Abstract
Neuroblastic tumours may occur in a predisposition context. Two main genes are involved: PHOX2B, observed in familial cases and frequently associated with other neurocristopathies (Ondine's and Hirschsprung's disease); and ALK, mostly in familial tumours. We have assessed the frequency of mutations of these two genes in patients with a presumable higher risk of predisposition. We sequenced both genes in 26 perinatal cases (prebirth and <1 month of age, among which 10 were multifocal), 16 multifocal postnatal (>1 month) cases, 3 pairs of affected relatives and 8 patients with multiple malignancies. The whole coding sequences of the two genes were analysed in tumour and/or constitutional DNAs. We found three ALK germline mutations, all in a context of multifocal tumours. Two mutations (T1151R and R1192P) were inherited and shared by several unaffected patients, thus illustrating an incomplete penetrance. Younger age at tumour onset did not seem to offer a relevant selection criterion for ALK analyses. Conversely, multifocal tumours might be the most to benefit from the genetic screening. Finally, no PHOX2B germline mutation was found in this series. In conclusion, ALK deleterious mutations are rare events in patients with a high probability of predisposition. Other predisposing genes remain to be discovered.
Keywords: ALK, neuroblastoma, predisposition
Introduction
Most cancers occurring in children are thought to be sporadic and a genetic predisposition is rarely evoked. However, some particular clinical features are more suggestive of a genetic susceptibility and justify further investigation. Familial aggregations of cancers are among the most suggestive situations, having lead, for example, to the identification of the Li-Fraumeni syndrome.1 However, random familial aggregations of sporadic cancers are not so unusual. Thus, the suspicion of familial susceptibility is more justified when several individuals develop the same malignancy. The paradigmatic example in paediatric cancers is the familial form of retinoblastoma due to hereditary mutations in RB1,2 in which multifocal disease is a common observation. Similarly, bilateral Wilms tumours are indicative of a predisposition, occasionally related to 11p15 abnormality with or without Beckwith-Wiedemann phenotype,3, 4 which is the paradigmatic imprinting defect associated with neoplasia.5 Therefore, a multifocal presentation is sufficient per se to suggest a predisposition syndrome, especially when it is observed with any kind of developmental disease.4 Finally, a young age at diagnosis is also indicative of a higher probability of carrying a germline mutation. Such a positive correlation between the presence of a germline mutation and the youngest age at diagnosis has been proven for several predisposing genes, such as RB1 in retinoblastoma, SMARCB1 in rhabdoid tumours. Another example come from the mutations in SUFU (a tumour suppressor gene controlling the Sonic hedgehog signalling) which most remarkably predispose to early onset desmoplastic medulloblastomas, before 3 years of age.6, 7
Peripheral neuroblastic tumours are tumours of neural crest origin that mostly affect children before 5 years of age. The young age at diagnosis lead Knudson and Strong8 to propose that neuroblastic tumours might frequently arise in a predisposition context. Accordingly, familial cases, although rare, confirmed the hypothesis of germline mutations being responsible for at least a subset of those tumours.9, 10 Recently, mutations in two genes have been identified as predisposing causes for neuroblastic tumours. First, mutations in PHOX2B, a gene expressed in the developing peripheral autonomous nervous system, were observed in children with neuroblastic tumours in a syndromic context of Ondine's curse and/or Hirschsprung disease.11, 12, 13 Subsequent studies showed that PHOX2B mutations could also be observed in non-syndromic familial cases, and even as de novo mutations in apparently sporadic tumours.14, 15, 16, 17 More recently, constitutional mutations in the ALK gene have been shown to be the predominant genetic cause of familial neuroblastomas.18, 19 Furthermore, somatically acquired ALK mutations are evidenced in ∼7% of sporadic tumours, but, again, some of those apparently sporadic tumours actually arise in a context of constitutional mutation, either inherited or de novo.20, 21, 22, 23 Hence, the actual incidence of PHOX2B and ALK germline mutations may be highly underestimated if one only considers familial recurrences of neuroblastic tumours to initiate a constitutional screening.
In the present work, we have addressed the frequency of ALK germline mutations in children who developed a neuroblastoma, a ganglioneuroblastoma or a ganglioneuroma with clinical features highly suggestive of a predisposition context. Based on previous experiences and literature review, we selected, as good candidates for having a predisposition, children with perinatal diagnosis (before birth and up to 1 month of age),24 multifocal disease,25 second malignancy,26 and familial recurrence of neuroblastic tumours. Our work aims at giving a better insight for genetic counselling and further document the incidence and the penetrance of ALK mutations in patients with neuroblastic tumours.
Materials and methods
Selection of the study population
We first retrospectively reviewed the clinical database from the tumour banks of Institut Curie and Institut Gustave Roussy; the study period was 1998–2010. We first selected patients in whom the diagnosis of a neuroblastic tumour was made either before birth by antenatal ultrasonography or within the first month of life (perinatal tumour) (Table 1). We also included patients harbouring a multifocal disease; the multifocal status of the disease consisted in (i) significant enlargement and/or tumour mass of both adrenal glands, (ii) synchronous tumours emerging from at least two distant sites of the autonomous nervous system, apart from the adrenal gland, and (iii) metachronous neuroblastic tumours occurring at different sites (Table 2).
Table 1. Results of ALK mutation screening in sporadic perinatal and multifocal postnatal cases.
| Localized | MS | M | ||||||
|---|---|---|---|---|---|---|---|---|
| Multi. | Uni. | Multi. | Uni. | Multi. | Uni. | Unknown | Total | |
| Perinatal | ||||||||
| Tum. | 1/4 | 1/15 | 0/6 | 0/4 | 0/0 | 1/2 | 0/5 | 2/36 |
| Const. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| >1 month | ||||||||
| Tum. | 0/3 | 2/3 | 0/0 | 2/6 | ||||
| Const. | 0 | 2 | 0 | 2 | ||||
| Total of const. mutations | ||||||||
| 1/7 | 0/15 | 2/9 | 0/4 | 0/0 | 0/2 | 0/5 | 3/42* | |
| 1/22 | 2/13 | 0/2 | 0/5 | |||||
Abbreviations: MS, Pepper syndrome; M, metastatic; Multi., multifocal; Uni., unifocal; Tum., mutation in the tumour; Const., germline mutation.
*Total of germline mutations only.
Table 2. Summary of clinical data for multifocal cases.
| Patient no. | Stage | Location | Histology | Occurrence | PHOX2B | ALK |
|---|---|---|---|---|---|---|
| Neonatal case | ||||||
| mNB1 | L | Adre x2+thor | NB+GNB | Syn | wt | wt |
| mNB2 | L | Adre x2+ cerv+thor | NB+NB+NB | Meta | wt | R1275Q* |
| mNB3 | L | Adre x2 | NB+NB | Meta | wt | wt |
| mNB4 | L | Adre x2+sub/c | NB + GN | Meta | wt | wt |
| mNB5 | MS | Adre x2 | NB, nos | Syn | wt | wt |
| mNB6 | MS | Adre x2 | NB, nos | Syn | wt | wt |
| mNB7 | MS | Adre x2 | NB, nos | Syn | wt | wt |
| mNB8 | MS | Adre x2 | NB, nos | Syn | wt | wt |
| mNB9 | MS | Adre x2 | NB, nos | Syn | wt | wt |
| mNB10 | MS | Adre x2 | NB, nos | Syn | wt | wt |
| Postnatal cases | ||||||
| mNB11 | L | Adre+thor | NB+NB | Syn | wt | wt |
| mNB12 | L | Adre+cerv | NB+GNB | Syn | wt | wt |
| mNB13 | L | Adre x2 | NB+GN | Syn | wt | wt |
| mNB14 | MS | Adre+sub/c | NB+GN | Meta | wt | R1192P* |
| mNB15 | MS | Pelv+sub/c | NB+GN | Meta | 676insG | wt |
| mNB16 | MS | Adrex2+retrop | NB+NB | Syn | wt | T1151R* |
Abbreviations: mNB, multifocal neuroblastoma; MS, Pepper syndrome; L, localized; Adre, adrenal gland; thor, thoracic; cerv, cervical; pelv, Pelvic; retrop, retroperitoneal; sub/c, subcutaneous; NB, neuroblastoma; GNB, ganglioneuroblastoma; GN, ganglioneuroma; nos, Not otherwise specified (no biopsy); Syn, synchronous; Meta, metachronous; wt, wild type.
*Mutation found in the germline.
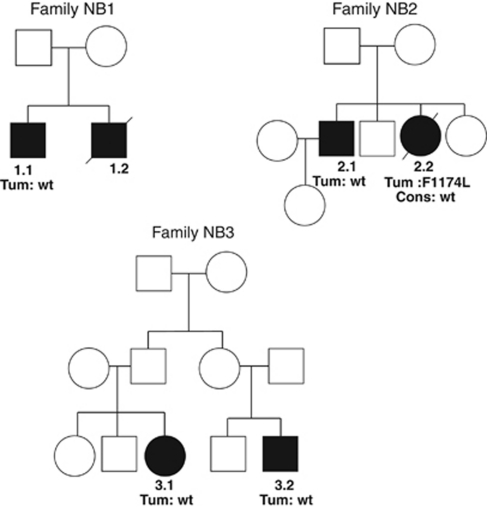
Familial cases were also explored. Three new French familial cases were identified in which siblings (families 1 and 2) or first cousins (family 3) were affected by neuroblastic tumours (Figure 3). In families 1 and 2, interestingly, the thoracic neuroblastic tumours of the two elder boys were fortuitously diagnosed on a chest X-ray performed for a febrile cough.
Finally, we included patients who developed multiple malignancies including one neuroblastic tumour (Table 3).
Table 3. Clinical and genetic features of patients with multiple neoplasia including a neuroblastic tumour.
| First tumour | Second tumour | Genetic results |
|---|---|---|
| Unilateral retinoblastoma, 16 months | Adrenal ganglioneuroblastoma, 9 years | ALK, RB1 wt |
| Unilateral nephroblastoma, 9 months | Adrenal neuroblastoma, 9 months | No 11p15 LOI, ALK, CDKN1C wt |
| Neuroblastoma, 3 years | Rhabdoid tumour, 22 years | ALK wt, SMARCB1 wt |
| Abdominal NB, 5 years | Desmoid tumour, 27 years | ALK wt |
| Melanoma, 22 years | Adrenal neuroblastoma, 30 years | ALK, CDK4, CDKN2A wt |
| Neuroblastoma, 9 months | Nerve sheath tumour, 28 years | ALK wt |
| Neuroblastoma, 8 years | Liposarcoma, 28 years | ALK wt |
| Abdominal neuroblastoma, 3 years | Medulloblastoma, 3 years | ALK wt |
Abbreviations: RB, retinoblastoma; wt, wild type; LOI, loss of imprinting.
Tumour and constitutional DNA analyses
We first assessed the status of ALK mutations in tumour DNAs. Mutation screening in the tumours were made according to the French Huriet law regarding the biological research on human tissue samples.
A consultation with a geneticist was proposed in case of a familial recurrence, multifocal disease and multiple malignancies; it was also proposed when a mutation in the ALK gene was observed in the tumour DNA. Informed consent was obtained from the parents or legal tutor of the affected children.
Tumour cell content was assessed by cryosection on frozen samples, to help in the interpretation of a minor allele in case of abundant stroma.
Direct sequencings of the 29 exons of ALK and the 3 exons of PHOX2B were performed using the classical Sanger procedures. Primers sequences and PCR conditions have been published elsewhere and are available on request.18, 27 Mutations were a priori considered deleterious when leading to obvious impairment of the protein (premature stop codon and frameshift) or when affecting the functional domains of the protein (tyrosine kinase of ALK, and homeodomain or polyalanine stretch of PHOX2B) on highly conserved residues (unique amino-acid substitution).
Familial analyses
Germline analyses of the relatives were investigated upon the parents' request and with the appropriate information to the siblings. Informed consents of the individuals or their legal tutors were obtained for all germline analyses. In case of a germline mutation in asymptomatic relatives of the index case, a chest X-ray and an abdominal ultrasonography were performed to evidence a silent benign neuroblastic tumour.
Array CGH on tumour DNA
Three tumours were analysed on a homemade BAC arrays. Subsequent analyses were performed on NimbleGen 4 × 72K arrays. Genomic DNA extracted with Phase Lock Gel Light (Eppendorf, Le Pecq, France) and was analysed versus normal reference DNA. In all, 700 ng of each sample was labelled and co-hybridized to the NimbleGen 4 × 72k arrays according to the manufacturer's protocol (Roche NimbelGen, Madison, WI, USA). Arrays were washed and then scanned on a GenePix 4000B scanner using GenePix 6.0 Software (Axon, Sunnyvale, CA, USA). Raw data were normalized using NimbleScan v2.5 Software (Roche NimbleGen) and then processed using NimbleScan Software using the segMNT algorithm to obtain data with a resolution of 100 pb. The data were then visualized with SignalMap v1.9 (Roche NimbelGen) before analysis. Normalized data are available on http://microarrays.curie.fr/.
Results
Perinatal (<1 month) cases
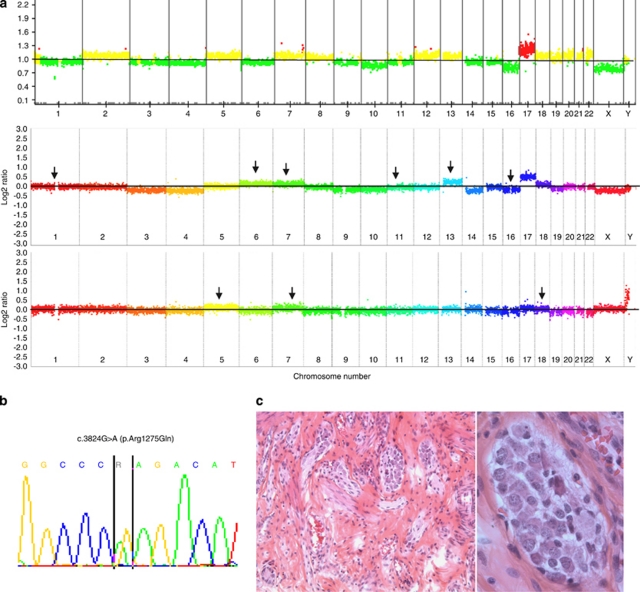
Thirty-six perinatal cases were analysed. Among those, ten newborns showed a multifocal disease, involving both adrenal glands in seven cases. None of the seven bilateral adrenal neuroblastomas showed a mutation. Conversely, a c.3824G>A (p.Arg1275Gln, R1275Q) mutation was found both in the tumours and in the germline of a child affected by multiple adrenal and extra-adrenal neuroblastomas (mNB2) (Figure 1b). This initially trifocal tumour (cervical, mediastinal, and adrenal) was revealed by dyspnoea at 2 weeks of age. After successful initial treatment, the child developed a controlateral adrenal tumour and another fifth abdominal retroperitoneal tumour at 9 months of age. Array CGH performed on three different tumours showed clearly distinct profiles (Figure 1a). Interestingly in this patient, the Meckel diverticulitis has been ressected for acute painful inflammation; an unexpected hyperplasia of myenteric plexus was noticed (Figure 1c), a phenomenon that could eventually be somewhat related to the sympathetic tumours given the neural crest origin of the myenteric plexus, despite the neurons harboured a normal morphology. The child is doing well without disease with a 10-year follow-up.
Figure 1.
Clinical and genetic features observed in neonatal case (mNB2) with a c.3824G>A (p.Arg1275Gln) mutation. (a) Array CGH on cervical (BAC array), adrenal and retrohepatic tumours (NimbleGen 4 × 72K) from top to bottom. On BAC arrays, yellow indicates normal copy number, red indicates a chromosomal gain, and green a chromosomal loss. On Nimblegen arrays, colours indicate each a different chromosome. Arrows show the chromosomal copy number statuses that differ between each of the two bottom arrays and the upper one. (b) Nucleotide sequence of exon 25 showing the c.3824G>A (p.Arg1275Gln). (c) Haematoxylin Eosin Safran (HES) on Meckel diverticle showing hyperplasia of the myenteric plexus (left panel), without neoplastic feature (right panel).
Apart from those 10 multifocal cases, 26 unifocal perinatal cases were also analysed (Table 1). Two other mutations were found in those latter cases: a p.Pro238del in frame deletion and a c.3824G>A (p.Arg1275Gln, R1275Q) mutation. None of these two variants were confirmed in the germline.
No PHOX2B mutation was found in any of those perinatal cases.
Multifocal postnatal (>1 month) cases
The main features of all multifocal cases are reported in Table 2. Among the six postnatal patients with multifocal tumours, ALK mutations were found in two tumours. For one patient, a c.3452C>G (p.Thr1151Arg, T1151R) was also present in the germline (mNB16). The girl developed a stage MS neuroblastoma; one adrenal gland was obviously enlarged, the second showed multiple calcifications compatible with tumour involvement and another bulky mass was seen in the paravertebral thoracic region. After initial treatment, the tumour relapsed with bone metastasis, leading to intensification of the treatment and autologous haematopoietic stem cells infusion. She is alive without disease with a 5-year follow-up.
The second patient had an adrenal stage M neuroblastoma diagnosed at 6 months, and developed multiple paravertebral and subcutaneous ganglioneuromas, arising from nervous ganglia and fibers at cervical, thoracic, and pelvic levels, in successive waves up to 6 years of age (mNB14). A c.3575G>C (p.Arg1192Pro, R1192P) ALK mutation was evidenced in all tumours tested and in the lymphocytes, thus confirming the germline predisposing context.
No ALK mutation was found in the other multifocal cases. However, in one patient with a pelvic stage M neuroblastoma, and multiple metachronous subcutaneous, para-uterine and intestinal ganglioneuromas (patient mNB15), the PHOX2B sequencing revealed a 676insG mutation. The variation was found in ganglioneuromas and neuroblastoma, but not in the lymphocytes, compatible with a mosaicism. Other tumours did not show any PHOX2B mutation.
Familial cases
Three familial tumours were analysed from three different pedigrees (Figure 2). One ALK c.3522C>A (p.Phe1174Leu, F1174L) was observed (patient F2.2) in the tumour DNA. This variant was not found at the germline level. No mutation in ALK was seen in the other patients. No mutation was found either in the three exons of PHOX2B.
Figure 2.
Pedigrees of familial neuroblastic tumours without neither PHOX2B nor ALK mutation. Tum, mutation found in the tumour DNA; Cons, status of ALK gene in the germline; wt, wild type.
Multiple cancers
Eight patients were tested because they have developed multiple cancers, including one neuroblastic tumour (Table 3). No mutation was found in ALK sequence, neither in PHOX2B.
ALK mutations in the relatives of germline mutation carriers with neuroblastoma
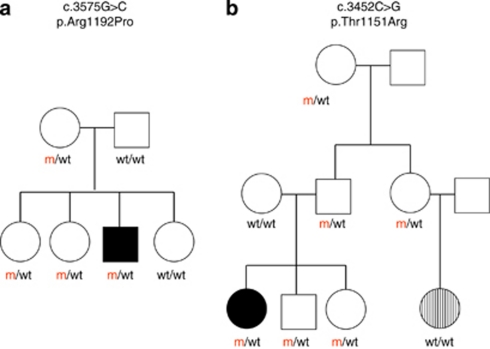
While the R1275Q was proven to be de novo, the R1192P and T1151R variants were inherited from asymptomatic parents and shared by three and five asymptomatic relatives, respectively (Figure 3). In particular, for the asymptomatic siblings of index cases with a germline mutation, ultrasonography and chest X-rays revealed no tumour so far.
Figure 3.
Pedigrees of index cases with inherited germline ALK mutations (mNB14 and mNB16): full dark indicates the children with neuroblastoma; dash indicates retinoblastoma that has been linked in this girl to a de novo constitutional RB1 mutation. m, mutated; wt, wild type.
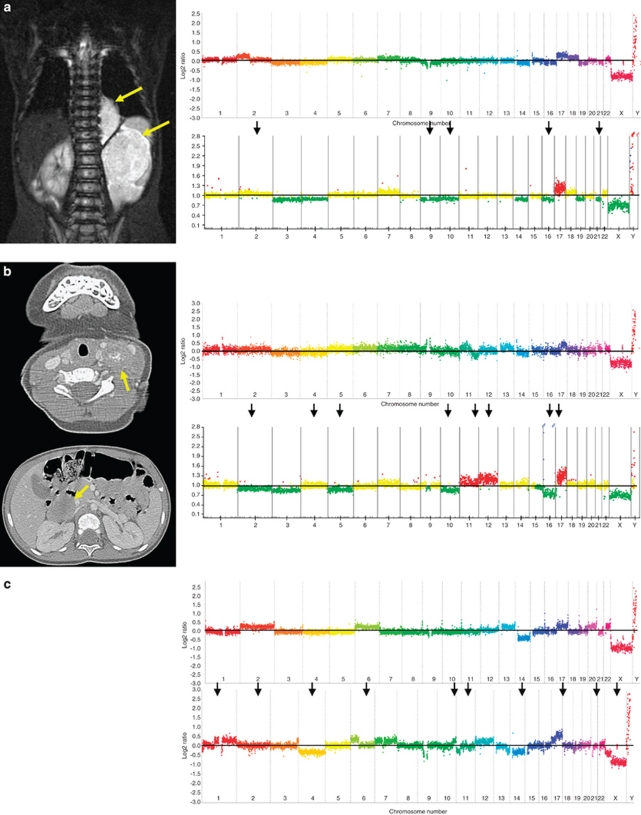
Array CGH on multifocal cases
To look for potential common chromosomal alterations indicating a putative predisposition locus, array CGH was performed on tumour DNA from distinct sites in three multifocal cases showing no germline ALK nor PHOX2B mutation (cases mNB3, mNB11, and mNB12; Figure 4). The different tumours arising in the same patient showed common gains and deletions but, more interestingly, also specific alterations, suggesting two independent evolutions. The coexistence of several neuroblastic tumours with different genomic background convincingly demonstrated the predisposition context for these patients. However, no remarkable alterations could straightforward orientate to any particular predisposition locus.
Figure 4.
Multifocal neuroblastomas without neither ALK nor PHOX2B mutations. (a) Frontal view on whole body MRI (patient mNB11) showing a thoracic paravertebral tumour and a large adrenal gland tumour. The two patterns obtained by array CGH showed some common alterations and several differences (arrows). On BAC arrays, yellow indicates a normal copy number; red indicates a chromosomal gain, and green a chromosomal loss. (b) Cervical and abdominal tumours in patient mNB12; the two array CGH show clearly distinct genotypes (arrows). (c) Array CGH from the right (upper panel) and left (lower panel) adrenal neuroblastomas of the patient mNB3 showing an asynchronous bilateral tumour.
Discussion
To delineate the proportion of those tumour mutations that are also present in the germline is of importance in clinical practice for proper genetic counselling; hence, finding relevant criteria to identify the tumours that arise in a predisposing context would be highly helpful. In the present study, we expected to identify the patients with apparently sporadic tumours who are the most prone to benefit from a germline analysis; with this aim, we specifically studied children showing multifocal neuroblastomas, early occurrence of the tumour or multiple malignancies. Our study concludes that this strategy does not dramatically increase the discovery rate of germline ALK or PHOX2B mutations.
Strikingly, previous results from non-familial neuroblastomas have shown a weak incidence of germline mutations in apparently sporadic cases: out of 51 patients showing an ALK mutation in the tumour that was also tested in the germline, 4 only have indeed an ALK-related predisposition.18, 19, 21, 23 These preliminary results suggested that no more than 8% of patients with a mutation in the tumour might have a predisposing context. In a recent large meta-analysis, the frequency of somatic ALK mutations was estimated to be 6.9% out of 709 neuroblastic tumours. Altogether, and although never properly assessed in a systematic screening, this suggests a very weak frequency for ALK germline mutations among all patients with sporadic neuroblastoma. Hence, the low incidence of ALK germline mutation that we have found in sporadic tumours in this study (3/42) is still to be interpreted in the light of the ratio extrapolated from published data. However, as previously observed for PHOX2B, we assume that ALK germline mutation might account for a very limited number of apparently sporadic cases.
In detail, only 1/36 perinatal cases showed an ALK germline mutation. In our cases of ALK germline mutation, neuroblastoma was diagnosed at 2 weeks, 3 months and 6 months of age, compared with 0.6, 1.222 and 8.219 years in the previously reported cases for which the age is available. Altogether, these observations suggest that young age at onset and perinatal tumour development in particular, is not a major relevant criterion to indicate a higher risk for ALK-related predisposition.
Moreover, this mutated neonatal case could be clearly distinguished from other perinatal patients because of multiple metachronous neuroblastomas. Considering that 3/16 multifocal apparently sporadic cases in our series show an ALK germline mutation, this particular clinical presentation might be considered as the most strongly suggestive of an ALK-related predisposition in absence of familial history. However, from a clinical point of view, making an indubitable difference between metastasis and multiple primary tumours is most often challenging. Although not categorical criterion (i) localization of the tumours strictly restricted to sympathetic structures with no localization in skin, liver, bone, or bone marrow which are the usual sites of metastases, (ii) metachronous development of the tumours and, when available, (iii) clear differences between the tumours' genomic profiles might help to specifically select the actual multifocal cases. The coexistence in the same patient of tumours with various histological types may also be taken into consideration.
Multiple malignancies also suggest a predisposition. Significantly high levels of ALK expression in nervous tissues (retina, retinoblastomas, or medulloblastomas, for example Janoueix-Lerosey et al18) made this gene a good candidate particularly in patients with multiple nervous tumours. Furthermore, very recent data support the involvement of ALK point mutations in non-neuroblastic cancers.28 Conversely, no patient with various nervous tumours showed ALK mutation and, reciprocally, none of our patients with germline mutation showed non-neuroblastic tumours. Hence, our results still suggest that the tumour spectrum of ALK predisposing syndrome is tightly restricted to neuroblastic tumours.
Finally, the absence of ALK mutations in three new pedigrees again emphasizes that other genes are most likely involved in the predisposition to neuroblastoma. Likewise, Mosse et al19 did not evidence any ALK mutation in several kindred, strongly suggesting at least one other predisposing gene. Interestingly, the somatically acquired ALK mutation in one familial case (Familial NB 2.2) indicates a probable synergistic effect between this potential unknown predisposing mutation and the secondary ALK activation.
Another interesting conclusion of our work is the relatively low penetrance of ALK mutations. Indeed, in 2/3 apparently sporadic multifocal cases, the germline mutation is shared by three and five unaffected members of the family, respectively. A precise penetrance of ALK mutation is hard to figure out, since many apparently unaffected carriers might in fact have had spontaneously regressive tumours, or asymptomatic stroma-rich tumours, as observed in two of the familial cases reported here (see Materials and Methods). Moreover, the penetrance is likely to be different from one specific mutation to another, although all occur within the kinase domain. Indeed, published data regarding affected and unaffected carriers suggest a 50% penetrance for R1275Q and R1192P ALK variants, the two main familial mutations.17, 18 However, in our present series, the R1192P variant was associated with a much lower penetrance and an apparently sporadic presentation. Point mutations affecting the T1151 residue has also been reported in one sporadic case,22 and our present case again suggest a weak penetrance for this mutation. Modifier genes might also partly explain the differences within families or within individuals in a same family. They might also influence the severity of the tumour phenotype, from one single neuroblastoma to multiple diffuse tumours. Recently described polymorphisms associated with a higher risk of neuroblastoma, such as BARD1 variants or copy number variations at 1q21, might have such a role. Since the resolution of our array did not allow to analyse these loci, specific investigations with SNP arrays might further document this hypothesis. Finally, it is striking that, although constituting 35% of somatic alterations,22 F1174 variants are again not observed in our germline analysis. This again illustrates that mutations that most strongly activate the tyrosine kinase domain might not be observed in patients without severe malformative phenotype.29
Altogether, our results show that germline ALK mutations are rarely involved in sporadic neuroblastomas. Thus, systematic screening may not be proposed in non-familial cases. Nevertheless, among sporadic presentations, patients with multifocal tumours might be the best candidates for germline screening. Whenever a variant is observed in the tumour and then confirmed in the germline, germline screening, and radiological assessment (chest X-ray and ultrasonography) may be proposed to parents and siblings, given the low penetrance and frequent asymptomatic stroma-rich tumours. For asymptomatic carriers, we are currently proposing abdominal ultrasonography and urinary catecholamines dosage every 3 months, plus chest X-ray every 6 months up to 6 years of age. However, the presumable benefit of presymptomatic screening for young relatives of a mutation carrier still warrants further prospective survey.
Acknowledgments
This work was supported by grants from the Institut National du Cancer (INCa) and Direction de l′Hospitalisation et de l′Organisation des Soins (DHOS), the Ligue Nationale contre le Cancer (Equipe labellise'e and CIT project), the APAESIC (Association des Parents et des Amis des Enfants Soignés à l'Institut Curie), the Association Hubert Gouin, les amis de Claire, Les Bagouz à Manon, la Hulotte and Enfance et Sante. We thank all the clinicians and pathologists from the French Society of Cancer in children (SFCE) who refer their tumours and clinical data. We thank Dr Virginie Verkarre for the picture of the myenteric plexus hyperplasia.
The authors declare no conflict of interest.
References
- Li FP, Fraumeni JF., Jr Rhabdomyosarcoma in children: epidemiologic study and identification of a familial cancer syndrome. J Natl Cancer Inst. 1969;43:1365–1373. [PubMed] [Google Scholar]
- Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Niemitz EL, McNeil DE, Brandenburg SA, Lee MP, Feinberg AP. Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith-Wiedemann syndrome with cancer and birth defects. Am J Hum Genet. 2002;70:604–611. doi: 10.1086/338934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RH, Douglas J, Baskcomb L, et al. Constitutional 11p15 abnormalities, including heritable imprinting center mutations, cause nonsyndromic Wilms tumor. Nat Genet. 2008;40:1329–1334. doi: 10.1038/ng.243. [DOI] [PubMed] [Google Scholar]
- Lim DH, Maher ER. Genomic imprinting syndromes and cancer. Adv Genet. 2010;70:145–175. doi: 10.1016/B978-0-12-380866-0.60006-X. [DOI] [PubMed] [Google Scholar]
- Brichard B, Heusterspreute M, De Potter P, et al. Unilateral retinoblastoma, lack of familial history and older age does not exclude germline RB1 gene mutation. Eur J Cancer. 2006;42:65–72. doi: 10.1016/j.ejca.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Garre ML, Cama A, Bagnasco F, et al. Medulloblastoma variants: age-dependent occurrence and relation to Gorlin syndrome--a new clinical perspective. Clin Cancer Res. 2009;15:2463–2471. doi: 10.1158/1078-0432.CCR-08-2023. [DOI] [PubMed] [Google Scholar]
- Knudson AG, Jr, Strong LC. Mutation and cancer: neuroblastoma and pheochromocytoma. Am J Hum Genet. 1972;24:514–532. [PMC free article] [PubMed] [Google Scholar]
- Maris JM, Kyemba SM, Rebbeck TR, et al. Molecular genetic analysis of familial neuroblastoma. Eur J Cancer. 1997;33:1923–1928. doi: 10.1016/s0959-8049(97)00265-7. [DOI] [PubMed] [Google Scholar]
- Maris JM, Kyemba SM, Rebbeck TR, et al. Familial predisposition to neuroblastoma does not map to chromosome band 1p36. Cancer Res. 1996;56:3421–3425. [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, et al. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Trochet D, Bourdeaut F, Janoueix-Lerosey I, et al. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. Am J Hum Genet. 2004;74:761–764. doi: 10.1086/383253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosse YP, Laudenslager M, Khazi D, et al. Germline PHOX2B mutation in hereditary neuroblastoma. Am J Hum Genet. 2004;75:727–730. doi: 10.1086/424530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis EM, Zhou L, Rand CM, Weese-Mayer DE. Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype. Am J Respir Crit Care Med. 2006;174:1139–1144. doi: 10.1164/rccm.200602-305OC. [DOI] [PubMed] [Google Scholar]
- McConville C, Reid S, Baskcomb L, Douglas J, Rahman N. PHOX2B analysis in non-syndromic neuroblastoma cases shows novel mutations and genotype-phenotype associations. Am J Med Genet A. 2006;140:1297–1301. doi: 10.1002/ajmg.a.31278. [DOI] [PubMed] [Google Scholar]
- Raabe EH, Laudenslager M, Winter C, et al. Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene. 2008;27:469–476. doi: 10.1038/sj.onc.1210659. [DOI] [PubMed] [Google Scholar]
- van Limpt V, Schramm A, van Lakeman A, et al. The Phox2B homeobox gene is mutated in sporadic neuroblastomas. Oncogene. 2004;23:9280–9288. doi: 10.1038/sj.onc.1208157. [DOI] [PubMed] [Google Scholar]
- Janoueix-Lerosey I, Lequin D, Brugieres L, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- Mosse YP, Laudenslager M, Longo L, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caren H, Abel F, Kogner P, Martinsson T. High incidence of DNA mutations and gene amplifications of the ALK gene in advanced sporadic neuroblastoma tumours. Biochem J. 2008;416:153–159. doi: 10.1042/bj20081834. [DOI] [PubMed] [Google Scholar]
- Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- De Brouwer S, De Preter K, Kumps C, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res. 2010;16:4353–4362. doi: 10.1158/1078-0432.CCR-09-2660. [DOI] [PubMed] [Google Scholar]
- George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshkow JE, Haller JO, Berdon WE, Sane SM. Hirschsprung's disease, Ondine's curse, and neuroblastoma--manifestations of neurocristopathy. Pediatr Radiol. 1988;19:45–49. doi: 10.1007/BF02388410. [DOI] [PubMed] [Google Scholar]
- Kushner BH, Gilbert F, Helson L. Familial neuroblastoma. Case reports, literature review, and etiologic considerations. Cancer. 1986;57:1887–1893. doi: 10.1002/1097-0142(19860501)57:9<1887::aid-cncr2820570931>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Abbaszadeh F, Barker KT, McConville C, Scott RH, Rahman N. A new familial cancer syndrome including predisposition to Wilms tumor and neuroblastoma. Fam Cancer. 2010;9:425–430. doi: 10.1007/s10689-009-9319-8. [DOI] [PubMed] [Google Scholar]
- Matera I, Bachetti T, Puppo F, et al. PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset Central Hypoventilation syndrome. J Med Genet. 2004;41:373–380. doi: 10.1136/jmg.2003.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 2011;71:4403–4411. doi: 10.1158/0008-5472.CAN-10-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pontual L, Kettaneh D, Gordon C, et al. Germline gain-of-function mutations of ALK disrupt central nervous system development. Hum Mutat. 2011;32:272–276. doi: 10.1002/humu.21442. [DOI] [PubMed] [Google Scholar]