scanned with an Agilent DNA Microarray Scanner (G2565BA). Log2 ratios were determined with Agilent Feature Extraction software (v 9.1.3.1). Results were visualized and analyzed with Agilent's Genomic Workbench 5.0 software. DNA sequence information was referred to the public UCSC (University of California Santa Cruz) database (Human Genome Browser, February 2009 assembly: hg 19, National Center for Biotechnology Information (NCBI) Build 37).
Fine characterization of ABCB4 intragenic deletion breakpoints
Long-range PCR was performed with the Expand 20 kb Plus PCR kit as recommended by the manufacturer (Roche Applied Science). The primer pairs and PCR conditions used to characterize the gross deletions are available upon request. The PCR products were sequenced with the ABI BigDye terminator sequencing kit (Applied Biosystems) on an ABI Prism 3130 automatic DNA sequencer (Applied Biosystems).
Results
In all, 102 index cases with LPAC and ICP/CIC were screened on the basis of their phenotype for mutations in the ABCB4 gene. A total of 32 ABCB4 heterozygous loss of function point or short insertion/deletion mutations (including non-sense, frameshift, splice, or previously reported/predicted to be deleterious missense mutations) were identified among the 102 tested index cases: in 37% (16/43) of LPAC patients and in 27% (16/59) of ICP/CIC patients.
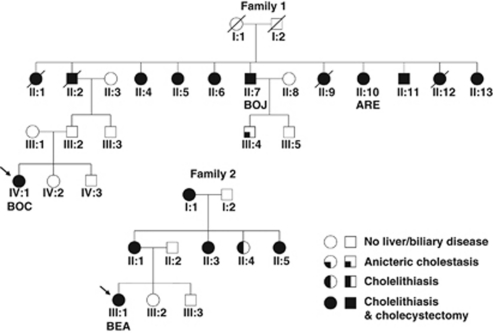
The 70 adult patients with no ABCB4 mutation found (27 with LPAC and 43 with ICP/CIC), were consequently screened for large rearrangements using MLPA. Four heterozygous deletions were identified with this approach in 7% (3/43) of the LPAC patients (BOC propositus of family 1, BEA propositus of family 2, and TIL) and in ∼2% (1/59) of the ICP/CIC patients (ROC) (Figure 1). The four deletions were confirmed using real-time PCR-based gene dosage. Clinical features of the individuals with ABCB4 gene deletions (family 1: patients BOC, ARE, and BOJ; family 2: patient BEA; patient ROC; patient TIL) are summarized in Table 1. Figure 2 shows families 1 and 2 pedigrees including 12 and 5 patients with LPAC syndrome, respectively.
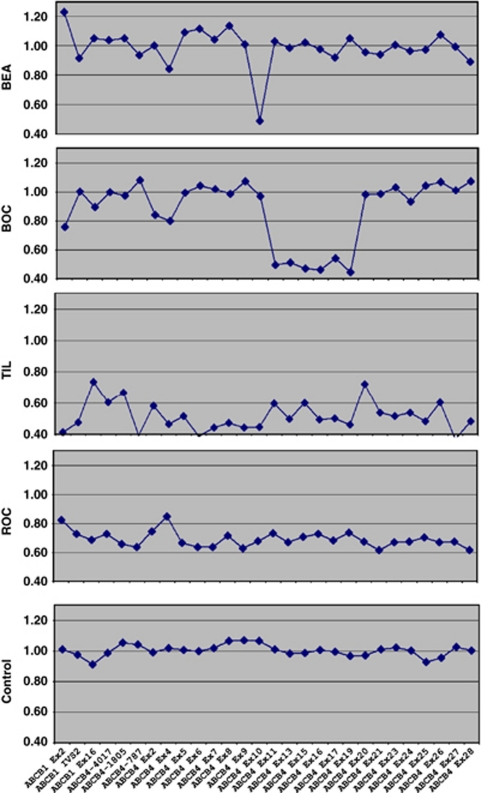
Figure 1.
ABCB4 SALSA MLPA kit P109 peak areas normalized ratio profiles of patients BEA, BOC, TIL, ROC, and one normal control DNA. Two partial intragenic deletions (patients BEA and BOC) and two whole-gene deletions (patients TIL and ROC) were identified. The mean of normalized ratios in patients without deletion was 1.02.
Table 1. Clinical and molecular characteristics of the patients carrying gross genomic deletions.
| Subject | Gender | Age at onset (years) | Features at presentation | Deletion length | Deleted genes |
|---|---|---|---|---|---|
| Family 1 | Familial history: see Figure 1 | 24720 bp | Deletion of ABCB4 exons 11–19 | ||
| BOC (propositus) | F | 24 | LPAC: intrahepatic cholelithiasis with biliary pain. Recurrence (biliary pain and elevated liver enzymes: AST=330 U/l, ALT=416 U/l, and GGT=616 U/) despite cholecystectomy. Free of symptom and normal liver enzymes under UDCA. | ||
| ARE | F | 60 | Recurrent cholelithiasis despite cholecystectomy. Free of symptom under UDCA. | ||
| BOJ | M | 60 | LPAC: cholangiopathy with intrahepatic cholelithiasis. Left hepatectomy because of liver atrophy with intrahepatic biliary dilatation and cholecystectomy. Liver biopsy: carcinoma in situ, biliary histology abnormalities with ductular proliferation, compatible with ABCB4 mutation. Free of symptom under UDCA. | ||
| Family 2 | Familial history: see Figure 1 | 998 bp | Deletion of ABCB4 exon 10 | ||
| BEA (propositus) | F | 30 | LPAC: cholelithiasis with biliary pain. Recurrence of biliary symptoms after cholecystectomy. Her mother, maternal grandmother and two maternal aunts had cholecystectomy before the age of 40 (Figure 1). | ||
| Patient TIL | M | 36 | LPAC with biliary pain. Recurrence (biliary pain and elevated liver enzymes: AST=473 U/l, ALT=722 U/l, and GGT=65 U/l) after cholecystectomy. Free of symptom and normal liver enzymes under UDCA. No evidence of familial history. | ∼339 kb | 4 genes: P53TG1, CROT, ABCB4, and ABCB1 |
| Patient ROC | F | 26 | CIC with biliary pain, jaundice and elevated liver enzymes (AST=1047 U/l, ALT=372 U/l, and GGT=63 U/l). Liver biopsy: biliary histology abnormalities with ductular proliferation and compatible with ABCB4 mutation. Free of symptom and normal liver enzymes in the absence of oral contraception. Normal pregnancy with delivery at term under UDCA therapy. | ∼5 Mb | 21 genes including ABCB4 (Supplemental Table 1) |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; U/l, units per liter.
The usual ranges for AST, ALT, and GGT are around 10–35 U/l, 10–45 U/l, and 10–55 U/l, respectively.
Figure 2.
Pedigrees of families 1 and 2. Squares and circles indicate males and females, respectively. Clear symbols indicate unaffected individuals. Arrows indicate propositus. Individuals II:4, II:7, II:10, and IV:1 in family 1 and individual III:1 in family 2 were molecularly tested. Individual II:2 in family 1 was diagnosed with cholangiocarcinoma at 71 years of age.
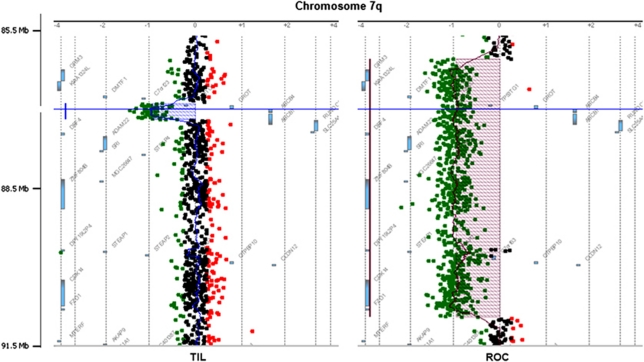
The four ABCB4 gross deletions were then accurately characterized. They included two whole-gene deletions (TIL and ROC), and two partial intragenic deletions (BEA: exon 10 deletion and BOC: exons 11–19 deletion). Figure 3 shows characteristic array-CGH profiles for the two complete ABCB4 deletions, as displayed by the workbench software. Deletion of patient ROC is flanked by last non-deleted centromeric probe at position chr7:86 068 686–86 068 745 (numbered as in build 37/hg19 assembly of the NCBI), and telomeric probe at chr7:91 075 851–91 075 910. Deletion of patient TIL is flanked by last non-deleted centromeric probe at chr7:86 907 515–86 907 574 and telomeric probe at chr7:87 246 524–87 246 583. Patient's ROC and TIL deletions were consequently estimated to be ∼5 Mb and ∼339 kb long, respectively, including 20 and 3 genes in addition to ABCB4 (Table 1; Supplementary Table 1). The 400K array-CGH data on these two patients have been deposited in NCBI's Gene Expression Omnibus (GEO) and are accessible through GEO accession number GSE28676 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE28676).
Figure 3.
Array-CGH profiles of the two partially characterized ABCB4 complete deletions: patients TIL and ROC. Zoom on ABCB4 region at chromosome 7q easily identified the deletions. Horizontal blue line shows ABCB4 position. Human Genome Browser, February 2009 assembly: hg 19, NCBI Build 37.
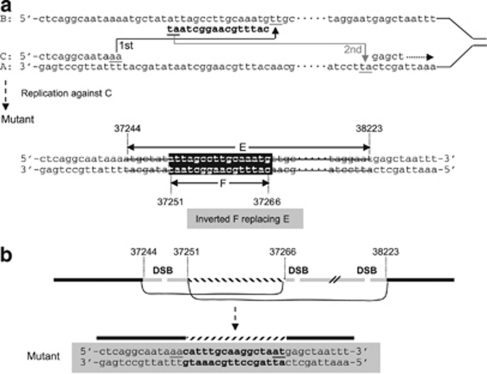
The breakpoints of the two intragenic deletions (patients BEA and BOC) were precisely determined by sequencing the junction long-range PCR products. Only DNA sample from BEA yielded a 3055-bp fragment with primers JONCTIONBEA-U (5′-TGTGACTCGGACTATGGATTGTT-3′) and JONCTIONBEA-L (5′-CCAAAACTGGATTCACACGCA-3′). Subsequent sequencing revealed that this deletion is in fact a complex rearrangement (Figure 4). A healthy control sample and BEA wild-type allele yielded a 4053-bp non-deleted fragment using the same primers. Only DNA sample from BOC (family 1 propositus) yielded a 2680-bp fragment with primers JONCTIONBOC-U (5′-AAAATAGACCCACTCAGGCAA-3′) and JONCTIONBOC-L (5′-ATGCTACATGCTTATCTAAAACCAT-3′). A healthy control sample yielded no PCR product, the wild-type allele being too large (∼27 kb) to be amplified. Sequencing the PCR product revealed a simple deletion of 24 720 bp (Supplementary Figure 1). This deletion was also detected in three other affected individuals with available DNA samples (Figure 2: individuals II:4, II:7, and II:10 of family 1).
Figure 4.
Illustration of two possible mechanisms underlying the complex rearrangement detected in patient BEA. (a) In the SRS model,42, 43 the newly synthesized leading strand (C) misaligned with the lagging template strand (B) through inverted short repeats (the first step of slippage); having synthesized a short DNA sequence tract, the C strand dissociated from B and re-annealed to its original template strand (A) via short repeats (the second step of slippage); continued DNA synthesis (right-handed dotted arrow). Then replication against the synthesized strand C resulted in the observed complex rearrangement. (b) In the non-homologous end-joining (NHEJ) model, at least two double-strand breaks (DSB) occurred within the sequences deleted. One short internal sequence was re-captured in an inverted orientation during the NHEJ repair. Numbers indicating nucleotide positions are in accordance with GenBank accession EF034088.1. Short repeats are underlined.
Discussion
In this study, we showed for the first time that a significant subset of patients with symptomatic cholestasis/cholelithiasis has underlying ABCB4 deletions. Partial or complete heterozygous ABCB4 deletions were found in 7% of the patients with LPAC and in ∼2% of the patients with CIC. A large family of 12 affected patients with severe LPAC and cholecystectomy (family 1) was notably reported (Table 1; Figure 2).
Recent gene dosage methodologies have allowed identification of these ABCB4 deletions. Our observations urge to systematically test the patients with LPAC for the presence of ABCB4 deletions. MLPA, a sensitive, rapid, and cost-effective approach seems particularly adapted to routine diagnosis in molecular genetics. We developed a molecular algorithm tailored to ABCB4 routine analysis that includes ABCB4 gene dosage by MLPA, in case of ABCB4 negative sequencing in patients with suggestive phenotype. MLPA allows a fast and inexpensive first-line screening for both the partial and complete ABCB4 deletions, complementary to a high-resolution technique such as array-CGH that can be used to characterize larger deletions. Real-time PCR-based gene dosage is useful for deletion's confirmation, particularly for the samples with ratio profiles considered as doubtful, that is, between 0.6 and 0.85 (patient ROC; Figure 1).
The two intragenic deletion breakpoints were cloned at the nucleotide level and no recurrent breakpoints were found. That no significant sequence similarity was found between the centromeric and telomeric breakpoints of both the deletions, effectively excluded homologous recombination as the underlying mutational mechanisms in both the cases. However, the presence of microhomology in each of the aberrant junctions is consistent with both the microhomology-dependent replication-based recombination (MMRDR) and non-homologous end-joining (NHEJ) mechanisms.40 Thus, the complex rearrangement detected in patient BEA can be perfectly explained by either serial replication slippage (SRS, a subpathway of MMRDR) or NHEJ repair of simultaneously generated double-strand breaks (Figure 4), while the simple 24 kb deletion (Supplementary Figure 1) is consistent with a single step of replication slippage (a subpathway of MMRDR), microhomology-mediated break-induced replication (MMBIR, a subpathway of MMRDR) or NHEJ.40
In this study, adult patients with LPAC were screened for the ABCB4 deletions. We assume that ABCB4 deletions may also be found in children with PFIC3. In some patients with PFIC3, only one ABCB4 mutation or no mutation has been previously reported while no ABCB4 protein was detected by immunohistochemistry analysis.19 Some of these genotype–phenotype discrepancies could be explained by the presence of ABCB4 deletions in the PFIC3 patients. However, in PFIC3 patient with no mutation found, a heterozygous ABCB4 deletion would not be sufficient to explain the phenotype because PFIC3 is an autosomal recessive disease. Our observations urge to reassess the ABCB4 molecular analysis in these patients using molecular tools allowing detection of rearrangements. PFIC3 patients who do respond to the UDCA therapy generally have a partial ABCB4 defect (missense mutations) and the residual phospholipid concentration in the bile combined with UDCA replacement, may be sufficient to reduce bile salt toxicity below a critical threshold.19 ABCB4 genotyping (including deletion's detection) should help to select those PFIC3 patients who could benefit from the UDCA therapy.
In clinical practice, the establishment of a comprehensive ABCB4 alteration-screening algorithm will permit the use of ABCB4 genotyping, to confirm the diagnosis of LPAC syndrome in young adults who present with a symptomatic cholelithiasis and allow familial testing. One argument in support of molecular testing for ABCB4 deletion is benefit of the UDCA therapy of both the symptomatic and asymptomatic cholelithiasis in patients with ABCB4 deficiency.18 Depending on the results, long-term curative or prophylactic UDCA therapy could be initiated early to prevent the occurrence or recurrence of syndromes and their potential severe complications. Patient ROC who suffered from CIC may also be susceptible to the development of ICP that carries a risk of premature delivery and sudden fetal death. Identification of the ABCB4 deletions may also benefit patients with CIC and ICP, as UDCA is recommended to reduce pruritus, and probably prematurity without adverse side effects.41 Management of ICP also includes close monitoring and early delivery for the fetus. All the patients with ABCB4 defect in this study have benefited from the UDCA treatment. These observations are remarkable examples of the inter-relationship between molecular biology and clinical medicine.
The extreme variability and the wide spectrum of ABCB4 alteration-related phenotypes make genotype–phenotype correlations difficult, although they are of crucial importance for the patients and their families. Further comparative studies of patients with well-characterized genotypes (including deletions) and phenotypes will help determine whether ABCB4 mutation types influence clinical outcomes. Three of the four patients with deletions (BEA, BOC, and TIL) developed LPAC that recurred despite cholecystectomy. Patient ROC presented a very large de novo ∼5 Mb deletion encompassing the ABCB4 locus and 20 additional genes including ABCB1 gene (Supplementary Table 1). Surprisingly, this patient presented a less severe phenotype only consisting in CIC, while being haploinsufficient for 21 genes. Unlike the ABCB4 gene, none of these 20 genes is known to be associated with an inherited human disease according to the OMIM database (http://www.ncbi.nlm.nih.gov/omim). Variable expressions of the liver diseases caused by ABCB4 mutations have previously been reported. Comorbidity factors, environmental influences, or unknown genetic modifiers may modulate these phenotypes.13, 19 Our observations reinforce the potential existence of these genetic modifiers.
Gene dosage technologies have allowed the identification of ABCB4 deletions in a significant subset (7%) of patient with LPAC syndrome. An early diagnosis of this biliary disease would be beneficial because of the potential preventive effect of UDCA on the biliary complications. These data must now be taken into account in patient diagnosis and follow-up.
Acknowledgments
We thank the patients for their participation. We thank all the clinicians from France, who provided the samples for this study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Oude Elferink RP, Paulusma CC, Groen AK. Hepatocanalicular transport defects: pathophysiologic mechanisms of rare diseases. Gastroenterology. 2006;130:908–925. doi: 10.1053/j.gastro.2005.08.052. [DOI] [PubMed] [Google Scholar]
- Lincke CR, Smit JJ, van der Velde-Koerts T, Borst P. Structure of the human MDR3 gene and physical mapping of the human MDR locus. J Biol Chem. 1991;266:5303–5310. [PubMed] [Google Scholar]
- Oude Elferink RP, Paulusma CC. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein) Eur J Physiol. 2007;453:601–610. doi: 10.1007/s00424-006-0062-9. [DOI] [PubMed] [Google Scholar]
- Lucena JF, Herrero JI, Quiroga J, et al. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology. 2003;124:1037–1042. doi: 10.1053/gast.2003.50144. [DOI] [PubMed] [Google Scholar]
- Kano M, Shoda J, Sumazaki R, Oda K, Nimura Y, Tanaka N. Mutations identified in the human multidrug resistance P-glycoprotein 3 (ABCB4) gene in patients with primary hepatolithiasis. Hepatol Res. 2004;29:160–166. doi: 10.1016/j.hepres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Jung C, Driancourt C, Baussan C, et al. Prenatal molecular diagnosis of inherited cholestatic diseases. J Pediatr Gastroenterol Nutr. 2007;44:453–458. doi: 10.1097/MPG.0b013e318036a569. [DOI] [PubMed] [Google Scholar]
- Jacquemin E, de Vree JM, Cresteil D, et al. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology. 2001;120:1448–1458. doi: 10.1053/gast.2001.23984. [DOI] [PubMed] [Google Scholar]
- Rosmorduc O, Hermelin B, Boelle PY, Poupon RE, Poupon R, Chazouillères O. ABCB4 gene mutations and primary sclerosing cholangitis. Gastroenterology. 2004;126:1220–1222. doi: 10.1053/j.gastro.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Ziol M, Barbu V, Rosmorduc O, et al. ABCB4 heterozygous gene mutations associated with fibrosing cholestatic liver disease in adults. Gastroenterology. 2008;135:131–141. doi: 10.1053/j.gastro.2008.03.044. [DOI] [PubMed] [Google Scholar]
- Denk GU, Bikker H, Lekanne Dit Deprez RH, et al. ABCB4 deficiency: a family saga of early onset cholelithiasis, sclerosing cholangitis and cirrhosis and a novel mutation in the ABCB4 gene. Hepatol Res. 2010;40:937–941. doi: 10.1111/j.1872-034X.2010.00698.x. [DOI] [PubMed] [Google Scholar]
- Poupon R, Arrive L, Rosmorduc O. The cholangiographic features of severe forms of ABCB4/MDR3 deficiency-associated cholangiopathy in adults. Gastroenterol Clin Biol. 2010;34:380–387. doi: 10.1016/j.gcb.2010.04.011. [DOI] [PubMed] [Google Scholar]
- De Vree JM, Jacquemin E, Sturm E, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo C, Vajro P, Degiorgio D, et al. Clinical features and genotype-phenotype correlations in children with progressive familial intrahepatic cholestasis type 3 related to ABCB4 mutations. J Pediatr Gastroenterol Nutr. 2011;52:73–83. doi: 10.1097/MPG.0b013e3181f50363. [DOI] [PubMed] [Google Scholar]
- Degiorgio D, Colombo C, Seia M, et al. Molecular characterization and structural implications of 25 new ABCB4 mutations in progressive familial intrahepatic cholestasis type 3 (PFIC3) Eur J Hum Genet. 2007;15:1230–1238. doi: 10.1038/sj.ejhg.5201908. [DOI] [PubMed] [Google Scholar]
- Deleuze JF, Jacquemin E, Dubuisson C, et al. Defect of multidrug-resistance 3 gene expression in a subtype of progressive familial intrahepatic cholestasis. Hepatology. 1996;23:904–908. doi: 10.1002/hep.510230435. [DOI] [PubMed] [Google Scholar]
- Maggiore G, Bernard O, Hadchouel M, Lemonnier A, Alagille D. Diagnostic value of serum gamma-glutamyl transpeptidase activity in liver diseases in children. J Pediatr Gastroenterol Nutr. 1991;12:21–26. doi: 10.1097/00005176-199101000-00005. [DOI] [PubMed] [Google Scholar]
- Rosmorduc O, Hermelin B, Poupon R. MDR3 gene defect in adults with symptomatic intrahepatic and gallbladder cholesterol cholelithiasis. Gastroenterology. 2001;120:1459–1467. doi: 10.1053/gast.2001.23947. [DOI] [PubMed] [Google Scholar]
- Rosmorduc O, Hermelin B, Boelle PY, Parc R, Taboury J, Poupon R. ABCB4 gene mutation-associated cholelithiasis in adults. Gastroenterology. 2003;125:452–459. doi: 10.1016/s0016-5085(03)00898-9. [DOI] [PubMed] [Google Scholar]
- Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis. 2010;30:134–146. doi: 10.1055/s-0030-1253223. [DOI] [PubMed] [Google Scholar]
- Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet. 1999;353:210–211. doi: 10.1016/S0140-6736(05)77221-4. [DOI] [PubMed] [Google Scholar]
- Dixon PH, Weerasekera N, Linton KJ, et al. Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking. Hum Mol Genet. 2000;9:1209–1217. doi: 10.1093/hmg/9.8.1209. [DOI] [PubMed] [Google Scholar]
- Gendrot C, Bacq Y, Brechot MC, Lansac J, Andres C. A second heterozygous MDR3 nonsense mutation associated with intrahepatic cholestasis of pregnancy. J Med Genet. 2003;40:e32. doi: 10.1136/jmg.40.3.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllenbach R, Linton KJ, Wiltshire S, et al. ABCB4 gene sequence variation in women with intrahepatic cholestasis of pregnancy. J Med Genet. 2003;40:e70. doi: 10.1136/jmg.40.5.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli-Magnus C, Kerb R, Fattinger K, et al. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2004;39:779–791. doi: 10.1002/hep.20159. [DOI] [PubMed] [Google Scholar]
- Keitel V, Vogt C, Häussinger D, Kubitz R. Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology. 2006;131:624–629. doi: 10.1053/j.gastro.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Wasmuth HE, Glantz A, Keppeler H, et al. Intrahepatic cholestasis of pregnancy: the severe form is associated with common variants of the hepatobiliary phospholipid transporter ABCB4 gene. Gut. 2007;56:265–270. doi: 10.1136/gut.2006.092742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JE. Liver disease in pregnancy. Hepatology. 2008;47:1067–1076. doi: 10.1002/hep.22130. [DOI] [PubMed] [Google Scholar]
- Schneider G, Paus TC, Kullak-Ublick GA, et al. Linkage between a new splicing site mutation in the MDR3 alias ABCB4 gene and intrahepatic cholestasis of pregnancy. Hepatology. 2007;45:150–158. doi: 10.1002/hep.21500. [DOI] [PubMed] [Google Scholar]
- Floreani A, Carderi I, Paternoster D, et al. Intrahepatic cholestasis of pregnancy; three novel MDR3 gene mutations. Aliment Pharmacol Ther. 2006;23:1649–1653. doi: 10.1111/j.1365-2036.2006.02869.x. [DOI] [PubMed] [Google Scholar]
- Floreani A, Carderi I, Paternoster D, et al. Hepatobiliary phospholipid transporter ABCB4, MDR3 gene variants in a large cohort of Italian women with intrahepatic cholestasis of pregnancy. Dig Liver Dis. 2008;40:366–370. doi: 10.1016/j.dld.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Bacq Y, Gendrot C, Perrotin F, et al. ABCB4 gene mutations and single-nucleotide polymorphisms in women with intrahepatic cholestasis of pregnancy. J Med Genet. 2009;46:711–715. doi: 10.1136/jmg.2009.067397. [DOI] [PubMed] [Google Scholar]
- Mazzella G, Rizzo N, Azzaroli F, et al. Ursodeoxycholic acid administration in patients with cholestasis of pregnancy: effects on primary bile acids in babies and mothers. Hepatology. 2001;33:504–508. doi: 10.1053/jhep.2001.22647. [DOI] [PubMed] [Google Scholar]
- Paus TC, Schneider G, Van De Vondel P V, Sauerbruch T, Reichel C. Diagnosis and therapy of intrahepatic cholestasis of pregnancy. Z Gastroenterol. 2004;42:623–628. doi: 10.1055/s-2004-813165. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Meier PJ. Hepatobiliary transporters and drug-induced cholestasis. Hepatology. 2006;44:778–787. doi: 10.1002/hep.21359. [DOI] [PubMed] [Google Scholar]
- Ganne-Carrié N, Baussan C, Grando V, Gaudelus J, Cresteil D, Jacquemin E. Progressive familial intrahepatic cholestasis type 3 revealed by oral contraceptive pills. J Hepatol. 2003;38:693–694. doi: 10.1016/s0168-8278(03)00049-7. [DOI] [PubMed] [Google Scholar]
- Lang C, Meier Y, Stieger B, et al. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet Genomics. 2007;17:47–60. doi: 10.1097/01.fpc.0000230418.28091.76. [DOI] [PubMed] [Google Scholar]
- Poupon R, Barbu V, Chamouard P, Wendum D, Rosmorduc O, Housset C. Combined features of low phospholipid-associated cholelithiasis and progressive familial intrahepatic cholestasis 3. Liver Int. 2010;30:327–331. doi: 10.1111/j.1478-3231.2009.02148.x. [DOI] [PubMed] [Google Scholar]
- Pasmant E, de Saint-Trivier A, Laurendeau I, Dieux-Coeslier A, Parfait B, Vidaud M, et al. Characterization of a 7.6-Mb germline deletion encompassing the NF1 locus and about a hundred genes in an NF1 contiguous gene syndrome patient. Eur J Hum Genet. 2008;16:1459–1466. doi: 10.1038/ejhg.2008.134. [DOI] [PubMed] [Google Scholar]
- Pasmant E, Sabbagh A, Masliah-Planchon J, et al. Detection and characterization of NF1 microdeletions by custom high resolution array CGH. J Mol Diagn. 2009;11:524–529. doi: 10.2353/jmoldx.2009.090064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Cooper DN, Férec C, Kehrer-Sawatzki H, Patrinos GP. Genomic rearrangements in inherited disease and cancer. Semin Cancer Biol. 2010;20:222–233. doi: 10.1016/j.semcancer.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Hardikar W, Kansal S, Oude Elferink RP, Angus P. Intrahepatic cholestasis of pregnancy: when should you look further. World J Gastroenterol. 2009;15:1126–1129. doi: 10.3748/wjg.15.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Chuzhanova N, Stenson PD, Férec C, Cooper DN. Complex gene rearrangements caused by serial replication slippage. Hum Mutat. 2005;26:125–134. doi: 10.1002/humu.20202. [DOI] [PubMed] [Google Scholar]
- Chen JM, Chuzhanova N, Stenson PD, Férec C, Cooper DN. Intrachromosomal serial replication slippage in trans gives rise to diverse genomic rearrangements involving inversions. Hum Mutat. 2005;26:362–373. doi: 10.1002/humu.20230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.