Abstract
Disorders of sex development (DSD) are congenital conditions where chromosomal, gonad or genital development is atypical. In a significant proportion of 46,XY DSD cases it is not possible to identify a causative mutation, making genetic counseling difficult and potentially hindering optimal treatment. Here, we describe the analysis of a 46,XY DSD patient that presented at birth with ambiguous genitalia. Histological analysis of the surgically removed gonads showed bilateral undifferentiated gonadal tissue and immature testis, both containing malignant germ cells. We screened genomic DNA from this patient for deletions and duplications using an Illumina whole-genome SNP microarray. This analysis revealed a heterozygous deletion within the WWOX gene on chromosome 16, removing exons 6–8. Analysis of parental DNA showed that the deletion was inherited from the mother. cDNA analysis confirmed that the deletion maintained the reading frame, with exon 5 being spliced directly onto exon 9. This deletion is the first description of a germline rearrangement affecting the coding sequence of WWOX in humans. Previously described Wwox knockout mouse models showed gonadal abnormalities, supporting a role for WWOX in human gonad development.
Keywords: disorders of sex development, copy number, WWOX, gonad, microarrays
Introduction
In the early stages of human embryogenesis the developing gonads are bipotent, being capable of forming either testes or ovaries. In males the expression of the Y chromosomal SRY gene initiates testis development, whereas ovarian development in principle occurs only in the absence of SRY (reviewed in Wilhelm et al1). Following the establishment of sex-specific expression of key regulatory genes in the gonad, gonadal differentiation results in development of the external genitalia. As a result there are two main stages in gonad formation, and disruption of either can lead to disorders of sex development (DSD). DSD are surprisingly common, with ambiguous genitalia estimated to occur with an incidence of 1 in 4500 live births.2
A number of genes important in the regulation of sex determination have been identified, yet in as many as 70% of 46,XY DSD cases no genetic cause has been identified. We have previously demonstrated the power of whole-genome copy number analysis with high-density microarrays to identify causative mutations in DSD.3, 4 Here, we describe the use of this approach to identify a multi-exon heterozygous deletion in the WWOX gene of a 46,XY DSD patient.
Materials and methods
Array hybridization and analysis
Genomic DNA was hybridized onto an Illumina 610-Quad microarray at the Australian Genome Research Facility (Melbourne, Australia) following the manufacturer's instructions. Data were analyzed using Genome Studio data analysis software (Illumina).
Multiplex ligation-dependent probe amplification (MLPA) analysis
Deletion screening of the WWOX gene was performed with MLPA. Probe design, the MLPA reaction and data analysis were performed as described previously.5
RNA extraction, cDNA generation and breakpoint PCR
RNA was extracted from lymphocytes obtained from the index case using standard procedures, with cDNA generated using random hexamers and the Transcriptor High Fidelity cDNA synthesis kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. The PCR amplification across the deletion used the following primers; F-5′-CGAAACCGCCAAGTCTTTT-3′, R-5′-CGTCTCTTCGCTCTGAGCTT-3′, and was run under the following conditions:
1 cycle: 60 s 95°C; 35 cycles: 30 s 95°C; 30 s 58°C; 60 s 72°C; 1 cycle: 20 min 72°C.
DNA sequencing
Sanger sequencing was conducted at the Department of Pathology, University of Melbourne, Australia.
Histological and immunohistochemical analysis
Research on human tissue samples was performed according to the Code for Proper Secondary Use of Human Tissue in the Netherlands, as developed by the Dutch Federation of Medical Scientific Societies (FMWV) version 2002 and approved by an Institutional Review Board (MEC 02.981). Immunohistochemical detection of formalin-fixed paraffin-embedded tissue was performed for SOX9 and FOXL2,6 OCT3/47 and KITL,8 as described previously.
Results
Physical examination of the index patient at the age of 10 days revealed unfused labioscrotal folds, impalpable gonads, clitoral hypertrophy 20 mm in size and a perineal urogenital sinus. Genitography demonstrated the presence of a vagina and underdeveloped uterus. Chromosomal analysis showed a 46,XY karyotype with no visible aberrations. Sequence analysis of the SRY and NR5A1 genes did not reveal any variants, and MLPA analysis with a commercially available kit (MLPA P185-B1) containing probes targeted at the WNT4, SRY, NR0B1, SOX9 and NR5A1 genes did not show any deletions or duplications.
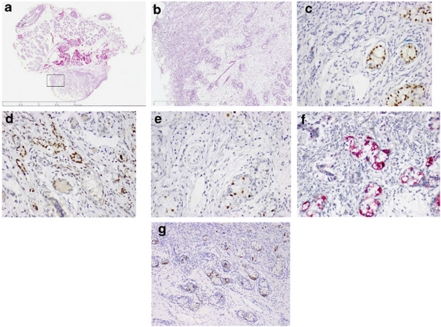
Small dysgenic gonads were present in the abdomen, and pathological analysis following complete removal at 2 years of age showed that they contained edematous infantile testicular parenchyma (Figure 1). The epididymis was completely separated from the rete testis on both sides, and tubular epithelium was identified at the left side. The left gonad consisted of centrally located edematous testicular tissue, positive for immunohistochemical detection of SOX9 (indicative for Sertoli cells) and negative for FOXL2 (a granulosa cell marker). A gradual transition toward undifferentiated gonadal tissue, containing both SOX9 and FOXL2-positive cells at the upper and lower poles were identified. Undifferentiated gonadal tissue is a gonadal pattern found specifically in patients with gonadal dysgenesis and is typically characterized by the combined expression of FOXL2 and SOX9 (usually with a preponderance of FOXL2), suggesting limited differentiation of the supportive cell lineage into pre-granulosa and pre-Sertoli cells9. Immature OCT3/4-positive germ cells were also found, either dispersed in ovarian-like stroma or organized together with Sertoli/granulosa cells in cord-like structures, reminiscent of sex cords. These structures have been recognized as the precursor lesion for gonadoblastoma10.
Figure 1.
Representative histological and immunohistochemical findings for the left gonad: (a) total overview of the gonad histology (H&E); (b) higher magnification ( × 2.5 magnification, indicated by square in (a); immunohistochemical detection of (c) SOX9, positive in the Sertoli cells; (d) FOXL2, positive in the granulosa cells; (e) OCT3/4; (f) TSPY; (g) KITL, all positive in the transformed germ cells. All immunohistochemical images are at the magnification of × 100, except G, being × 50.
In spite of the presence of ovarian-like stroma, no ovarian follicles were present, and therefore the histology did not allow the diagnosis of ovarian differentiation. The right gonad displayed a similar morphology, with predominant, SOX9-positive testicular differentiation, except for the upper pole, where a large area of FOXL2-positive undifferentiated gonadal tissue was seen, also containing scarce SOX9-positive cells. On the basis of morphological criteria and immunohistochemical analysis for OCT3/4 and TSPY (showing co-expression of both markers), presence of pre-malignant germ cells was diagnosed. The co-expression is assumed to identify the earliest pathogenetic changes in the genesis of malignant germ cell tumors. That the germ cells are indeed transformed was confirmed by staining for KITL. A limited hormonal work-up was carried out in the neonatal period, with normal values for ACTH, cortisol and 17 hydroxy progesterone. At 28 weeks, serum values were obtained for LH and FSH and were 1.0 and 5.2 U/l, respectively. Although FSH levels were not measured before this, the relatively high value at this age suggests a higher value (in the range of gonadal dysgenesis) during full mini-puberty. A HCG test performed at 18 months showed a moderate rise of testosterone (testosterone 231 ng/dl after HCG, 500 U, 2 × /week for 3 weeks).
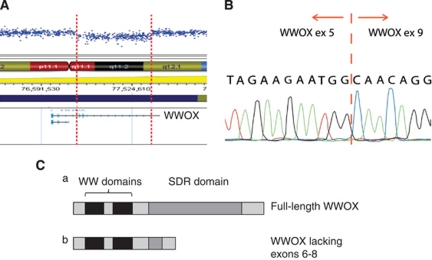
Microarray analysis did not reveal deletions or duplications covering any genes already known to be involved in DSD. The most promising candidate rearrangement was a 767-kb deletion on chromosome 16 (chr16:76956767-77723905; hg18) (Figure 2a). This deletion was located within the WWOX gene, and removed exons 6–8. MLPA probes for each of the nine WWOX exons were designed, and MLPA analysis confirmed that the deletion was restricted to these three exons (data not shown). The deletion is predicted to generate an alternative, in-frame mRNA product, with exon 5 being spliced directly onto exon 9. This alternative transcript was verified by sequencing cDNA derived from matched lymphocytes (Figure 2b).
Figure 2.
Molecular analysis of DNA from the 46,XY DSD patient. (A). The deletion identified by microarray analysis, removing 767 kb of genomic DNA on chromosome 16. Data are plotted along the chromosome, with each point representing the copy number estimate of an individual probe. The breakpoints of the deletion are shown by the broken vertical lines. (B) PCR analysis of the deletion. cDNA was derived from lymphocytes of the index patient, and primers were designed to amplify a PCR product across the predicted breakpoint. Sequence analysis shows that exon 5 is spliced directly onto exon 9. (C) Effect of the deletion on the WWOX protein. The full-length WWOX protein has two WW domains at the N-terminal, and a SDR domain at the C-terminal. A deletion of exons 6–8 is predicted to result in an in-frame but shortened product, with the SDR domain largely missing.
MLPA analysis of parental DNA samples revealed that the deletion was maternally inherited, with no evidence of mosaicism.11 The clinical history of the mother included a relatively late menarche (16 years) with irregular menstruation up to the first pregnancy. Before this pregnancy she had received hormonal stimulation, but this was stopped before conception. Four subsequent pregnancies occurred naturally. DNA analysis of the children (two brothers and two sisters, all unaffected) showed that none had inherited the deletion. Analysis of the WWOX gene in the index case did not show any sequence variants.
Discussion
Here, we report the first germline rearrangement affecting the coding sequence of the WWOX gene. WWOX consists of nine exons, and is >1 Mb in size. It is located on the long arm of chromosome 16 in a known fragile site frequently rearranged in a wide range of cancers,12 and is a suspected tumor suppressor gene.13
WWOX encodes a 414 amino acid, 46 kDa protein.14 At the amino terminus there are two WW domains, with a short chain oxidoreductase (SDR) domain within the central portion of the protein.15 The WW domains are believed to be involved in protein–protein interactions, including a number of transcription factors.16, 17 The SDR region is thought to have a role in steroid metabolism,18 with the exon 6–8 deletion identified in this study maintaining the reading frame but effectively ablating the SDR domain from the predicted protein (Figure 2c).
Two different Wwox knock-out mouse models have show gonadal abnormalities, including defects of Leydig cell function in the testis.18, 19 There are various possible mechanisms for WWOX-mediated testicular dysfunction. WWOX expression inhibits the Wnt/β-catenin pathway in a dose-dependent manner, although this inhibition is reduced if the SDR domain is removed.20 The Wnt/β-catenin pathway is involved in normal ovarian development, and increased Wnt/β-catenin activity in the developing gonad through compromised WWOX activity may interfere with normal testis development. Indeed, expression of a stabilized form of β-catenin in the somatic cells of XY mice leads to male to female sex reversal.21
WWOX expression is high in endocrine tissues such as pituitary, testis and ovary,22 and it may have a role in gonadotrophin or sex-steroid biosynthesis. It has been suggested that a truncated WWOX protein consisting of both WW domains but without a functional SDR domain may act as a competitor of the full-length protein. The truncated WWOX could bind to WWOX-interacting proteins via the WW domains, but would not have any SDR activity.20
Although the mother of the index case carried the same deletion there was a milder gonadal phenotype, with late menarche. An increase in activity of the Wnt/β-catenin pathway due to reduced WWOX activity would not necessarily be expected to have an effect on ovarian development, as this pathway is already active during this process. There may, however, be a disturbed ovarian function due to impaired steroidogenesis, as was observed in one of the mouse models.18
In conclusion, we have identified a multi-exon deletion in WWOX in a 46,XY DSD patient with ambiguous genitalia. This finding is the first germline rearrangement affecting WWOX coding sequence described in humans, and implicates WWOX in normal gonadal development.
Acknowledgments
We thank the patient and family members for contributing to this research. This study was financially supported by the National Health and Medical Research Council of Australia (334314 to PK, VH and AS, 546478 and 491293 to SW) and the Victorian Government's Operational Infrastructure Support Program.
The authors declare no conflict of interest.
References
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Lee PA, Houk CP, Ahmed SF, Hughes IA. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006;118:e488–e500. doi: 10.1542/peds.2006-0738. [DOI] [PubMed] [Google Scholar]
- White SJ, Ohnesorg T, Notini A, et al. Copy number variation in patients with disorders of sex development due to 46,XY gonadal dysgenesis. PLoS One. 2011;6:e17793. doi: 10.1371/journal.pone.0017793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton E, Hughes J, White SJ, et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest. 2011;121:328–341. doi: 10.1172/JCI42580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SJ, Breuning MH, den Dunnen JT. Detecting copy number changes in genomic DNA: MAPH and MLPA. Methods Cell Biol. 2004;75:751–768. doi: 10.1016/s0091-679x(04)75032-3. [DOI] [PubMed] [Google Scholar]
- Hersmus R, Kalfa N, de Leeuw B, et al. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD) J Pathol. 2008;215:31–38. doi: 10.1002/path.2335. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- Stoop H, Honecker F, van de Geijn GJ, et al. Stem cell factor as a novel diagnostic marker for early malignant germ cells. J Pathol. 2008;216:43–54. doi: 10.1002/path.2378. [DOI] [PubMed] [Google Scholar]
- Cools M, Wolffenbuttel KP, Drop SL, Oosterhuis JW, Looijenga LH. Gonadal development and tumor formation at the crossroads of male and female sex determination. Sex Dev. 2011;5:167–180. doi: 10.1159/000329477. [DOI] [PubMed] [Google Scholar]
- Cools M, Drop SL, Wolffenbuttel KP, Oosterhuis JW, Looijenga LH. Germ cell tumors in the intersex gonad: old paths, new directions, moving frontiers. Endocr Rev. 2006;27:468–484. doi: 10.1210/er.2006-0005. [DOI] [PubMed] [Google Scholar]
- Notini AJ, Craig JM, White SJ. Copy number variation and mosaicism. Cytogenet Genome Res. 2008;123:270–277. doi: 10.1159/000184717. [DOI] [PubMed] [Google Scholar]
- Paige AJ, Taylor KJ, Taylor C, et al. WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci USA. 2001;98:11417–11422. doi: 10.1073/pnas.191175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Trapasso F, Hussain S, et al. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci USA. 2007;104:3949–3954. doi: 10.1073/pnas.0609783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3–24.1, a region frequently affected in breast cancer. Cancer Res. 2000;60:2140–2145. [PubMed] [Google Scholar]
- Del Mare S, Salah Z, Aqeilan RI. WWOX: its genomics, partners, and functions. J Cell Biochem. 2009;108:737–745. doi: 10.1002/jcb.22298. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res. 2004;64:8256–8261. doi: 10.1158/0008-5472.CAN-04-2055. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Pekarsky Y, Herrero JJ, et al. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci USA. 2004;101:4401–4406. doi: 10.1073/pnas.0400805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, HaganJP, de Bruin A, et al. Targeted ablation of the WW domain-containing oxidoreductase tumor suppressor leads to impaired steroidogenesis. Endocrinology. 2009;150:1530–1535. doi: 10.1210/en.2008-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludes-Meyers JH, Kil H, Nunez MI, et al. WWOX hypomorphic mice display a higher incidence of B-cell lymphomas and develop testicular atrophy. Genes Chromosomes Cancer. 2007;46:1129–1136. doi: 10.1002/gcc.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouteille N, Driouch K, Hage PE, et al. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene. 2009;28:2569–2580. doi: 10.1038/onc.2009.120. [DOI] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez MI, Ludes-Meyers J, Aldaz CM. WWOX protein expression in normal human tissues. J Mol Histol. 2006;37:115–125. doi: 10.1007/s10735-006-9046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]