Abstract
In mammalian females, one of the two X chromosomes is inactivated in early embryonic life. Females are therefore mosaics for two cell populations, one with the maternal and one with the paternal X as the active X chromosome. A skewed X inactivation is a marked deviation from a 50:50 ratio. In populations of women past 55–60 years of age, an increased degree of skewing (DS) is found. Here the association between age-related skewing and mortality is analyzed in a 13-year follow-up study of 500 women from three cohorts (73–100 years of age at intake). Women with low DS had significantly higher mortality than the majority of women who had a more skewed DS (hazard ratio: 1.30; 95% CI: 1.04–1.64). The association between X inactivation and mortality was replicated in dizygotic twin pairs for which the co-twin with the lowest DS also had a statistically significant tendency to die first in the twin pairs with the highest intra-pair differences in DS (proportion: 0.71; 95% CI: 0.52–0.86). Both results suggest that lower DS is associated with higher mortality. We therefore propose that age-related skewing may be partly due to a population selection with lower mortality among those with higher DS.
Keywords: aging, sex, mortality
Introduction
The biological phenomenon of X-chromosome inactivation is observed in females across a variety of mammalian species. Females have two X chromosomes, of which either the maternal or the paternal X is inactivated in early embryonic life, at about the time of implantation.1 Females are therefore mosaics for two cell populations, one with the maternal and one with the paternal X as the active X chromosome. Early in life the two cell populations are normally distributed around a 50:50 ratio.2, 3 In some females, one of the two cell populations is more abundant than the other and the value of the larger proportion is called the degree of skewing (DS), with DS≥50. DS=50 is therefore a complete random X inactivation and DS=100 is a completely skewed X inactivation. X inactivation is believed to occur randomly for each cell and to be permanent for all descendants of the cell. For women younger than 55–60 years of age, DS does not increase significantly with age in populations2, 3 or within the same individual.4 However, DS increases considerably with age in cross-sectional studies of populations older than 55–60 years and this age association continues to increase in populations at the most advanced ages.2, 3, 5 Most X-inactivation studies have been performed on peripheral blood cells, but a higher frequency of skewed X inactivation in elderly women has also been found in buccal swab cells.6, 7
Most X-inactivation studies use an indirect assay targeted to a methylation site and polymorphic sequence named the HUMARA assay. The representativeness of this indirect method for studying X inactivation has been questioned. Recently, however, skewing of X inactivation in aging women was confirmed by two transcriptional-based assays.8
There are two main hypotheses as to why DS increases with age in cross-sectional studies. One is that DS increases with age within the same individual and the other is that DS is associated with life expectancy in women. Only weak evidence has been published for the first hypothesis, while the latter hypothesis has not been studied previously. In this study, we aim to test the survival hypothesis by investigating whether DS is associated with mortality. We therefore conducted a 13-year follow-up study of women aged 73–100 years to investigate the relationship between DS and mortality registered in a nationwide database register. In addition, the study was replicated by the analysis of a subset of the sample using the statistically powerful twin intra-pair analysis, which also controls for genetic and familial environmental factors.
Materials and methods
Study population
The subjects were female participants of three population-based nationwide surveys conducted at the University of Southern Denmark: The Longitudinal Study of Aging Danish Twins (LSADT), The Danish 1905 Cohort Study and the Danish Centenarian Study.
LSADT is a longitudinal study of Danish twins aged 70 years and older. The study was initiated in 1995, and the survey was repeated in 1997, 1999, 2001, 2003 and 2005. The subjects in this study were participants from the 1997 survey, in which full blood samples were taken, and included 78 monozygotic (MZ) and 92 dizygotic (DZ) twin pairs.
The Danish 1905 Cohort Study is a prospective investigation of an entire birth cohort. The survey was initiated in 1998, when the participants were 92–93 years old and comprised of follow-up studies in 2000, 2003 and 2005. Of the 3600 individuals still alive at intake, 118 women provided full blood samples in 1998 and were included in this study. Each survey of the LSADT and the 1905 Cohort Study comprises multidimensional face-to-face interviews focusing on health and lifestyle issues, as well as objective assessments of cognitive and physical abilities.9, 10, 11
The Danish Centenarian Study is a nationwide epidemiological survey of all persons living in Denmark who celebrated their 100th birthday during a 14-month period from 1995 to 1996. A year and a half later, 21 participants were still alive and were revisited. These were included in this study. All three studies were approved by the Danish Scientific–Ethical Committees.
X-chromosome inactivation
DNA was extracted from peripheral blood using standard procedures.
The X-chromosome inactivation pattern has been determined previously by a PCR analysis of a polymorphic CAG repeat and a methylation-sensitive site in the androgen receptor gene.3, 5, 12 The DS was defined as a number between 50 and >95, where 50 indicates a random X-inactivation pattern and >95 an extremely skewed pattern, where most or all cells had either the maternal or the paternal X as the active X chromosome. The most common thresholds of X inactivation are a DS above 75, 80 or 90. However, using a predefined grouping of a quantitative measure forces the outcome a priori to be similar in the groups, despite what the data supports. Hence, we grouped DS into quartiles, which is a more flexible way to analyze the association between X inactivation and mortality having no previous assumption of the association.
Survival
Each participant was followed from the baseline interview date until 31 December 2009, in the Danish Civil Registration system, which registers date of death.13 Hence, there was no loss to follow-up as none of the participants emigrated.
Statistical analyses
Cox's proportional hazards models were performed to study the association between DS and mortality. A linear model was applied with DS as a continuous variable, and in order to take into account potential nonlinearity, DS was divided into quartiles. Since the mortality was similar in the second, third and fourth quartile, we also analyzed the association between mortality and DS divided into the first quartile and the others. Those in the first lower quartile (50≤DS≤63) are referred to as low DS and those in the upper three quartiles (DS>64) as skewed X inactivation. In all models, age was the underlying timescale and risk was assessed from the age at blood sampling to age at death or end of follow-up at the end of 2009, whatever came first, thereby adjusting for age.14 Alternative models also adjusted for physical and cognitive functioning measures, which are strong predictors of survival among elderly and the oldest-old.15
As the data partly pertain to twin pairs, and because observation within twin pairs might be correlated, the Cox's proportional hazards models were performed using the robust estimator of variance, assuming independence between pairs.16 The proportional hazard assumption underlying the Cox models was tested using the Schoenfeld residual test and fulfilled the requirements for the Cox model.
Intra-pair analyses were performed by calculating the proportion of times the co-twin who had the lowest DS died first. This proportion was compared to the null hypothesis of equality (50/50) by using the standard binomial test. We analyzed trends with a conditional logistic regression to study the association between increasing intra-pair DS and survival, and tested for interaction terms between twin pairs age, zygosity and intra-pair DS. Statistical analyses were carried out using Stata 10.1 (STATA Corp., College Station, TX, USA).
Results
The LSADT twins, the 1905 Cohort members and the centenarians comprised together 500 female participants. After the follow-up period of 13 years, 223 of 361 (62%) of the twins and all except two of the 118 (98%) from the 1905 Cohort Study were deceased. After 6 years of follow-up, all 21 centenarians were deceased. In total, 72% of the 500 participants died during follow-up (Table 1).
Table 1. Descriptive characteristics of age, mortality and X inactivation.
| LSADT | 1905 cohort | Centenarians | Combined | |
|---|---|---|---|---|
| Number of individuals | 361 | 118 | 21 | 500 |
| Age | ||||
| Median (range) | 77.6 (73.2–94.3) | 92.9 (92.2–93.8) | 101.5 | 83.3 (73.2–101.5) |
| X inactivation | ||||
| Extremely skewed no. ind. (%)a | 38 (10.5) | 12 (10.1) | 5 (23.8) | 55 (11.0) |
| Skewed no. ind. (%)b | 131 (36.3) | 54 (45.7) | 13 (61.8) | 198 (39.6) |
| Mean (SD) | 73.6 (13.9) | 75.6 (14.4) | 82.1 (12.5) | 74.4 (14.1) |
| Median | 72 | 78 | 84 | 74 |
| Number of deaths (%) | 223 (61.8) | 116 (98.3) | 21 (100.0) | 360 (72.0) |
| Mortality rate per 100 person-year | 7.0 | 24.9 | 59.6 | 9.8 |
Individuals for whom more than 95% or more of the blood cells had either the maternal or the paternal X as the active X-chromosome.
Individuals for whom more than 80% or more of the blood cells had either the maternal or the paternal X as the active X-chromosome.
The hazard ratio of DS estimated as a linear effect showed tendencies to lower hazard ratio by approximately 4% per 10 DS (hazard ratio: 0.96; 95% CI: 0.89–1.02). However, as Table 2 shows, women with low DS (1 quartile) had a higher hazard compared to women in the other quartiles, which indicate that the association is properly not linear in the entire DS range. Women with low DS had a significantly higher hazard compared with the majority of women in the three top quartiles who had a more skewed X inactivation (hazard ratio: 1.30; 95% CI: 1.04–1.64), that is, women with low DS had a 30% higher mortality risk during the 13-year follow-up period. The hazard ratios were similar and the analysis of low DS versus a more skewed X inactivation was still statistically significant after adjustment for cognitive and physical abilities.
Table 2. Mortality (HR) and degree of skewing of X inactivation in all 500 female participants, all above 73 years of age.
| Degree of skewing | Min.–Max. value | No. ind. | Adjusted for age | Adjusted for age, cognitive and physical abilities | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| First quartile | 50–63 | 131 | 1 (ref.) | — | — | 1 (ref.) | — | — |
| Second quartile | 64–74 | 121 | 0.67 | 0.50–0.92 | 0.012 | 0.67 | 0.48–0.93 | 0.016 |
| Third quartile | 75–87 | 128 | 0.87 | 0.66–1.13 | 0.29 | 0.81 | 0.63–1.05 | 0.11 |
| Fourth quartile | 88 to >95 | 120 | 0.77 | 0.58–1.02 | 0.065 | 0.74 | 0.56–0.98 | 0.036 |
| Low DS | 50–63 | 131 | 1.30 | 1.04–1.64 | 0.020 | 1.35 | 1.08–1.69 | 0.009 |
| Skewed X inactivation | 64 to >95 | 369 | 1 (ref.) | — | — | 1(ref.) | — | — |
Abbreviation: HR, hazard ratio.
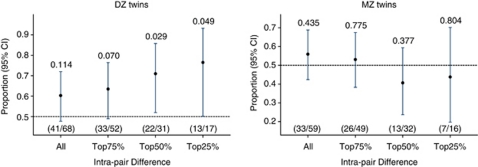
Of the 170 twin pairs, where both co-twins participated, 10 pairs were discarded from further analysis because their DS was identical. For 127 of the remaining 160 pairs, one or both of the twins had died, and of these 59 were MZ and 68 were DZ twin pairs. Figure 1 shows that for DZ twins with a difference in DS of more than 13 (top 50%), the twin with the lowest DS died first in 71% of the pairs (proportion: 0.71; 95% CI: 0.52–0.86). Regression analysis showed that increase in intra-pair difference of DS was associated with increased mortality risk (P=0.019). When all 68 DZ twin pairs were included, the twin with the lowest DS died first in 60% of the pairs (proportion: 0.60; 95% CI: 0.48–0.72). For MZ twins, there was no evidence that it was more common that the twin with the lowest DS died first (proportion: 0.56; 95% CI: 0.42–0.69) and in agreement regression analysis showed significant interaction between zygosity and DS (P=0.038), suggesting that genetic factors could be the basis for the DS–mortality association seen in twins.17
Figure 1.
Proportion of twin pairs aged 73–94 years, where the twin with the lowest DS died first during a 13-year follow-up period. The figure shows the proportion of twin pairs, where the twin with the lowest DS died first according to the magnitude of the intra-pair difference. The proportions are also displayed as fractions within the parenthesis. Each line indicates the 95% CI of the proportions. Above each line is the P-value from the binomial test, which tests if the proportion equals to 0.5.
Discussion
The life expectancy for both women and men has increased in developed countries over the past two centuries. Twin studies have shown that genetic factors account for approximately 25% of the variance in human lifespan. The genetic influences on lifespan are minimal before the age of 60 years, but increase thereafter and appear to be biggest at the most advanced ages.18, 19 Presently, the ɛ4 allele of the APOE gene is the only known common variant with a substantial effect on survival at advanced age,20 whereas others, such as the FOXO3A gene, have a modest effect.21 Telomere length is also associated with both age-related changes and mortality, but is not a strong predictor for the remaining lifespan.22 These genetic and epigenetic factors are mostly identical for women and men.22, 23 Uniquely for women, life expectancies could be as a result of the mosaicism caused by X inactivation, as they have a choice between two cell populations.24
In this study, a 13-year follow-up study of 73–101-year-old participants in the three cohorts showed that mortality was higher for females with a low DS (50≤DS≤63) compared to females with a more skewed pattern (DS>63). As our analyses were carried out without an a priori hypothesis of association with mortality, and our analysis did not include correction for multiple testing, it cannot be ruled out that our finding is caused by a Type 1 error. However, the evidence was strengthened by the association between mortality and lower DS in an intra-pair analysis using the most discordant DZ twin pairs, although the association was not found in MZ twin pairs. Previous twin studies have shown that DS intra-class correlation is significantly higher for MZ twins than for DZ twins, indicating a genetic effect on DS, and X-inactivation pattern has a heritability of 0.63 in young and 0.58 in elderly twins.3 This heritability indicates that the age-related skewing of X inactivation could be determined by a selection for or against cells with a particular X chromosome as the active X chromosome.
There are many clinical examples of selective advantages in X-linked disorders. In the X-linked adrenal leukodystrophy, there is a selective advantage of the cell carrying the mutated gene on the active X chromosome.25 In most cases, the cell carrying the wild-type gene on the active X has a selective advantage, leading to a totally skewed X inactivation in the carrier, for example, as reported for Wiskott–Aldrich syndrome.26 However, in these examples selection probably starts very early in life. A different situation is found in some X-linked anemias, where the age-related skewing leads to the manifestation of the disease in elderly female carriers.27, 28, 29
These selection processes in X-linked disorders do not explain why the frequency of the age-related skewed X inactivation has been shown to be higher in females >55–60 years of age in several cross-sectional studies.2, 3, 5 Likewise, these processes do not explain why females with low DS have higher mortality compared to those with a more skewed pattern. It is possible that one or more genes affect both the DS and longevity, for example, because association between DS and mortality was marked in intra-pair analyses of DZ twin compared to MZ twins. In our earlier cross-sectional study, only 7% of women 19–65 years of age had DS above 80 compared with 67% of centenarians.5 This substantial difference in the proportion of women with high DS cannot be explained by the 30% higher mortality risk observed in the current follow-up study for women with DS in the lowest quartile, because mortality is not associated with increased DS beyond the lowest quartile; that is, there is no mortality difference between second, third and fourth quartile (Table 2). However, we cannot exclude that our study does not have the power to detect such an association. The age-related increase is likely to be caused mainly by individual increases in DS with age. However, the reduced number of elderly with low DS (lowest quartile) could at least partly be due to population selection by mortality. Few studies of longitudinal measurements of X inactivation in the same females over time have been reported. X-inactivation pattern analyzed in peripheral granulocytes, monocytes and T cells in 35 blood donors aged 20–75 years was not significantly different in repeated measures of samples drawn 18 months later.30 The authors concluded that no fluctuation of X-inactivation pattern occurred in these blood cells in this time period. Similarly, no differences were found between most of the samples taken about two decades apart from 178 females aged 5–75 years. Only in the few samples where the age at the time of the second sampling was more than 60 years, a tendency to a more skewed X inactivation in the second sample was found.4 Thus, there is little evidence that skewing of X inactivation in individuals with age is a common phenomenon, at least before the age of 60 years. Individual changes of skewing of X inactivation over time at more advanced ages thus remains to be confirmed in longitudinal studies.
Our results should be replicated; however, we propose that age-related skewed X inactivation may be partly due to population selection with lower mortality among those with higher DS. Since this selection scenario is unique for women, it would be most relevant to study skewed X inactivation and its relation to common age-related diseases. Little is known about X inactivation and common diseases, except for breast cancer, which has been widely studied, but with inconsistent results.31, 32, 33, 34, 35
Acknowledgments
The study was supported by US National Institute on Aging Research Grant NIA-PO1-AG08761. The Danish Aging Research Center is supported by a grant from the VELUX Foundation and from The National Program for Research Infrastructure 2007 from the Danish Agency for Science Technology and Innovation.
Author contributions
KC initiated this study and JMF primarily wrote this paper, and MT contributed with statistical analysis and discussions. Other co-authors contributed with discussions and writing.
The authors declare no conflict of interest.
References
- van den Berg IM, Laven JS, Stevens M, et al. X chromosome inactivation is initiated in human preimplantation embryos. Am J Hum Genet. 2009;84:771–779. doi: 10.1016/j.ajhg.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busque L, Mio R, Mattioli J, et al. Nonrandom inactivation patterns in normal females: lyonization ratios vary with age. Blood. 1996;88:59–65. [PubMed] [Google Scholar]
- Kristiansen M, Knudsen GP, Bathum L, et al. Twin study of genetic and aging effects on X chromosome inactivation. Eur J Hum Genet. 2005;13:599–606. doi: 10.1038/sj.ejhg.5201398. [DOI] [PubMed] [Google Scholar]
- Sandovici I, Naumova AK, Leppert M, Linares Y, Sapienza C. A longitudinal study of X-inactivation ratio in human females. Hum Genet. 2004;115:387–392. doi: 10.1007/s00439-004-1177-8. [DOI] [PubMed] [Google Scholar]
- Christensen K, Kristiansen M, Hagen-Larsen H, et al. X-linked genetic factors regulate hematopoietic stem-cell kinetics in females. Blood. 2000;95:2449–2451. [PubMed] [Google Scholar]
- Knudsen GP, Pedersen J, Klingenberg O, Lygren I, Ørstavik KH. Increased skewing of X chromosome inactivation with age in both blood and buccal cells. Cytogenet Genome Res. 2007;116:24–28. doi: 10.1159/000097414. [DOI] [PubMed] [Google Scholar]
- Bolduc V, Chagnon P, Provost S, et al. No evidence that skewing of X chromosome inactivation patterns is transmitted to offspring in humans. J Clin Invest. 2008;118:333–341. doi: 10.1172/JCI33166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busque L, Paquette Y, Provost S, et al. Skewing of X-inactivation ratios in blood cells of aging women is confirmed by independent methodologies. Blood. 2009;113:3472–3474. doi: 10.1182/blood-2008-12-195677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Christensen K. Social activity and healthy aging: a study of aging Danish twins. Twin Res Hum Genet. 2007;10:255–265. doi: 10.1375/twin.10.2.255. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Gaist D, Petersen HC, et al. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet Epidemiol. 2002;23:110–122. doi: 10.1002/gepi.1127. [DOI] [PubMed] [Google Scholar]
- Christensen K, Frederiksen H, Vaupel JW, McGue M. Age trajectories of genetic variance in physical functioning: a longitudinal study of Danish twins aged 70 years and older. Behav Genet. 2003;33:125–136. doi: 10.1023/a:1022501817781. [DOI] [PubMed] [Google Scholar]
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- Nybo H, Petersen HC, Gaist D, et al. Predictors of mortality in 2,249 nonagenarians – the Danish 1905 Cohort Survey. J Am Geriatr Soc. 2003;51:1365–1373. doi: 10.1046/j.1532-5415.2003.51453.x. [DOI] [PubMed] [Google Scholar]
- Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- McGue M, Osler M, Christensen K. Perspectives on causal inference an observational research: the utility of twins. Perspect Psychol Sci. 2010;5:546. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- vB Hjelmborg J, Iachine I, Skytthe A, et al. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet. 2006;7:436–448. doi: 10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliebe A, Kleindorp R, Blanché H, et al. No or only population-specific effect of PON1 on human longevity: a comprehensive meta-analysis. Ageing Res Rev. 2010;9:238–244. doi: 10.1016/j.arr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford A, Dorling D, Davey Smith G, Shaw M. Life expectancy: women now on top everywhere. BMJ. 2006;332:808. doi: 10.1136/bmj.332.7545.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008;20:91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon BR, Moser HW, Moser AB, Axelman J, Sillence D, Norum RA. Adrenoleukodystrophy: evidence for X linkage, inactivation, and selection favoring the mutant allele in heterozygous cells. Proc Natl Acad Sci USA. 1981;78:5066–5070. doi: 10.1073/pnas.78.8.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon ER, Kohn DB, Winkelstein JA, Vogelstein B, Blaese RM. Carrier detection in the Wiskott–Aldrich syndrome. Blood. 1988;72:1735–1739. [PubMed] [Google Scholar]
- Cazzola M, May A, Bergamaschi G, Cerani P, Rosti V, Bishop DF. Familial-skewed X-chromosome inactivation as a predisposing factor for late-onset X-linked sideroblastic anemia in carrier females. Blood. 2000;96:4363–4365. [PubMed] [Google Scholar]
- Au WY, Ma ES, Lam VM, Chan JL, Pang A, Kwong YL. Glucose 6-phosphate dehydrogenase (G6PD) deficiency in elderly Chinese women heterozygous for G6PD variants. Am J Med Genet A. 2004;129A:208–211. doi: 10.1002/ajmg.a.30213. [DOI] [PubMed] [Google Scholar]
- Au WY, Pang A, Lam KK, et al. G6PD deficiency from lyonization after hematopoietic stem cell transplantation from female heterozygous donors. Bone Marrow Transplant. 2007;40:677–681. doi: 10.1038/sj.bmt.1705796. [DOI] [PubMed] [Google Scholar]
- van Dijk JP, Heuver L, Stevens-Linders E, et al. Acquired skewing of lyonization remains stable for a prolonged period in healthy blood donors. Leukemia. 2002;16:362–367. doi: 10.1038/sj.leu.2402379. [DOI] [PubMed] [Google Scholar]
- Buller RE, Sood AK, Lallas T, Buekers T, Skilling JS. Association between nonrandom X-chromosome inactivation and BRCA1 mutation in germline DNA of patients with ovarian cancer. J Natl Cancer Inst. 1999;91:339–346. doi: 10.1093/jnci/91.4.339. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Langerød A, Knudsen GP, Weber BL, Børresen-Dale AL, Orstavik KH. High frequency of skewed X inactivation in young breast cancer patients. J Med Genet. 2002;39:30–33. doi: 10.1136/jmg.39.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen M, Knudsen GP, Maguire P, et al. High incidence of skewed X chromosome inactivation in young patients with familial non-BRCA1/BRCA2 breast cancer. J Med Genet. 2005;42:877–880. doi: 10.1136/jmg.2005.032433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struewing JP, Pineda MA, Sherman ME, et al. Skewed X chromosome inactivation and early-onset breast cancer. J Med Genet. 2006;43:48–53. doi: 10.1136/jmg.2005.033134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lose F, Duffy DL, Kay GF, et al. Skewed X chromosome inactivation and breast and ovarian cancer status: evidence for X-linked modifiers of BRCA1. J Natl Cancer Inst. 2008;100:1519–1529. doi: 10.1093/jnci/djn345. [DOI] [PubMed] [Google Scholar]