Abstract
Steroid hormones are made from cholesterol, primarily derived from lipoproteins that enter cells via receptor-mediated endocytosis. In endo-lysosomes, cholesterol is released from cholesterol esters by lysosomal acid lipase (LAL; disordered in Wolman disease) and exported via Niemann-Pick type C (NPC) proteins (disordered in NPC disease). These diseases are characterized by accumulated cholesterol and cholesterol esters in most cell types. Mechanisms for trans-cytoplasmic cholesterol transport, membrane insertion, and retrieval from membranes are less clear. Cholesterol esters and “free” cholesterol are enzymatically interconverted in lipid droplets. Cholesterol transport to the cholesterol-poor outer mitochondrial membrane (OMM) appears to involve cholesterol transport proteins. Cytochrome P450scc (CYP11A1) then initiates steroidogenesis by converting cholesterol to pregnenolone on the inner mitochondrial membrane (IMM). Acute steroidogenic responses are regulated by cholesterol delivery from OMM to IMM, triggered by the steroidogenic acute regulatory protein (StAR). Chronic steroidogenic capacity is determined by CYP11A1 gene transcription. StAR mutations cause congenital lipoid adrenal hyperplasia, with absent steroidogenesis, potentially lethal salt loss, and 46,XY sex reversal. StAR mutations initially destroy most, but not all steroidogenesis; low levels of StAR-independent steroidogenesis are lost later due to cellular damage, explaining the clinical findings. Rare P450scc mutations cause a similar syndrome. This review addresses these early steps in steroid biosynthesis.
Keywords: cytochrome P450, mitochondria, Niemann-Pick disease, Wolman disease, lipoid adrenal hyperplasia, steroidogenesis, steroidogenic acute regulatory protein
Steroidogenesis is the complex multienzyme process by which cholesterol is converted to biologically active steroid hormones. The enzymology of steroidogenesis from cholesterol has been reviewed in detail recently (1). However, steroidogenesis may also be conceptualized as beginning with the cellular importation of cholesterol and entailing its subsequent journey to the mitochondria, where the first enzymatic conversion (to pregnenolone) occurs. This review considers these “upstream events” in steroidogenesis; much has been learned since this area was reviewed by Jefcoate et al. 20 years ago (2). Steroidogenesis is largely confined to the adrenal cortex, testicular Leydig cells, ovarian granulosa and theca cells, and placental syncytiotrophoblast cells. However, most work elucidating these “upstream events” prior to cholesterol's arrival at the mitochondria have been studied in nonsteroidogenic systems (e.g., liver and macrophages), so that the applicability of these data to steroidogenic cells is somewhat inferential. Thus, we emphasize principles that are known or thought to be germane to steroidogenesis and have not attempted to review all aspects of cholesterol trafficking.
CHOLESTEROL IN MEMBRANES
Cellular bilayer membranes are mostly composed of phospholipids arranged with their hydrophilic head groups facing the aqueous medium and their fatty acyl tails forming a hydrophobic membrane core. The principal membrane phospholipids are the glycerol lipids cardiolipin, phosphotidylcholine (PC), phosphotidylethanoloamine (PE), and phosphatidylserine (PS), and a sphingolipid, sphingomyelin. PC is the major plasma membrane lipid in most cells, with the other lipids varying substantially among different cell types (3, 4). Cholesterol (ergosterol in fungi) is an essential and tightly regulated constituent of membranes. The cellular pathways of cholesterol biosynthesis and the human disorders that result from mutations in this pathway have been elegantly reviewed by Porter and Herman (5), and they are not addressed here. Cholesterol is essentially insoluble in water, having a critical micellar concentration of ∼25-40 μM (6); hence, intracellular cholesterol is mainly found in membranes, with smaller amounts bound to proteins. The amount of cholesterol in human fibroblasts remains constant during months in culture (7), and the several pathways that regulate cell cholesterol have been studied extensively (8–10). How cells determine the amount of cholesterol they need and how they sense transient changes in the amount of cellular cholesterol is beginning to be understood. Plasma membrane sterols form complexes with phospholipids in the polar bilayer, mostly phospholipids, with diverse affinities. The polar lipids bind the cholesterol in the plasma membrane; cholesterol intercalated between the phospholipid molecules of the lipid membrane maintains membrane fluidity. Cholesterol in excess of the complexing capacity of the membrane lipids is the “active” cholesterol, which can then move to intracellular membranes, restoring plasma membrane cholesterol to its resting level (11). This cholesterol is sometimes referred to as “free” cholesterol; it is not “free” in the sense that it is in solution but only in the sense that it is accessible and moveable. The pool of “active” or “free” cholesterol can be distinguished kinetically, but not chemically, from the cholesterol tightly bound to the membrane (11). This “active” cholesterol is the substrate for steroidogenesis.
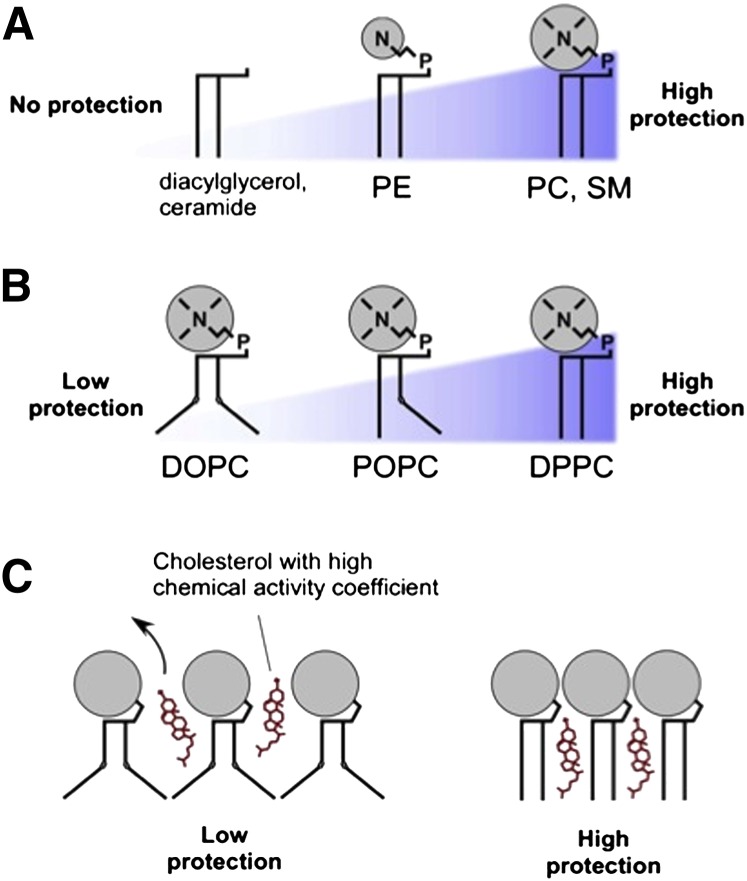
The cholesterol-binding capacity of a membrane, as well as the ability of cholesterol to enter and exit the membrane, depends on the chemical nature of the membrane phospholipids. Studies with model systems in vitro indicate that the head group of the phospholipid constitutes an “umbrella” that protects the cholesterol in the membrane; the size of this polar head group determines the size of the umbrella and the degree of protection (12) ( Fig. 1 ). Phospholipids with large head groups, such as PC or sphingomyelin, are predicted to protect the buried membrane cholesterol, whereas phospholipids with small head groups, such as PE, provide less protection of membrane cholesterol from cytoplasmic cholesterol-binding proteins, and diacylglycerol or ceramide provide no protection (13). The number of double bonds (degree of unsaturation) of the phospholipid's acyl chain will affect cholesterol-binding capacity (14). The saturated acyl chains of dipalmitoyl phosphatidylcholine are essentially straight, permitting them to associate closely, leaving little room for cholesterol to be intercalated between these phospholipid molecules. By contrast, unsaturated bonds, such as in dioleoyl phosphatidylcholine, result in bent acyl chains, providing room for intercalated cholesterol. The cholesterol binds with its 3β-hydoxyl group oriented toward the external hydrophilic region of the membrane with its hydrophobic side chain pointed toward the hydrophobic membrane core. In many eukaryotes, the aminoglycerophospholipids PS, PE, and PC comprise 75–80% of the total glycerophospholipids found within the cell (4). As mitochondria have the capacity to synthesize the entire PE pool required for cell growth (15), the flux of PS into the mitochondria and its subsequent decarboxylation and export as PE can account for the biosynthesis of the majority of glycrophospholipids present in all cell membranes.
Fig. 1.
Interactions between cholesterol and membrane lipids, as inferred from model systems in vitro. (A) Lipids that have a large polar head group (N) protect the space between the phospholipids from water. Diacyl glycerol and ceramide lack head groups and, hence, provide no protection; as the size of the head group increases to phosphatidyl ethanolamine (PE) and to phosphatidyl choline (PC) or sphingomyelin (SM), the “umbrella” of protection increases. (B) Bent acyl chains decrease protection. The level of acyl chain saturation alters the shape of the phospholipid; acyl chains containing carbon-carbon double bonds (e.g., dioleoyl phosphatidylcholine, DOPC) create a “bend” in the acyl chain, causing it to take up more space than phospholipids with saturated chains (e.g., palmitoyl-oleoyl-phosphatidylcholine, POPC, or dipalmitoyl phosphatidyl choline, DPPC), affording more exposure to water. (C) Poorly protected cholesterol (e.g., cholesterol in a DOPC-rich bilayer) can readily leave the membrane, so that it has high chemical activity, whereas well-protected cholesterol (e.g., in a membrane rich in phosphatidyl choline) is less readily available. Reproduced from Ref. 13 with permission.
INTRACELLULAR DISTRIBUTION OF CHOLESTEROL
Cholesterol is transported within a cell by vesicular and nonvesicular means (16). Membrane cholesterol can facilitate protein-protein interactions and protein trafficking by forming lipid/protein rafts (17–19), which are primarily a means of protein transport (20). “Vesicular transport” typically refers to portions of a donor bilayer membrane budding off to form a vesicle, which then fuses with an acceptor membrane, thereby delivering membrane lipids and membrane-bound cholesterol as well as vesicular cargo; by contrast, “nonvesicular transport” involves cholesterol binding to proteins (21, 22). Cholesterol reduces the mobility of phospholipid acyl chains, increasing membrane rigidity (23). Interaction of phospholipid acyl chains with cholesterol forces neighboring hydrocarbon chains into a more extended conformation, increasing membrane thickness and promoting segregation of vesicles through hydrophobic mismatch (24). Both vesicular and nonvesicular transport helps to maintain the appropriate amount of lipids in an organelle or domain of an organelle (22). Compared with vesicular trafficking, an obvious advantage of nonvesicular trafficking is that it can rapidly move lipids between specific cellular compartments without also having to transfer integral membrane proteins. Cells use a number of mechanisms to decrease cholesterol levels rapidly when they are too high: cells can export cholesterol to external lipoproteins, and they can esterify cholesterol to cholesterol esters, which are then stored in lipid droplets. Nonvesicular transport of cholesterol may provide a process to move cholesterol quickly and efficiently to cholesterol-modifying enzymes (22, 25–27), such as those involved in steroidogenesis.
The lipid and protein composition of membranes differs among cellular organelles, depending on their specific functions. Cholesterol, which represents about 30-40% of total cellular lipid, is unevenly distributed among cellular membranes (26–28). Although various studies differ in the exact numbers, all show that the plasma membrane is highly enriched in cholesterol relative to other cellular membranes (28, 29). The endoplasmic reticulum (ER) contains about 0.5-1% of total cellular cholesterol (30), even though the surface area of the ER is greater than that of the plasma membrane. Many aspects of cholesterol regulation are under tight feedback control and are sensitive to the concentration of cholesterol in the ER. Cholesterol concentrations are higher in the Golgi apparatus and highest in the plasma membrane, which contains about 60-80% of total cellular cholesterol (31). The endocytic recycling compartment, which contains recycled membrane proteins and lipids (32), also contains substantial amounts of cholesterol (33). Cholesterol is more abundant in the plasma membrane than in other cellular membranes (4, 13). The concentrations of sphingolipids and sterols increase along the protein biosynthetic pathway from the ER to the trans-Golgi network (34, 35). Membranes are fluid and, hence, can contribute cholesterol and other membrane constituents by vesicular transport, so that the composition of the two mitochondrial membranes is not fixed (36). Instead, phospholipid transport between the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM) has been proposed to occur at contact sites between the two membranes (37, 38). Experiments have identified a mutant CHO cell with a lesion in phosphatidylserine transport between the OMM and IMM (39). Several mechanisms have been found for the transport of phospholipids (and possibly cholesterol) from specialized fractions of the ER that are in close proximity to the mitochondria, termed mitochondrial-associated membranes (MAM) (40). Therefore, the lipid composition of mitochondria is variable.
STEROL REGULATORY ELEMENT BINDING PROTEINS
The amounts of fatty acids and cholesterol present in intracellular membranes are under constant surveillance and are coordinated with lipid biosynthesis and import. De novo biosynthesis is coordinated by sterol regulatory-element binding proteins (SREBPs), a family of basic helix-loop-helix leucine zipper transcription factors that regulate key genes for lipid metabolism and adipocyte differentiation (41). There are three isoforms of mammalian SREBP: SREBP-1a and SREBP-1c are encoded by a single gene utilizing different promoters and alternative splicing, whereas SREBP-2 is encoded by a different gene (42). SREBP-1c targets genes that are involved in fatty acid metabolism (43–45). SREBP-2 is responsible for cholesterol-related genes, such as 3-hydroxy 3-methylglutaryl coenzyme A (HMG-CoA) reductase, which catalyzes the rate-limiting step in cholesterol biosynthesis, and the low-density lipoprotein-receptor (LDLR), which imports circulating cholesterol as LDL particles (29). SREBP-1a targets both sets of genes (45). SREBP-1a and SREBP-2 are the predominant isoforms of SREBP in most cultured cell lines, whereas SREBP-1c and SREBP-2 predominate in the liver and most steroidogenic tissues. Large amounts of SREBP-1a and SREBP-1c are also found in mouse adrenal gland and adipose tissues and in human liver and adrenal gland (44).
SREBPs have a large C-terminal domain containing two closely spaced membrane-spanning helices that line up in the ER membrane in a hairpin configuration. This juxtaposes the N-terminal domain containing the transcriptional activation and DNA-binding domains with the C-terminal regulatory domain on the cytoplasmic side of the membrane (46). The C-terminal domain of SREBPs from yeast to humans interacts with the SREBP cleavage activating protein (SCAP). When intracellular sterol levels are low, SCAP escorts SREBP from the ER to the Golgi via vesicles containing the coat protein II complex (COPII), where SREBP is cleaved sequentially in a two-step proteolytic process (47). The “mature” or “nuclear” N-terminal portion of SREBP migrates to the nucleus where it functions as a transcription factor, binding to sterol-regulatory elements in the promoters of target genes, thus ultimately increasing sterol accumulation by both increased synthesis and increased import (10). SCAP is an 8-transmembrane protein that senses cholesterol in the ER. The 245 amino acid loop 1, which projects into the lumen of the ER, contains the cholesterol-binding site of SCAP, and the 78 amino acid loop 6, which projects into the cytosol, interacts with COPII proteins. Recombinant loop 1 protein binds sterols with the same specificity as the whole SCAP membrane domain. Mutation of Tyr 234 to Ala in loop 1 causes cytosolic loop 6 to assume its cholesterol-bound conformation, even in sterol-depleted hamster SRD-13A cells, a mutant that lacks SCAP (48), so that full-length SCAP (Y234A) cannot mediate SREBP processing in transfected cells (48). Thus, cholesterol binding to SCAP loop 1 controls the conformation of loop 6, thereby controlling interactions with COPII and hence the transport of the active fragment of SREBP to the nucleus, determining whether cells produce cholesterol (49).
Metabolic factors regulate the SREBP system. Incubating human retinoic pigment epithelial cells with insulin or constitutively activating Akt kinase rapidly induces nuclear accumulation of SREBP-1a and SREBP-1c and the expression of lipogenic genes (50). The mechanism by which insulin activates SREBP-1 is unknown. Insulin and other growth factors increase Akt kinase activity, which activates mTORC1 by directly phosphorylating the tuberous sclerosis complex 2 (TSC 2) and PRAS40 (51). Inhibition of mTOR1 with rapamycin blocks Akt-induced SREBP-1 nuclear localization, the expression of lipogenic genes, and the production of various classes of lipids (50). Uncontrolled signaling caused by mutant proteins involved in PI3K kinase and Ras signaling is commonly observed in tumors and serves as a key signaling mechanism to the activation of SREBP-1 and de novo lipogenesis (45).
The tissue distributions of SREBP1a and SREBP1c differ. SREBP1c is the predominant isoform in vivo, being most abundant in liver and adipose tissue, but it is also found in the adrenal, brain, kidney, muscle, and pancreas (44). SREBP1a is the predominant isoform in cells in vitro and in rapidly proliferating tissues such as spleen, testis, and ileum. The ratio of SREBP1c to SREBP1a gene expression varies in different tissues, with SREBP1c mRNA being 3- and 9-fold higher than SREBP1a in adipose tissue and liver, respectively (44). As the transcriptional activity of SREBP1a is higher than that of SREBP1c, the baseline ratio of SREBP1a and SREBP1c may be particularly important in regulating gene expression. Introns within the SREBP-1 and 2 genes also encode the microRNA miR-33, which has the opposite effect (52, 53); miR-33 reduces the amount of mRNA encoding ABC1 (ATP-binding cassette 1), a transporter in the plasma membrane that regulates cholesterol efflux from cells (54). When cellular cholesterol is depleted, both the transcription of SREBPs and miR-33 rise moderately. SREBPs may be involved in the hypertriglyceridemia of the metabolic syndrome (55), which is characterized by insulin resistance, obesity, hyperinsulinemia, hyperglycemia, fatty liver, and increased plasma triglycerides secondary to elevated very low density lipoproteins (VLDL), and decreased plasma high density lipoproteins (HDL) (56). The hypertriglyceridemia of the metabolic syndrome appears to be caused by the insulin-induced increases in SREBP-1c mRNA and protein.
STEROIDOGENIC CHOLESTEROL: LDL, LYSOSOMAL ACID LIPASE AND WOLMAN DISEASE
Steroidogenic cells can employ four potential sources of cholesterol for steroidogenesis: i) de novo synthesis in the ER; ii) cholesterol stored in lipid droplets as cholesterol esters; iii) uptake of circulating HDL via scavenger receptor B1 (SR-B1); and iv) uptake of LDL via receptor-mediated endocytosis. Although steroidogenic cells can synthesize cholesterol de novo in the ER (5, 57), the high demand for cholesterol in steroidogenic cells requires efficient import of cholesterol found in circulating lipoproteins, primarily derived from dietary sources (58). Thus, steroidogenesis requires coordinated regulation of the cellular uptake, transport, and utilization of cholesterol followed by a series of unique biosynthetic steps. It is likely that these steps are coordinated by the SREBP system, but this has not yet been investigated in detail in steroidogenic systems. Adequate concentrations of LDL will suppress HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis (59). Cholesterol associated with LDL is taken up by LDL receptors on the cell surface, providing the primary source of intracellular sterol for most mammals (60–63). Patients with low LDL cholesterol secondary to congenital abetalipoproteinemia have normal basal cortisol concentrations and mildly impaired cortisol secretion in response to adreno-corticotropic hormone (ACTH) (64), and those treated with high doses of statins have no impairment in cortisol secretion (65), suggesting that endogenous synthesis can suffice (66). Rodents differ from most other mammals in using cholesterol from circulating HDL taken up by SR-B1 as their principal source of cholesterol for steroidogenesis; HDL and SR-B1 appear to play minor roles in human steroidogenesis (67–69) ( Fig. 2 ).
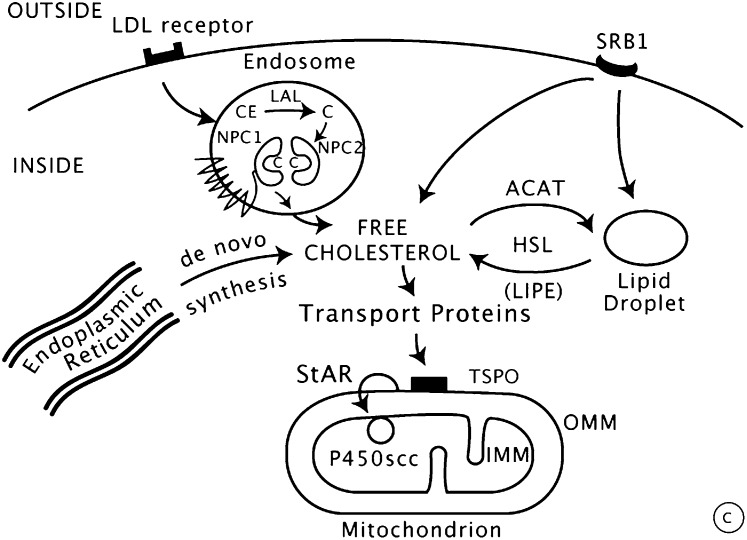
Fig. 2.
Import and transfer of cellular cholesterol. Human steroidogenic cells take up circulating low-density lipoproteins (LDL) by receptor-mediated endocytosis, directing the cholesterol to endosomes; by contrast, rodent cells bind high-density lipoproteins (HDL) via scavenger receptor B1 (SRB1). Cholesterol can also be synthesized de novo in the endoplasmic reticulum. In endo-lysosomes, cholesterol esters are cleaved by lysosomal acid lipase (LAL) to yield free cholesterol, which is then bound by NPC2, transferred to NPC1, and exported. The MLN64/MENTHO system resides in the same endosomes as the NPC system, but their roles in cholesterol trafficking remain uncertain. Cholesterol can be reesterified by acyl-CoA:cholesterol transferase (ACAT) and stored in lipid droplets as cholesterol esters. Free cholesterol may be produced by hormone-sensitive lipase (HSL), encoded by the LIPE gene. Cholesterol may reach the outer mitochondrial membrane (OMM) by vesicular traffic (not shown) or by nonvesicular means by cholesterol-binding transport proteins, such as those with START domains. Movement of cholesterol from the OMM to the inner mitochondrial membrane (IMM) requires an OMM protein complex (represented by the black box), including the ubiquitously expressed TSPO protein. In the adrenals and gonads, the steroidogenic acute regulatory protein (StAR) is responsible for the rapid movement of cholesterol from the OMM to the IMM, where it can be converted to pregnenolone by the cholesterol side-chain cleavage enzyme P450scc. Copyright © W. L. Miller.
LDL cholesterol esters are taken up by receptor-mediated endocytosis, and then stored directly or converted to free cholesterol and used for steroid hormone synthesis (70). LDL particles bind to specific receptors on the plasma membrane and are internalized by endocytosis; the resulting endocytic vesicles then fuse with lysosomes, where the protein portion of the LDL is hydrolyzed to free amino acids, and cholesterol esters are hydrolyzed to free cholesterol by a lysosomal acid lipase (LAL), encoded by the LIPA gene on chromosome 10q23.31. LAL is one of three structurally related acid lipase isozymes (71, 72). Unesterified cholesterol can then be used as substrate for steroidogenesis or be reesterified for storage in lipid droplets by acyl-CoA:cholesterol acyl transferase (ACAT, also known as sterol O-acetyltransferase, or SOAT1), whose mRNA is increased in the presence of surplus cholesterol (73). By contrast with the endocytic pathway from LDL receptors, HDL (and LDL) cholesterol esters that enter the cell via SR-B1 are acted on by hormone-sensitive neutral lipase (HSL) (67), encoded by the LIPE gene on chromosome 19q13.1-q13.2 (74). HSL is widely expressed, especially in adipose tissues, where it is involved in hydrolyzing triglycerides and phospholipids (75). Testicular HSL differs from that of adipose tissue with regard to structure and regulation during development (76, 77). ACAT, HSL, and LAL are thus in an equilibrium, regulating the relative distribution of cholesterol into its free and esterified forms. ACTH acting on the adrenal and luteinizing hormone (LH) in the gonad elaborate intracellular cAMP, stimulating HSL and inhibiting ACAT, thus increasing the availability of free cholesterol for steroid hormone synthesis. ACTH and LH also stimulate the activity of HMG-CoA reductase, LDL receptors, and uptake of LDL cholesterol (78, 79). When intracellular cholesterol concentrations are high, the genes for the LDL receptor, HMG-CoA reductase, and LAL are repressed, whereas ACAT is induced, thereby decreasing cholesterol uptake, synthesis, and deesterification; conversely, when intracellular cholesterol concentrations are low, this process is reversed (80). In addition to HSL, microsomal neutral cholesterol ester hydrolase 1 (Nceh1) may also play a physiologic role in cholesterol deesterification, at least in mice, but a role has not been established for this enzyme in human steroidogenic tissues (81, 82).
Mutations in the LIPA gene cause Wolman disease and its milder variant, cholesterol ester storage disease (83–88). Wolman disease is characterized by accumulated cholesteryl esters and triglycerides in the liver, spleen, lymph nodes, and other tissues, consistent with the broad expression of LAL. In the adrenal, the deficient lysosomal acid lipase activity results in insufficient free cholesterol available for steroidogenesis, causing adrenal insufficiency. Affected infants are normal at birth but develop hepatospenomegaly, vomiting, jaundice, anemia, diarrhea, failure to thrive, developmental delay, and malabsorptive malnutrition, sometimes beginning in the first week of life. Wolman disease is rare, causing about 3% of cases of primary adrenal insufficiency in children (89). The diagnosis can be suggested by a characteristic pattern of subcapsular adrenal calcification that outlines the adrenals, and it is established by finding deficient lysosomal acid lipase activity in leukocytes, cultured fibroblasts, or cultured amniocytes (for prenatal diagnosis). The disease is less severe than congenital lipoid adrenal hyperplasia (discussed in the section Disordered StAR: Congenital Lipoid Adrenal Hyperplasia) with respect to steroidogenesis, but as all cells store and utilize cholesterol, it affects all tissues and is relentless and fatal. Treatment by transplanting bone marrow or other hematopoetic cells appears to ameliorate the course of the disease in about half of cases, but the mechanism of this effect is unclear (90–92). In cholesterol ester storage disease, hypercholesterolemia is common, and accumulated neutral fats and cholesterol esters in the arteries predispose to atherosclerosis. Massive hepatomegaly and hepatic fibrosis may lead to esophageal varices.
In contrast to LAL, no human disease has been associated with HSL deficiency. HSL knockout mice are phenotypically normal except that the males are oligospermic and sterile, but they do not have hypogonadism or adrenal insufficiency. The testes of these mice lack detectable HSL activity and contain increased amounts of cholesterol esters. HSL activities were completely absent from both brown and white adipose tissue, and the adipocytes were substantially enlarged, apparently with accumulated triglycerides (93). However, in contrast to the normal adrenal glucocorticoid levels in the knockout mice, adrenal cell cultures from these mice had a 50% reduction in corticosterone production (94). Thus, HSL participates in, but is not absolutely essential for, the delivery of cholesterol to the steroidogenic machinery in rodents; an essential role is not established in human steroidogenesis.
CHOLESTEROL EFFLUX FROM ENDOSOMES: NPC PROTEINS AND NIEMANN-PICK TYPE C DISEASE
The mechanisms by which cholesterol and/or cholesterol esters enter and exit endosomes and lipid droplets are incompletely understood. Much recent work has concerned four proteins: MLN64 (also known as StarD3), MENTHO (also known as STARD3NL), and the two NPC proteins, so-named because their disruption causes Niemann Pick type C (NPC) disease. An essential role is established only for the NPC proteins.
Niemann-Pick disease type C disease is a rare autosomal recessive neurovisceral disorder of cholesterol trafficking (95, 96), characterized by endosomal accumulation of LDL-cholesterol and glycosphingolipids, leading to progressive neurodegeneration and death and having an incidence of about 1/150,000 (97). NPC disease is distinct from Niemann-Pick disease types A and B, which have similar neurohistopathology but are caused by deficient sphingomyelinase activity. Classical NPC disease manifests in early infancy and results in death within the first decade of life, although the age of onset and clinical course may be variable, so that infantile, juvenile, and adult forms are described clinically. Although the clinical phenotype is highly variable, patients typically appear neurologically normal during infancy but develop ataxia, dementia, vertical supranuclear gaze palsy, loss of speech, and spasticity between ages 2 and 4 years. Seizures, gelastic cataplexy, and psychiatric manifestations can develop. Hepatosplenomegaly, liver dysfunction, and cholestatic jaundice may develop early but resolve. The brain pathology is characterized by accumulation of cholesterol and other lipids, progressive loss of neurons, especially Purkinje cells, and robust glial infiltration. Characteristic foamy Niemann-Pick cells and “sea-blue” histiocytes are found in the bone marrow. Patients with classic NPC disease typically die in their second decade of life, although some patients have a mild disease presenting insidiously as adult neuropsychiatric illness (98). Two cholesterol oxidation products, cholestane-3β,5α,6β-triol and 7-ketocholesterol, accumulate markedly in the blood of patients with NPC disease (99), offering promise of improved diagnosis and a potential tool for following experimental treatments (100).
A spontaneously occurring BALB/c mouse mutant presenting clinical and biochemical features of NPC disease helped to delineate its biochemistry and genetics (101). Cell fusion studies using primary cultures of patients’ skin fibroblasts and a cell line derived from the spontaneously occurring mouse model of NPC identified NPC1 and NPC2 as genetic complementation groups (102). Biochemical studies of this mouse identified early peroxisomal impairment (by 18 days) in the development of its disease (103). The responsible locus in the mouse was localized to chromosome 18 (104), and linkage was then established in the syntenic region of human chromosome 18q11-q12 (105). Based on these data, the human disease-causing NPC1 gene was identified by linkage analysis and complementation studies (106). Three years later, the gene responsible for NPC2 was identified (107). Approximately 95% of NPC patients have mutations in the gene encoding NPC1, and 5% have mutations in the gene for NPC2; these two groups are clinically and biochemically indistinguishable (107).
There are two NPC proteins: NPC1 and NPC2. NPC1, a 170-190 kDa polytopic glycoprotein containing 1,278 amino acids, is found in lysosomal membranes, contains 13 transmembrane domains, and binds both cholesterol and oxysterols (108). NPC2, a soluble 18 kDa glycoprotein containing 132 amino acids, processed from a precursor of 151 amino acids, is found in the lysosomal lumen (107); in vitro, NPC2 binds cholesterol and dehydroergosterol with high affinity (109, 110) and binds fatty acids with lower affinity (110). NPC2 contains a domain similar to the extracellular adaptor protein MD-2, which is similar to that present in various lipid-binding proteins (111). Cholesterol binds to the soluble N-terminal domain of NPC1, which extends into the lysosomal lumen (112). In contrast to NPC2, the N-terminal domain of NPC1 binds both cholesterol and its oxygenated derivatives, 25-OH- and 27-OH-cholesterol. Crystallographic studies show that NPC1 and NPC2 bind cholesterol in opposite orientations. When bound by NPC2, the side-chain of cholesterol is buried in a hydrophobic sterol-binding pocket with the polar 3βOH group exposed to the surface, so that NPC2 may bind cholesterol esters prior to their cleavage by LAL (109, 114). By contrast, when cholesterol is bound by the N-terminal domain of NPC1, the 3βOH group is coordinated by polar residues in the otherwise hydrophobic sterol-binding pocket, and the side chain is partially exposed (115). Consistent with this binding mechanism, NPC1 does not bind sterols with modified 3βOH positions, such as cholesterol sulfate or epicholesterol ( Fig. 3 ).
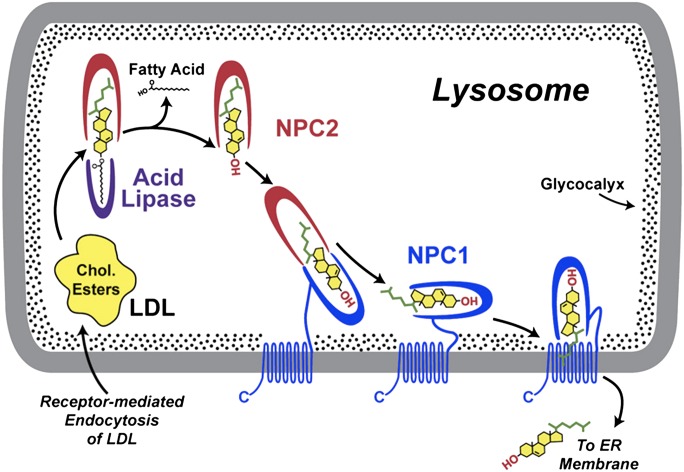
Fig. 3.
Cell biology of NPC proteins in the endo-lysosome. Circulating LDL is taken up by receptor-mediated endocytosis, and the resulting endosomes fuse with lysosomes containing lysosomal acid lipase (LAL) and NPC proteins. Intra-lysosomal NPC2 binds cholesterol with the side-chain buried in the NPC2 protein and the ester attached to the 3β-hydroxyl group extending into the lysosomal matrix. LAL appears to be able to cleave the ester moieties from the cholesterol either before or after binding to NPC2. NPC2 with bound deesterified cholesterol interacts with the C-terminal cholesterol-binding domain of the NPC1 protein, so that the 3β-hydroxyl group is now buried and the cholesterol side-chain is exposed. The precise mechanism by which NPC1 then inserts the cholesterol into the lysosomal membrane and the means by which that cholesterol is taken up by other proteins remain under investigation. Cholesterol exiting the endo-lysosome may reach the ER or other cellular membranes. Reproduced from Ref. 115 with permission.
NPC2 binds cholesterol with micromolar affinity, resulting in rapid bidirectional transfer of cholesterol between lipid bilayers in vitro, particularly in the acidic environment of lysosomes (116). By contrast, NPC1 binds cholesterol with nanomolar affinity (108) and mediates the transfer of cholesterol between liposomes more slowly than NPC2. However, cholesterol movement between NPC1 and liposomes is enhanced >100-fold when NPC2 is present (117). The cholesterol-binding pocket of the NPC1 N-terminal domain contains 18 cysteine residues, all of which participate in disulfide bonds, and the structures of the apoprotein and cholesterol-bound form show little movement of the α-carbon backbone, suggesting that the N-terminal domain of NPC is fairly rigid (115). Transfer of cholesterol from NPC2 to NPC1 requires the movement of two helices in this domain, enlarging the opening to permit cholesterol entry. It appears that the interaction of NPC2 with NPC1 results in a rearrangement of helix 7, helix 8, and the loop between helix 8 and strand 7, thus permitting cholesterol transfer. Alanine scanning mutagenesis of NPC2 identified V81, L175, and L176 as essential for this process (118). Thus, NPC2 and NPC1 appear to act in tandem so that NPC2 delivers cholesterol to NPC1 for subsequent egress from the lysosome or late endosome. The handoff from NPC2 to NPC1 apparently then permits NPC1 to insert cholesterol into the lysosomal membrane with the hydrophobic side-chain going in first, whereas direct membrane insertion from NPC2 would be energetically unfavorable because the 3βOH group would have to go in first.
There is no FDA-approved treatment for NPC disease; however, four proposed approaches to treatment are of interest. The first is N-butyldeoxynojirimycin (NB-DNJ, miglustat), a 219 Da imino sugar that inhibits glucosylceramide synthase (119), which catalyzes the first step in the biosynthesis of glycosphingoloipids; NB-DNJ targets the accumulation of the glycosphingolipids by reducing availability of substrate for their synthesis. NB-DNJ has been approved for use in the European Union and elsewhere for substrate reduction therapy in mild to moderate forms of lysosomal storage diseases in which glycosphingolipids accumulate, but clinical efficacy in NPC disease is modest (120). Second, the neurosteroid allopregnanolone (ALLO), delivered by a single subcutaneous injection at seven days (well before onset of symptoms), increased Purkinje cell survival, reduced accumulation of gangliosides, slowed the progression of neurodegeneration, and doubled life span in Npc1−/− mice (121, 122). The ALLO was delivered via hydroxypropyl-β-cyclodextrin (HPBCD), but treatment with HPBCD alone had no effect in these studies. ALLO appears to exert neuroprotective actions by mechanisms other than a direct effect on lipid trafficking, including activating GABA receptors (121) and pregnane X receptors (123) and by reducing the accumulation of reactive oxygen species (124). Third, it has been reported that HPBCD alone accounts for the salutary effects initially seen with ALLO treatment (125, 126). This model is mechanistically attractive, as cyclodextrins can act as scavengers of cholesterol and gangliosides, and in vitro data indicate that HPBCD provided in cell culture medium reaches the lysosomal storage organelles of human fibroblasts deficient in either NPC1 or NPC2 and lowers intracellular cholesterol accumulation (127). It is difficult to reconcile these two very different views, especially because the Npc1−/− mice appear to behave differently, with dramatically different, untreated survivals in these different laboratories, but it would seem parsimonious to suggest that both a lipid-scavenging action of HPBCD and an antineuroinflamatory action of ALLO contribute to these promising results. Fourth, several inhibitors of histone deacetylases reduce the accumulation of cholesterol in two human fibroblast cell lines carrying mutations in NPC1 but not in a cell line carrying a mutation in NPC2; however, the mechanism of this effect is uncertain (128). Potential impacts on steroidogenesis have not been reported for these experimental treatments.
MLN64 AND MENTHO
Metastatic lymph node, clone 64 (MLN64) is a cholesterol-binding protein that colocalizes with the NPC proteins, but its precise role remains unclear. MLN64 was identified in a screen of clones prepared from the metastatic lymph nodes of women with ductal breast carcinomas (129). The gene for MLN64 is located on chromosome 17q12-21, close to two other genes involved in breast cancer: it is distal to the c-erbB2 gene and proximal to the BRCA1 gene. Whether MLN64 has a role in breast cancer remains unclear. MLN64 and NPC1 colocalize in late endosomes (130), suggesting that MLN64 may function downstream from NPC1 to facilitate cholesterol egress from endosomes, but this remains unproven and further work is needed.
MLN64 contains 445 residues divided into two domains: the N-terminal half contains four apparent transmembrane regions and targets the protein to the membranes of late endosomes (131), and the carboxy-terminal half is structurally related to the steroidogenic acute regulatory protein (StAR, discussed below) (132) ( Fig. 4 ). The N-terminal domain (residues 1-230) with its four transmembrane domains is structurally similar to a subsequently cloned late-endosomal protein called MENTHO. It is termed the MENTAL domain (for MLN64 N-terminal domain homolog) (133). MENTHO and the MENTAL domain of MLN64 share 70% identity and 83% similarity (133). The carboxy-terminal half of MLN64 is structurally related to StAR (134, 135), a mitochondrial protein that regulates the acute production of steroids in the adrenal and gonads in response to tropic hormones (136). This carboxy-terminal domain of MLN64 (residues 231-445) is called the StAR-related lipid transfer (START) domain (133, 137) because it is 35% identical and 54% similar to the putative lipid-binding domain of StAR (138, 139). The MENTAL domains of MENTHO and MLN64 can interact to form homo- and heterodimers and to bind cholesterol, suggesting a role in endosomal cholesterol transport (140). The gene structures of MLN64, MENTHO, and StAR are closely related. Exons 2-7 of MLN64 encoding the MENTAL domain have an identical organization to the corresponding exons encoding the MENTAL domain of MENTHO. Exon 8 of the MLN64 gene encodes a nonconserved linker region joining the MENTAL and START domains. The boundaries of the exons encoding the START domains of StAR and MLN64 are also conserved (141, 142). However, whereas the START domain of StAR is encoded by five exons (exons 3-7), the START domain of MLN64 is encoded by seven exons (exons 9-15). Interestingly, the two additional introns present in the MLN64-START domain genomic sequence are also conserved. Because of these similarities, MLN64 is also termed StarD3.
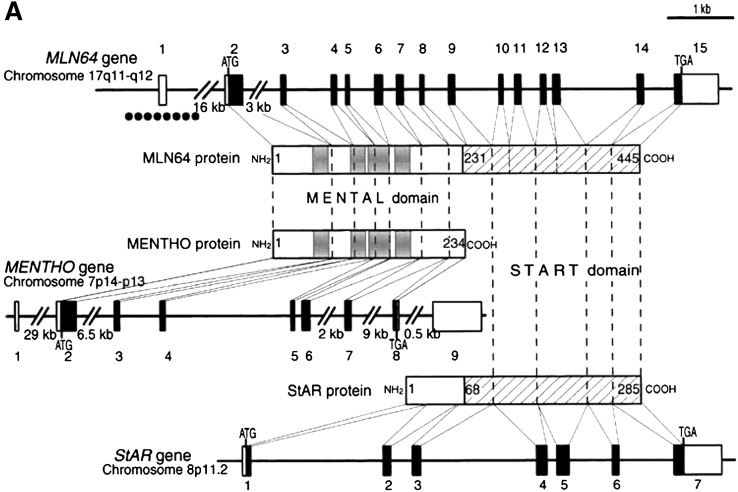
Fig. 4.
Structural relationships among MLN64, MENTHO, and StAR. The figure shows the human genes for MLN64 (top), MENTHO (middle), and StAR (bottom) as lines with the numbered boxes representing exons (open boxes, noncoding regions; black boxes, coding regions). The encoded proteins are represented as rectangles with light lines connecting to the corresponding coding exon. Numbers in the protein domains designate amino-acid positions. The four helices of the MENTAL domain of MLN64 and MENTHO that traverse the membrane are represented as gray boxes. The START domains of MLN64 and StAR are indicated by hatched lines. The location of a CpG island in the 5′ region of the MLN64 gene is indicated by a dotted line below exon 1. Reproduced from Ref. 132 with permission.
Consistent with these structural similarities, when expressed in nonsteroidogenic cells, full-length MLN64 lacks StAR-like activity, but MLN64 lacking the 234 N-terminal amino acids of the MENTAL domain (N-234 MLN64) has about 50-60% of StAR-like activity in vitro (143). These conserved START domains of both MLN64 and StAR can bind cholesterol, but whereas StAR acts as a cholesterol transporter in mitochondria (144), MLN64 participates in cholesterol transport from endosomes to cytoplasmic acceptor(s) (131). The mechanism of action of full-length MLN64 remains unclear, although the START domain of MLN64 is found as a cleavage protein in the placenta (143), and a recent report suggests that the START domain of MLN64 interacts with a cytoplasmic 60 kDa heat shock protein (HSP60) to stimulate steroidogenesis in placental mitochondria (145). Another recent study identified MLN64 as a lutein-binding protein in the retina and, hence, potentially involved in preventing night blindness (146).
The structures, subcellular location, and cholesterol-binding properties of MLN64 and MENTHO suggest an action downstream from NPC1, so that one might predict that their disruption would result in a similar phenotype. However, disruption of the cholesterol-binding START domain of MLN64 by homologous recombination in mice yielded no obvious phenotype. Mice homozygous for the MLN64 mutant allele were viable, neurologically intact, and fertile (147). There were no significant changes in plasma lipid levels, the content and distribution of hepatic lipids, or in the expression of genes involved in sterol metabolism, except for an increase in sterol ester storage in mutant mice fed a high-fat diet. Embryonic fibroblasts transfected with the cholesterol side-chain cleavage system and primary cultures of granulosa cells from MLN64 mutant mice showed defects in sterol trafficking as reflected in reduced conversion of endogenous cholesterol to steroid hormones. These observations suggest that the MLN64 START domain is largely dispensable for sterol metabolism in mice (147), and no human genetic disorders of MLN64 or MENTHO have been described. However, under basal conditions in NPC1-deficient cells, endosomal cholesterol accumulation leads to increased MLN64-mediated transport of cholesterol to mitochondria and a buildup of cholesterol in the outer mitochondrial membrane (148). Thus, cholesterol transport from endosomes to mitochondria may involve MLN64.
TRANSPORT OF CHOLESTEROL TO MITOCHONDRIA: CHOLESTEROL-BINDING PROTEINS
Both vesicular and nonvesicular means of cholesterol transport appear to be important in steroidogenic cells. Nonvesicular cholesterol transport may involve protein-protein interactions between lipid droplets and other organelles, including mitochondria (149–153). These proteins include NSF, α-SNAP, SNARE, SNAP23, syntaxin-5, and VAMP4. Steroidogenic cells express high levels of some members of the SNARE protein family, such as Syntaxin-17, SNAP23, and SNAP25; these are hormonally regulated in ovarian granulosa cells (154–158). SNARE proteins may promote functional interactions between lipid droplets and mitochondria, facilitating the transport of cholesterol substrate from lipid droplets to steroidogenic mitochondria.
Alternatively, cholesterol may be transported from lipid droplets to the OMM by nonvesicular transport processes involving high-affinity cholesterol-binding proteins (9, 13, 159). Early studies suggested that sterol carrier protein-2 (SCP2), a nonspecific lipid transfer protein, mediates cholesterol transport to steroidogenic mitochondria and stimulates steroid hormone biosynthesis (159). However, SCP2 appears to function mainly as a carrier for fatty acids, fatty acyl CoAs, lysophosphatidic acid, phosphatidyl inositol, and sphingolipids; it facilitates branched-chain fatty acid oxidation, but it has a low affinity for cholesterol and appears to play a minor role in cholesterol trafficking (160, 161). The crystal structure of rabbit SCP2 determined at 1.8 Å resolution (162), and the NMR structure of human SCP2 at 2.2 Å resolution both show a tunnel-like cavity lined primarily by hydrophobic residues, providing an environment for the binding hydrophobic ligands (163). Knockout of the mouse SCP2 gene affects peroxisomal function secondary to the loss of SCP-x, but the animals are phenotypically and reproductively intact and their cells do not accumulate cholesterol (164, 165).
A family of lipid-binding proteins termed StarD4, StarD5, and StarD6 (166, 167) is structurally related to StAR (StarD1), which is the prototype of the START domain protein superfamily (167–169) (MLN64 is StarD3). This superfamily is evolutionarily conserved in plants and animals (137, 138). Fifteen human proteins contain a START domain, either alone or in association with other domains. START domain proteins can bind a variety of lipids: StAR and MLN64 bind cholesterol; StarD5 binds cholesterol and 25-hydroxycholesterol; phosphatidylcholine-transfer protein (PCTP; StarD2) binds phosphatidylcholine; StarD10 binds phosphatidylcholine and phosphatidylethanolamine; and ceramide-transport protein (CERT) binds ceramides. The oxysterol-binding protein (OSBP)-related proteins (ORP) are conserved from yeast to man and are implicated in regulating sterol pathways (170–173). OSBP and other ORPs have been suggested as candidates for oxysterol transport to other membrane compartments (174).
StarD4 and StarD5 are widely expressed in steroidogenic cells, whereas StarD6 expression appears to be mostly restricted to the testicular germ cells (169). In contrast to StarD1 and StarD3 (MLN64), StarD4, StarD5, StarD6, StarD10, and CERT lack N-terminal sequences, suggesting they are cytosolic proteins like StarD2 (PCTP) (175), but direct evidence for this is lacking in most cases. StarD4 and StarD5 bind cholesterol with high affinity and specificity, facilitate cholesterol transport through an aqueous environment, and appear to play important roles in cellular cholesterol homeostasis (176, 177). StarD4 and StarD5 have negligible StAR-like activity to stimulate the movement of cholesterol from the OMM to the IMM; by contrast, StarD6 has robust StAR-like activity and physicochemical properties that closely resemble StAR (StarD1) (169). StarD7 is a phosphatidyl choline-binding protein (178) abundantly expressed in trophoblast cells (179). In THP-1 macrophages, StarD4 is found in cytoplasm and colocalized with ACAT in the ER, where it appears to increase ACAT activity, suggesting that StarD4 participates in directional, nonvesicular cholesterol transport (180). Because StarD4 and StarD5 resemble a StAR molecule lacking its mitochondrial leader peptide and because such leaderless StAR molecules are fully active in transfected cells (144, 181) it seemed that StarD4 and/or StarD5 might function to “load” cholesterol into the OMM, where it could be acted upon by StAR itself. However, knockout of StarD4 in the mouse resulted in minimal changes in weight and serum lipids and no changes in steroidogenesis, so that any role played by StarD4 appears to be compensated by other factors (182). Human disorders of the StarD proteins, other than StAR itself (see below), have not been reported.
ACUTE VERSUS CHRONIC REGULATION OF STEROID BIOSYNTHESIS
Steroid secretion is constitutively linked to steroid synthesis. Cells that produce polypeptides for export (e.g., insulin, growth hormone, digestive enzymes, etc.) first synthesize and then store large amounts of the protein in secretory vesicles, ready for rapid release. By contrast, steroidogenic cells store very little steroid hormone, so a rapid steroidogenic response (e.g., adrenal secretion of cortisol in the classic “fight or flight” response) requires rapid synthesis of new steroid (183–185). The regulation of steroidogenesis is best described in the adrenal, but similar principles apply in the gonad. ACTH acting on the adrenal (and LH acting on the gonad) promotes steroidogenesis at three distinct levels with substantially different timing. First, over the course of months, long-term exposure to ACTH promotes adrenal growth: ACTH via its second messenger, cAMP, promotes the intra-adrenal synthesis of insulin-like growth factor 2 (186, 187), basic fibroblast growth factor (188), and epidermal growth factor (189), promoting cellular hypertrophy and hyperplasia. Second, ACTH, acting through cAMP in the adrenal zona fasciculata (and angiotensin II acting through the calcium/calmodulin pathway in the zona reticularis), promotes transcription of genes encoding the steroidogenic enzymes, especially the CYP11A1 gene encoding the enzymatically rate-limiting enzyme P450scc (190, 191). Third, ACTH rapidly stimulates the production and activation of StAR. In response to ACTH, cAMP stimulates phosphorylation of StAR at Ser195 almost immediately, thus doubling its activity (192), and stimulates transcription of the StAR gene within minutes (193). StAR increases the flow of cholesterol from the OMM to IMM, where it becomes substrate for P450scc, thus mediating the acute steroidogenic response. It was the physiologic observation that ACTH exerts distinct acute and chronic actions that led to the discovery and characterization of StAR. The regulation of StAR expression is controlled by multiple factors (194).
Studies in the 1960s showed that the acute steroidogenic response could be blocked by inhibitors of protein synthesis, such as puromycin or cycloheximide, indicating that a short-lived protein species mediates this process (183–185). Two-dimensional gel electrophoresis first showed that this acute steroidogenic response was accompanied by the rapid synthesis of a group of proteins ultimately shown to be derived from a 37 kDa phosphoprotein (195–198), leading to its cloning and designation as the steroidogenic acute regulatory protein, StAR (199). The history of the discovery of StAR as this long-sought acute trigger of steroidogenesis was reviewed in detail shortly after its discovery (136). StAR is synthesized as a 37 kDa protein that has a typical mitochondrial leader sequence that directs it to the mitochondria; upon mitochondrial entry, this leader peptide is cleaved off to yield the 30 kDa intramitochondrial protein. In some cell types, this cleavage takes place in two steps, yielding a 32 kDa intermediate. Overexpression of StAR in mouse Leydig MA-10 cells increased their basal rate of steroidogenesis (199), and cotransfection of vectors expressing StAR and a fusion protein of the P450scc enzyme system in nonsteroidogenic COS-1 cells augmented pregnenolone synthesis above that obtained with the P450scc system alone (200), providing evidence that StAR is the acute regulator of steroidogenesis. The definitive proof of StAR's essential role came when it was shown that StAR mutations cause congenital lipoid adrenal hyperplasia, in which very little steroid is made (200, 201).
Some steroidogenesis is independent of StAR. Nonsteroidogenic cells transfected with the P450scc enzyme system can convert some cholesterol to pregnenolone in the absence of StAR (202, 203). Cotransfection with a vector that expresses StAR shows that this StAR-independent steroidogenesis occurs at about 14% of the StAR-induced rate (200, 201). In vivo, the human placenta utilizes mitochondrial P450scc to initiate steroidogenesis (191) but does not express StAR (141), although the placentae of pigs and cattle express low levels of StAR (204). The mechanism of StAR-independent steroidogenesis is unclear; it may occur without a triggering protein, or some other protein may exert StAR-like activity to promote cholesterol flux but without StAR's rapid kinetics. Some data suggest that a 30 kDa proteolytic cleavage product of MLN64 exerts StAR-like activity in the placenta and elsewhere (143, 145).
STRUCTURE AND MECHANISM OF StARrsquoS ACTION
The mechanism of StAR's action has been studied extensively, but it remains incompletely understood (205, 206). It was initially noted that the 37 kDa cytoplasmic form possessing the mitochondrial leader sequence had a short half-life of less than 15 min, suggesting it was a “precursor,” whereas the 30 kDa intramitochondrial “mature” form of StAR had a half-life of several hours. It was first suggested that importation of StAR into mitochondria formed “contact points” between the OMM and IMM, permitting cholesterol to flow down a chemical concentration gradient (136, 199), and others concluded that the 30 kDa “mature” StAR is the biologically active moiety, functioning as a cholesterol shuttle in the intramembranous space (207). However, deletion of the mitochondrial leader has no effect on StAR's activity in transfected cells (181), and both the 37 and 30 kDa forms of StAR are equally active when expressed in the cytoplasm or added to mitochondria in vitro (144). Immobilizing StAR on the cytoplasmic surface of the OMM, in the intramembranous space, or on the matrix side of the IMM showed that StAR was constitutively active on the OMM, but it was inactive when localized to the intramembranous space or to the IMM (144) ( Fig. 5 ). Thus StAR acts exclusively on the OMM (144, 181). Similarly, constructs that could either speed up or slow down the entry of StAR into the mitochondria showed that faster import decreased activity, whereas slower import increased activity (144). Thus, StAR's activity to promote steroidogenesis is proportional to its residency time on the OMM, and it is StAR's cellular localization, and not its cleavage from 37 to 30 kDa, that determines its activity.
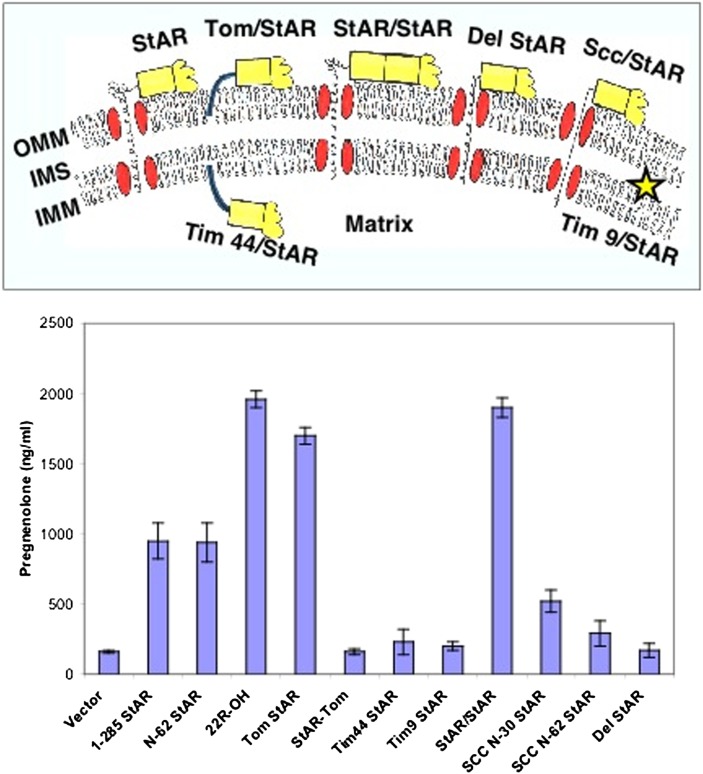
Fig. 5.
StAR acts on the outer mitochondrial membrane (OMM). The upper panel shows the experimental design (144), depicting the OMM, intramembranous space (IMS), inner mitochondrial membrane (IMM), and various StAR constructs designed to test mitochondrial import and organellar localization. The first 62 residues of StAR, which include the mitochondrial leader and pause sequences, are represented by the corkscrew line, the protease-resistant domain (residues 63-188) by the yellow box, and the protease-sensitive carboxyl-terminal domain by the yellow blob. Mitochondrial protein-import proteins are represented by red ovals. Fusion of N-62 StAR to the C terminus of Tom20, an OMM protein, generates the Tom/StAR construct, which immobilizes StAR on the OMM. Tim9/StAR, a fusion of StAR to an IMS protein, immobilizes StAR in the IMS, and Tim44/StAR, a fusion of StAR to an IMM protein, immobilizes StAR on the matrix side of the IMM. StAR/StAR is a construct that fuses 1-188 StAR to N-62 (63-285) StAR, thus dimerizing the protease-resistant domain (residues 63-188); Del StAR retains residues 1-30 but deletes a “pause” sequence at residues 31-62, and Scc/StAR replaces the StAR leader (either 1-30 or 1-62) with the 39 amino acid leader sequence of human P450scc. The lower panel shows the activity of the constructs illustrated in the upper panel, when transfected into COS-1 cells cotransfected with the F2 fusion of the cholesterol side-chain cleavage system. A low level of StAR-independent steroidogenesis is seen with the vector control, but full-length (1-285) or N-62 StAR increase steroidogenesis 6-fold. The hydroxysterol 22R-OH-cholesterol bypasses the action of StAR, providing an index of the maximal level of steroidogenesis that can be achieved in this system. Transfection with the Tom/StAR fusion or the StAR/StAR dimer achieves this maximal level of steroidogenesis. By contrast, when StAR is placed at the N-terminus rather than the C terminus of Tom20 (StAR/Tom), when it is localized to the matrix side of the IMM (Tim44/StAR), or when it is confined to the IMS (Tim9/StAR), it is inactive. Replacing StAR's leader peptide with that of P450scc also reduces activity. Cell-free transcription/translation linked to mitochondrial import assays show that the level of activity seen with the leader mutants is inversely proportional to their speed of import (144). Copyright © W. L. Miller.
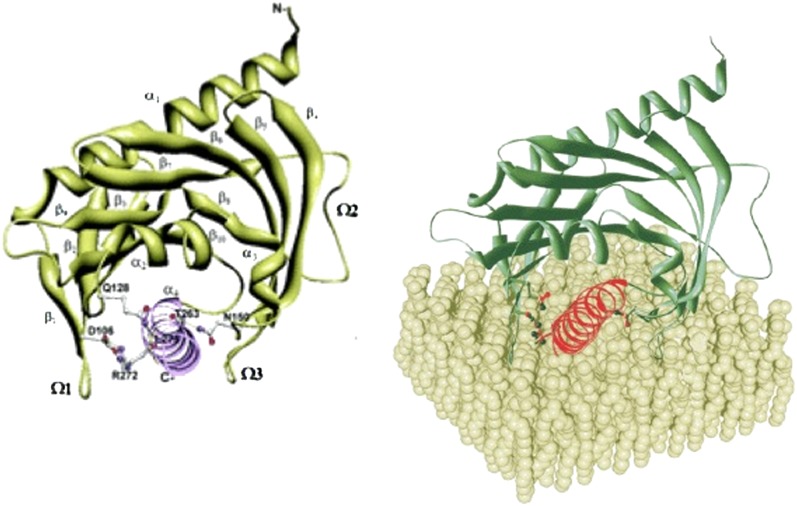
The three-dimensional structure of StAR has been deduced from the crystal structure of the START domain of MLN64 (MLN64 lacking its N-terminal 218 residues). This protein (N-218 MLN64) is 37% identical and about 50% similar to the sequence of StAR (134). N-218 MLN64 has about half of the activity of StAR to promote steroidogenesis, both in transfected cells and when the bacterially expressed, purified protein is added to steroidogenic mitochondria in vitro (135, 143). The crystal structure of N-216 MLN64 shows a globular protein with an α/β helix-grip fold and an elongated hydrophobic pocket measuring about 26 Å deep and 10 Å across at its widest diameter (207). Modeling indicates that N-216 MLN64 accommodates a single molecule of cholesterol in this pocket, with the 3β-OH group coordinated by the two polar residues at the bottom of the pocket. This structure, the crystal structure of the closely related protein StarD4 (208), and several computational models of StAR (209–211) are all very similar. Each is characterized by two long α-helixes at the N- and C-termini, two short α-helixes, and a set of nine antiparallel β sheets that form a helix-grip fold ( Fig. 6A ). A recent, low-resolution (3.4 Å) structure of human StAR has confirmed the results of these models (212).
Fig. 6.
Model of N-62 StAR. (Left) Ribbon diagram showing the N terminus in the upper right-hand corner and the C-terminal helix in the lower center, extending out of the plane of the diagram. Residues that contribute to the associations between this C-terminal helix and adjacent structures are shown as ball-and-stick representations: carbon atoms are in white, nitrogen are in blue, oxygen in red, and hydrogen bonds in green. Reprinted from (210) with permission. (Right) Model showing the orientation of StAR as it interacts with the OMM (cover picture of Ref. 210).
To determine which domains of the StAR protein interact with the OMM, bacterially expressed human StAR was incubated with small unilamellar vesicles having a lipid composition designed to model that of the OMM. The complex was then proteolyzed, and the StAR peptides protected from proteolysis were identified and characterized by mass spectrometry (210). Only the exterior surface of the C-terminal α-helix and small segments of the adjacent Ω-loops were protected by the synthetic membrane, indicating these domains probably interact with the OMM (Fig. 6B). The Ω-loops form hydrogen bonds with the C-terminal helix; when these bonds are intact, the C-helix obstructs access of cholesterol to StAR's hydrophobic cholesterol-binding pocket. Molecular Dynamics simulations indicated that the C-helix can “swing open,” especially when the surface residues are protonated, as would be expected when StAR interacts with the OMM (213). It is presumed that the interaction of StAR with the charged phospholipid head groups on the OMM disrupts these hydrogen bonds, permitting the C-helix to swing open and closed, governing access of cholesterol to the sterol-binding pocket. To test the role of the movement of the C-helix, residues were computationally identified that could be changed to Cys without altering the protein's conformation. Immobilizing the C-helix by forming disulfide bonds with the adjacent loops ablated activity, whereas reducing these artificial disulfide bonds restored activity, showing that it was the immobilization of the C-helix, and not the amino acid changes, that were responsible for the lost activity (213). Thus, the activity of StAR on the OMM requires an acid-induced disruption of hydrogen bonds and a consequent conformational change in StAR to permit it to bind and release cholesterol.
StAR has a hydrophobic pocket that binds one molecule of cholesterol (207, 213). The interaction of StAR with the OMM involves conformational changes (213–218) that are needed for it to accept and discharge cholesterol molecules. StAR can transfer cholesterol between membranes in vitro (219), suggesting that other protein molecules are not needed for its action; however, the mutant R182L, which is biologically inactive (201, 220), can also transfer cholesterol to membranes in vitro (221), and other proteins are known to interact with StAR. Thus, StAR's action to promote steroidogenesis is distinct from its cholesterol-binding and cholesterol-transfer activity; cholesterol binding is necessary but not sufficient for StAR activity.
StAR appears to interact with a protein complex on the OMM (222); different experiments have identified multiple StAR “partner proteins,” whose roles remain under study. It has long been known that the peripheral benzodiazepine receptor (PBR) plays a key role in the hormonally induced entry of cholesterol into steroidogenic cells (223), and several lines of evidence now indicate that this 18 kDa protein (now also called the mitochondrial transporter protein, TSPO) (224) acts downstream of StAR on the outer mitochondrial membrane (225–227). PBR/TSPO, which is localized to the OMM and binds benzodiazepines and the test drug PK11195, has a cytoplasmic domain containing a cholesterol recognition amino acid consensus (CRAC) domain (228). PBR/TSPO ligands stimulate steroid synthesis and promote translocation of cholesterol from OMM to the IMM in several steroidogenic cell types (229–231). Mutagenesis of the CRAC domain interferes with cholesterol binding and transfer of cholesterol to the IMM (228), and blocking the binding of cholesterol to the CRAC domain prevents steroidogenesis (232). Targeted deletion of the TSPO gene in a Leydig cell line blocked cholesterol transport into the mitochondria and reduced steroid production; reintroduction of TSPO into the deficient cell line restored steroidogenic capacity (228).
It has long been known that PBR/TSPO interacts with the voltage-dependent anion channel (VDAC) on the OMM, which in turn interacts with the adenine nucleotide transporter (ANT) on the IMM (233). More recent data showed that PBR/TSPO is a component of a 140-200 kDa multiprotein complex consisting of PBR/TSPO itself, the 34 kDa VDAC1, the 30 kDa ANT, a 10 kDa protein (pk 10), the TSPO-associated protein-1 (PRAX-1), and the TSPO and protein kinase A (PKA) regulatory subunit RIα-associated protein 7 (PAP7, also known as acyl-CoA binding domain-containing protein 3, or ACBD3) (234). MA-10 Leydig cells that overexpress a fusion protein that affixes StAR to the OMM (Tom20/StAR) and, hence, have maximal StAR activity, produced steroids at a maximal level, but these cells lost their steroidogenic capacity if exposed to PBR/TSPO-antisense oligonucleotides (226). However, whereas the functional interaction with PBR/TSPO appears clear, a physical interaction has not been demonstrated. Instead, physical interactions with VDAC1 and phosphate carrier protein (PCP) have been described by protein cross-linking studies (222), and VDAC1 also appears to interact with PBR/TSPO (227, 235). The role of VDAC-1 is unclear; VDAC-1 has a ring-like structure with a hydrophilic interior that is well-suited to anion transport, but it is a seemingly poor candidate to form a channel for hydrophobic cholesterol (236, 237).
The phosphorylation of StAR on Ser 195 approximately doubles StAR's activity (192), but the precise mechanism of this phosphorylation remains under investigation. StAR has been reported to interact with PAP7 and be phosphorylated by cAMP-dependent protein kinase regulatory subunit 1α (PKA-RIα) (227, 235), but more recent data indicate that the PKA anchor protein AKAP121, one of four AKAP proteins arising from the alternately spliced AKAP1 gene (238), recruits the type II PKA regulatory subunit α (PKAR2A), which phosphorylates StAR, whereas the type I kinase drives StAR transcription (239, 240). A physical interaction between the 37 kDa cytoplasmic form of StAR and HSL has also been reported (241). StAR is recycled, with each molecule moving hundreds of molecules of cholesterol into the mitochondria before StAR is imported into the mitochondria and hence inactivated (242). Low levels of steroidogenesis will persist in the absence of StAR at about 14% of the StAR-induced rate (200, 201), accounting for the steroidogenic capacity of tissues that lack StAR (e.g., the human placenta). Physical studies and partial proteolysis indicate that residues 63-193 of StAR are protease resistant and constitute a “pause-transfer” sequence, which permits the bioactive loosely folded carboxy-terminal molten globule domain to have increased interaction with the outer mitochondrial membrane (214).
DISORDERED StAR: CONGENITAL LIPOID ADRENAL HYPERPLASIA
Congenital lipoid adrenal hyperplasia (lipoid CAH) is a very severe disorder of steroidogenesis, characterized by minimal or absent serum concentrations of all steroids; high basal ACTH and plasma renin activity; absent steroidal responses to long-term treatment with high doses of ACTH or chorionic gonadotropin; and grossly enlarged adrenals filled with cholesterol and cholesterol esters (243–246). Because these findings indicate a lesion in the conversion of cholesterol to pregnenolone, lipoid CAH was initially thought to result from a disorder in an enzyme involved in this conversion; hence, before the role of P450scc was understood, lipoid CAH was mis-termed 20,22-desmolase deficiency (246–251). However, the CYP11A1 gene for P450scc is not mutated in these patients (251), and placental steroidogenesis persists in lipoid CAH, permitting the synthesis of the progesterone needed for term gestation; this would not be expected to happen if P450scc were involved (252). The normally functional P450scc system in conjunction with the accumulation of cholesterol esters in the affected adrenal suggested that the lesion lay in an upstream factor involved in cholesterol transport into mitochondria (251). Mutations in the gene for PBR/TSPO were then sought but not found (253). The identification of disease-causing StAR mutations in lipoid CAH proved StAR's indispensable role in adrenal and gonadal steroidogenesis. Thus, lipoid CAH is the StAR gene knockout experiment of nature (254), and the initial studies of lipoid CAH showed that StAR promotes steroidogenesis by increasing the movement of cholesterol into mitochondria (200, 201, 255, 256). The observation that cells could make some steroids in a StAR-independent fashion led to the two-hit model of lipoid CAH (201) ( Fig. 7 ). The first hit is the loss of StAR activity, resulting in a loss of most, but not all steroidogenesis. Diminished steroidogenesis causes a compensatory rise in ACTH and gonadotropins, eliciting increased intracellular cAMP, which increases biosynthesis of LDL receptors, the uptake of LDL cholesterol, and de novo synthesis of cholesterol. Consequently, in the absence of StAR, intracellular cholesterol accumulates, causing the second hit, which is the loss of all steroidogenic capacity caused by mitochondrial and cellular damage resulting from the accumulated cholesterol, cholesterol esters, and their auto-oxidation products (201).
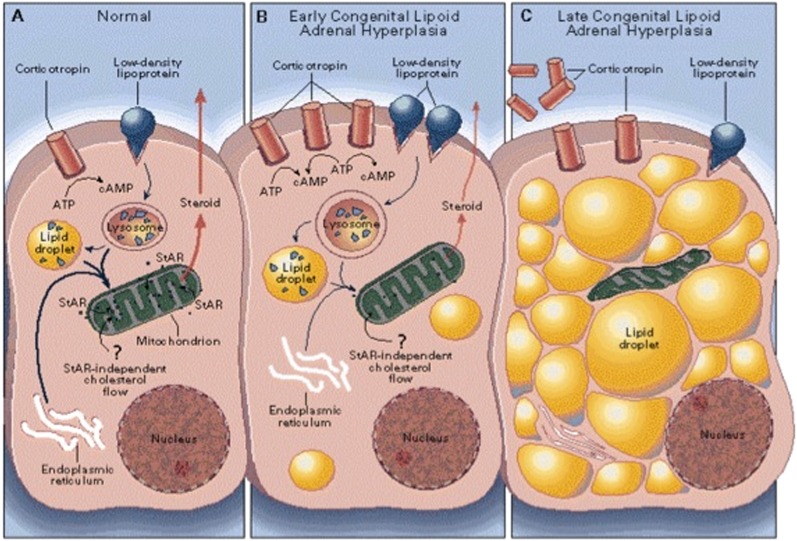
Fig. 7.
Two-hit model of lipoid CAH. (A) In a normal human adrenal cell, cholesterol is primarily derived from low-density lipoproteins. The rate-limiting step in steroidogenesis is the flow of cholesterol from the outer to the inner mitochondrial membrane. (B) In early lipoid CAH, StAR-independent mechanisms can still move some cholesterol into the mitochondria, yielding a low level of steroidogenesis. ACTH secretion increases, stimulating further accumulation of cholesterol esters in lipid droplets. (C) As lipid droplets accumulate, they engorge and damage the cell through physical displacement and by the action of cholesterol auto-oxidation products. Steroidogenic capacity is destroyed, and tropic stimulation continues. In the ovary, follicular cells remain unstimulated and undamaged until puberty, when small amounts of estradiol are produced, as in panel B, causing partial feminization, with infertility and hypergonadotropic hypogonadism. Modified from Ref. 201, with permission.
The two-hit model (128), which has been confirmed clinically (257, 258) and in StAR knockout mice (259, 260), explains the findings in lipoid CAH. The same abnormal physiology seen in adrenal cells occurs in fetal testicular Leydig cells, which normally make the large amounts of testosterone needed for development of male external genitalia. Consequently, an affected 46,XY fetus cannot produce testosterone and is born with female external genitalia. The Sertoli cells are undamaged and produce Müllerian inhibitory hormone normally, so that the phenotypically female 46,XY fetus lacks a cervix, uterus, or fallopian tubes. The steroidogenically active fetal zone of the adrenal is similarly affected early in development, disrupting its biosynthesis of dehydroepiandrosterone, which is converted to estriol by the placenta, so that midgestation maternal and fetal estriol levels are very low (252). By contrast, the fetal adrenal produces very little aldosterone because fetal salt and water balance are governed by the placenta, so that stimulation of the aldosterone-producing zona glomerulosa generally does not begin until birth. Consistent with this, many newborns with lipoid CAH do not have a salt-wasting crisis until after several months of life, when chronic stimulation then leads to cellular damage (201, 261).
The two-hit model explains the perplexing adolescent feminization of affected 46,XX females who received steroid replacement therapy in infancy and hence survived (257, 258). Unlike the fetal testis, the fetal ovary makes little or no steroids (262). In the absence of tropic hormone induction of steroidogenesis, the affected ovary remains undamaged until it is stimulated by gonadotropins. At the onset of puberty, the ovary in lipoid CAH produces small amounts of estrogen by StAR-independent steroidogenesis. However this low level of steroidogenesis cannot generate adequate progesterone in response to the midcycle LH surge, and continued stimulation results in cholesterol accumulation and cellular damage, so that biosynthesis of progesterone in the latter part of the cycle is impaired. Gonadotropin stimulation only recruits individual follicles and does not promote steroidogenesis in the whole ovary; hence, most follicles remain undamaged and available for future cycles. Monthly cyclicity is determined by the hypothalamic-pituitary axis, and remains normal. A new follicle is recruited with each successive cycle, and more estradiol is produced by StAR-independent steroidogenesis. Although ovarian steroidogenesis is impaired, enough estrogen is produced to induce breast development; hence, the patient experiences general feminization, monthly estrogen withdrawal, and cyclic vaginal bleeding (201, 257). Because little progesterone is produced in the latter half of the cycle, the cycles are anovulatory. Measurements of estradiol, progesterone, and gonadotropins throughout the cycle in affected adult females with lipoid CAH confirm this model (258). Similarly, examination of the ovaries of StAR-knockout mice confirms the two-hit model (260). Understanding this physiology has permitted successful in vitro fertilization in affected female patients (263, 264).
Lipoid CAH is a rare disorder in most populations, but is especially common in Japan and Korea. Many StAR mutations have been found in lipoid CAH patients, but about 65-70% of affected Japanese alleles and virtually all affected Korean alleles carry the mutation Q258X (200, 201, 220, 246, 265–267). The Q258X carrier frequency in these countries is about 1 in 300 (201, 266, 267) so that 1 in every 250,000 to 300,000 newborns is affected, or about 500 patients in Japan and Korea. Other genetic clusters are found among Palestinian Arabs, carrying the mutation R182L (201), in eastern Saudi Arabia, carrying R182H (261), and in parts of Switzerland, carrying the mutation L260P (268).
An attenuated form of the disease called “non-classic lipoid CAH” is caused by mutations that retain about 10-25% of normal StAR activity (269, 270). These patients typically experience adrenal insufficiency several years after infancy; the 46,XY individuals may masculinize normally, and mineralocorticoid secretion may be affected minimally. The most common mutation causing this phenotype is R188C. Thus the spectrum of clinical presentation of mutations in the StAR protein is substantially broader than that of classic lipoid CAH, and many affected patients may not be diagnosed correctly (270).
STEROIDOGENESIS AND CYTOCHROME P450
Once StAR and its interacting partners deliver cholesterol to the IMM, the cholesterol can be used as the substrate for steroidogenesis. Most steroidogenic enzymes are isoforms of “cytochrome P450,” a term that designates a group of oxidative enzymes sharing similar chemical properties: all have about 500 amino acids, contain a single heme group, and absorb light at 450 nm in their reduced states (271). P450 means pigment 450, because of their yellow color in solution. The human genome encodes 57 cytochrome P450 enzymes; this is a small number: Drosophila have 83 functional P450 genes (272), mice have 102 (273), and rice has 356 (273). These genes are formally termed CYP genes; the encoded proteins may be given the same name without the use of italics (thus, the CYP11A1 gene encodes CYP11A1), but most biochemists and endocrinologists continue to use the traditional P450 names for the proteins (thus the CYP11A1 gene encodes P450scc). Seven human cytochrome P450 enzymes are targeted to the mitochondria and are termed “Type 1”; the other 50 human P450 enzymes are targeted to the endoplasmic reticulum and are termed “Type 2.” All P450 enzymes activate molecular oxygen using their heme center and electrons donated by NADPH. The two types of P450 enzymes are distinguished by the different mechanisms by which they receive electrons from NADPH, as well as by their intracellular locations. Type 1 enzymes lie at the end of an electron transfer chain consisting of a flavoprotein termed ferredoxin reductase (also termed adrenodoxin reductase) (274) and a small iron-sulfur protein termed ferredoxin (also termed adrenodoxin) (275) ( Fig. 8 ). Type 2 P450 enzymes receive electrons via a single 2-flavin protein termed P450 oxidoreductase (POR) (213). Each P450 enzyme can catalyze multiple reactions, often with chemically different substrates; hence, they are designated by their P450 name (e.g., P450c21 or CYP21A1) rather than by a reaction-based name (e.g., 21-hydroxylase).
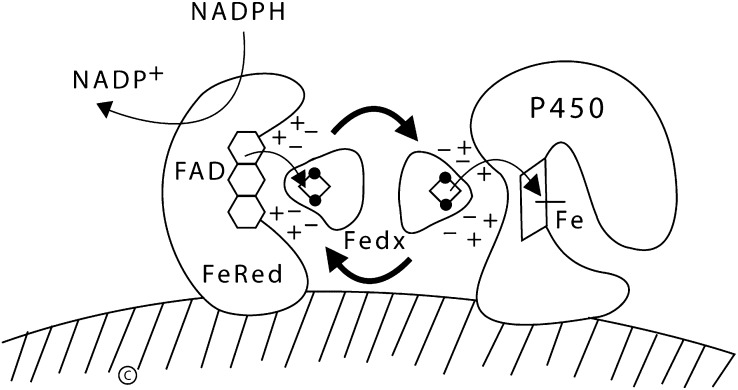
Fig. 8.
Functional organization of mitochondrial P450 enzymes. NADPH first donates electrons to the FAD moiety of ferredoxin reductase (FeRed); positively charged residues in ferredoxin reductase interact with negatively charged residues in ferredoxin (Fedx), permitting the electrons to be transferred to the Fe2S2 center (ball and stick diagram). Ferredoxin then dissociates from ferredoxin reductase and diffuses through the mitochrondrial matrix. The same surface of ferredoxin that received the electrons from ferredoxin reductase then interacts with the redox-partner binding site of a mitochondrial P450, such as P450scc. The electrons from the Fe2S2 center of ferredoxin travel to the heme ring of the P450. The heme iron then mediates catalysis with substrate bound in the P450. Copyright © W. L. Miller.
Six P450 enzymes are involved in steroid hormone biosynthesis (1). The first of these is mitochondrial P450scc, which converts insoluble cholesterol to soluble pregnenolone by removing the hydrophobic 6-carbon acyl chain by cleaving the 20,22 bond. A cell is said to be “steroidogenic” if it expresses P450scc and hence can catalyze this first and rate-limiting reaction; many cells can transform steroids produced in other cells, but only cells producing P450scc are steroidogenic. Pregnenolone may be converted to progesterone by a non-P450 enzyme, 3β-hydroxysteroid dehydrogenase (3βHSD, HSD3B) in association with the inner mitochndrial translocase Tim50 (276), or it may exit the mitochondrion and be acted on by P450c17 (CYP17A1) in the endoplasmic reticulum, which catalyzes both 17α-hydroxylase and 17,20 lyase activities. Pregnenolone appears to exit the mitochondrion unaided; no transport protein has been found, and physiologic evidence does not suggest the presence of such a transporter. Following the activities of 3βHSD and P450c17, P450c21 (CYP21A1) catalyzes the 21-hydroxylation of both glucocorticoids and mineralocorticoids. The final steps in the synthesis of both glucocorticoids and mineralocorticoids again takes place in the mitochondria, where the two isozymes of P450c11, P450c11β (11β-hydroxylase, CYP11B1) and P450c11AS (aldosterone synthase, CYP11B2) reside. P450c11β catalyzes the 11β-hydroxylation of 11-deoxycortisol to cortisol, and P450c11AS catalyzes the 11β-hydroxylase, 18-hydroxylase, and 18-methyl oxidase activities needed to convert deoxycorticosterone to aldosterone. Another type 2 enzyme, P450aro, catalyzes aromatization of androgens to estrogens. Histologic and electron microscopic examination of steroidogenic cells suggests that the smooth endoplasmic reticulum containing the type 2 steroidogenic P450 enzymes comes into physical contact with the OMM during hormonally induced steroidogenesis (222), forming a steroidogenic complex, so that the movement of steroidal intermediates from mitochondrion to endoplasmic reticulum involves very small distances and should not be visualized as steroid hormones having to travel great distances across the cytoplasm. The biochemistry, genetics, human physiology, and disease states of all these enzymes have been reviewed in detail recently (1).
CHOLESTEROL SIDE-CHAIN CLEAVAGE ENZYME P450scc
Conversion of cholesterol to pregnenolone by mitochondrial P450scc is the first, rate-limiting, and hormonally regulated step in the synthesis of all steroid hormones (277–279). This process involves three chemical reactions, the 22-hydroxylation of cholesterol, 20-hydroxylation of 22(R)-hydroxycholesterol, and oxidative scission of the C20-22 bond of 20(R),22(R)-dihydroxycholesterol (the side-chain cleavage event), to yield pregnenolone and isocaproaldehyde. Of these three reactions, the binding of cholesterol and 22-hydroxylation are rate limiting, as the efficiencies (kcat/Km ratios) are much higher for the subsequent reactions, and the high KD of ∼3000 nM drives the dissociation of pregnenolone from P450scc (280, 281). Nevertheless, soluble hydroxysterols such as 22(R)-hydroxycholesterol can enter the mitochondrion readily, bypassing the action of StAR and the cholesterol-import machinery (282), providing a useful experimental tool for studying these processes (200, 202). The overall side-chain cleavage reaction catalyzed by P450scc is slow, with a net turnover number of about 6 (283) to 20 (281) molecules of cholesterol per molecule of P450scc per second. Because 20-hydroxycholesterol, 22-hydroxycholesterol, and 20,22-hydroxycholesterol can all be isolated from bovine adrenals in significant quantities and because 3 mol of NADPH are required per mole of cholesterol converted to pregnenolone, it was initially thought that three separate enzymes were involved. However, protein purification and reconstitution of enzymatic activity in vitro showed that a single protein, P450scc, converted cholesterol to pregnenolone (284, 285). These three reactions occur on a single active site that is in contact with the inner mitochondrial membrane; the crystal structures of bovine (286) and human (287) P450scc, the latter in complex with its electron donor, ferredoxin, have been determined recently.
The cDNA (288) and gene (289) for human P450scc were cloned in the mid 1980s, showing that the 9-exon gene lies on chromosome 15q23-q24, spanning about 20 kb. The P450scc mRNA encodes a preprotein of 521 amino acids, including a 39 amino acid mitochondrial leader peptide that is cleaved off during its entry into mitochondria. A spontaneously occurring deletion of the gene for P450scc in the rabbit (290), knockout of P450scc in the mouse (291), and rare patients with P450scc gene mutations (292–294) result in the loss of all steroidogenesis, showing that all steroidogenesis is initiated by this one enzyme. Thus, it is the presence of P450scc that renders a cell “steroidogenic” and able to make steroids de novo, as opposed to modifying steroids produced elsewhere, which occurs in many types of cells. Transcription of the CYP11A1 gene determines the amount of P450scc synthesis and, hence, the steroidogenic capacity of a cell. CYP11A1 transcription is regulated by tissue-specific and hormonally responsive factors, and it can be induced by both the PKA and PKC second messenger systems acting through different CYP11A1 promoter sequences (190). The expression of P450scc and other steroidogenic enzymes in the adrenal and gonad requires the action of steroidogenic factor 1 (SF1) an orphan zinc-finger transcription factor (295, 296). By contrast, the expression of P450scc in the human placenta is constitutive (191) and independent of SF1; however, it requires members of the CP2 (graineyhead) family of transcription factors (also known as LBP proteins) (297–300) and TreP-132 (301, 302). Thus, long-term cellular stimulation over the course of days will increase the content of P450scc and the level of basal steroid produced, as well as the capacity of the cell to mount a steroidogenic response.
ELECTRON TRANSFER TO P450scc: FERREDOXIN AND FERREDOXIN REDUCTASE
P450scc and other mitochondrial P450 enzymes are terminal oxidases in an electron transport system consisting of two proteins, ferredoxin and ferredoxin reductase (210). Electrons from NADPH are accepted by ferredoxin reductase (also known as adrenodoxin reductase), a flavoprotein bound to the inner mitochondrial membrane, which then transfers the electrons to ferredoxin (adrenodoxin), an iron/sulfur protein found in the mitochondrial matrix or loosely adherent to the inner mitochondrial membrane; ferredoxin then transfers the electrons to P450scc (Fig. 8). Ferredoxin forms a 1:1 complex with ferredoxin reductase, then dissociates, then subsequently reforms an analogous 1:1 complex with a mitochondrial P450 such as P450scc, thus functioning as an indiscriminate diffusible electron shuttle mechanism. The relative abundances of ferredoxin reductase and ferredoxin are critical for determining the catalytic activity of P450scc (202, 303). Mutations in the human genes for ferredoxin and ferredoxin reductase have not been described, and mouse knockouts have not been reported, but mutation of the Drosophila ferredoxin reductase homolog dare causes developmental arrest and degeneration of the adult nervous system due to the loss of ecdysteroid production (304).
The human FDXR gene (305) on chromosome 17q24-q25 (306) yields two alternatively spliced mRNA species differing by 18 bp (274), but only the ferredoxin reductase encoded by the shorter mRNA is active (307). This alternative splicing may be regulated in some tumor tissues (308). Ferredoxin reductase mRNA is found in all tissues examined, but its abundance is two orders of magnitude higher in steroidogenic tissues (309). Ferredoxin reductase is a 54.5 kDa flavoprotein consisting of two domains, each comprising a β-sheet core surrounded by α-helices (310). The NADP(H)-binding domain is compact, whereas the more open FAD domain binds the dinucleotide portion of FAD across a Rossman fold with the redox-active flavin isoalloxazine ring abutting the NADP(H) domain. By analogy to the structures of glutathione reductase and thioredoxin reductase, the nicotinamide ring of NADPH appears to lie adjacent to the flavin ring in a position to transfer electrons to the FAD. Thus, intramolecular electron transfer occurs in the cleft formed by the angled apposition of these two domains. This cleft is characterized by basic residues that interact with acidic residues on ferredoxin (311, 312).
The FDX1 gene (313) on chromosome 11q22 (306) encodes ferredoxin (also termed Fdx1), a 14 kDa, soluble, iron/sulfur (Fe2S2) electron shuttle protein that resides either free in the mitochondrial matrix or loosely bound to the inner mitochondrial membrane (314). There are two human ferredoxins, Fdx1 and Fdx2, but only Fdx1 appears to support mitochondrial P450 enzymes that participate in steroidogenesis, whereas Fdx2 participates in the synthesis of heme and Fe/S cluster proteins (315). Ferredoxin consists of a core region and an interaction domain (316). The core region contains the four cysteines that tether the Fe2S2 cluster, which lies in a protuberance at the junction of its two domains. The interaction domain contains a helix with numerous charged residues, giving ferredoxin a highly negatively charged surface above the Fe2S2 cluster that interacts with P450scc (317). Thus, the same surface of ferredoxin interacts with both ferredoxin reductase and the P450 (311, 318).
Despite this constraint, creation of a three-component fusion protein of the general structure H2N-P450scc-FerredoxinReductase-Ferredoxin-COOH (termed F2) increases the enzyme's maximum velocity (Vmax) (202). Other fusion proteins have been tested, but catalysis appears to require that the ferredoxin moiety be tethered to the carboxy-terminus by a hydrophilic linker that permits rotational freedom, so that the same surface of the ferredoxin moiety can access both the P450 and the ferredoxin reductase (202, 203, 319). The requirement for ferredoxin reductase and ferredoxin is not absolute, as fusion of P450scc to microsomal P450 oxidoreductase and targeting of this fusion to the mitochondria yields an active enzyme (203). However, when P450scc, the F2 fusion, or other P450scc constructs are targeted to the ER, they are wholly inactive (203). Inactivity in the ER is unrelated to cholesterol delivery by StAR and availability of substrate, as P450scc constructs targeted to the ER remain inactive when soluble 22(R)-hydroxycholesterol is provided as substrate. Thus, the mitochondrial location is essential for the enzymatic activity of P450scc (320). The F2 fusion protein has been invaluable in studies of StAR, as it fixes the molar ratio of P450scc, ferredoxin reductase, and ferredoxin at 1:1:1, thus eliminating variation in cofactor availability as a potential confounding factor when assessing steroidogenic capacity.
HUMAN P450scc DEFICIENCY
Progesterone is needed to suppress uterine contractility during pregnancy in all mammals; absence of progesterone or interference with its action causes abortion (1). In some animals, including rodents and rabbits, the progesterone needed to maintain pregnancy is produced by the mother's corpus luteum, an ovarian steroidogenic structure that develops as a consequence of pregnancy. However, primates, ungulates, and most other mammals undergo a “luteo-placental shift” at the end of the first trimester, wherein the maternal corpus luteum involutes and placental steroidogenesis takes over as the source of progesterone. Because the placenta is a fetal tissue, one would expect that human P450scc deficiency would be incompatible with life, as it would prevent placental progesterone production, causing spontaneous abortion around the middle of pregnancy. Nevertheless, a few patients have now been described with mutations in P450scc that ablate virtually all P450scc activity (292, 294, 321, 322). It is most likely that these few fetuses with P450scc mutations reached late gestation because of unusually protracted maintenance of the maternal corpus luteum of pregnancy, which normally involutes in the second trimester. However, this has not been investigated directly. As these patients lack P450scc, they cannot produce any steroids. Absence of fetal testosterone results in 46,XY genetically male fetuses being born with female-appearing external genitalia, although the internal reproductive structures are male, not female, because the action of the anti-Mülarian hormone remains normal. Absence of glucocorticoids (cortisol) may result in poor glycemic control and poor response to stress, but it is generally compatible with life. Absence of mineralocorticoids (aldosterone) results in loss of sodium and retention of potassium and hydrogen ions, leading to hyponatremia, hyperkalemia, acidosis, dehydration, and death within a month or two of birth, if not diagnosed. Appropriate steroid hormone replacement therapy leads to long-term survival. A milder “nonclassical form” of P450scc deficiency caused by missense mutations that retain 10-20% of normal activity has been described recently (323, 324).
CONCLUSIONS AND FUTURE DIRECTIONS
Because atherosclerotic disease is so common and devastating, most research concerning cholesterol has centered on inhibiting its biosynthesis and understanding its plasma carrier proteins and their cellular uptake. Understanding the intracellular cholesterol economy, especially in steroidogenic cells, is taking longer, but the study of rare diseases, such as Wolman disease, Niemann-Pick type C disease, and congenital lipoid adrenal hyperplasia, has contributed substantially to this effort by leading to the discovery of StAR, START-domain proteins, and their relatives. The precise roles for many of the START-domain proteins, especially StarD4 and StarD5, remain under investigation, as do the molecular mechanisms by which StAR and its partners work at the OMM or by which MLN64 and its partners work at the endosomal membrane. Such an understanding is needed to permit a description of the molecular itinerary of a cholesterol molecule as it makes its way from an imported lipoprotein molecule to the guillotine of P450scc, permitting the initiation of the steroidogenic processes required for life and reproduction.
Acknowledgments
The authors thank Drs. Forbes D. Porter and Jerome F. Strauss III for their comments on the manuscript.
Footnotes
Abbreviations:
- ACTH
- adreno-corticotropic hormone
- CAH
- congenital adrenal hyperplasia
- CERT
- ceramide-transfer protein
- CRAC
- cholesterol recognition amino acid consensus domain
- CYP11A1
- cytochrome P450scc
- ER
- endoplasmic reticulum
- F2
- fusion protein 2
- HSL
- hormone-sensitive lipase
- IMM
- inner mitochondrial membrane
- LAL
- lysosomal acid lipase
- LH
- luteinizing hormone
- MENTAL
- MLN64 N-terminal
- MENTHO
- MLN64 N-terminal domain homologue
- MLN64
- metastatic lymph node, clone 64
- NPC
- Niemann-Pick type C (disease)
- OMM
- outer mitochondrial membrane
- OSBP
- oxysterol binding protein
- ORP
- oxysterol-related protein
- P450scc
- cytochrome P450 specific for cholesterol side-chain cleavage
- PAP7
- PBR-associated protein 7
- PBR
- peripheral benzodiazepine receptor
- PC
- phosphatidyl choline
- PCTP
- phosphatidyl choline transfer protein
- PE
- phosphatidyl ethanolamine
- PS
- phosphatidyl serine
- SF1
- steroidogenic factor 1
- SR-B1
- scavenger receptor B1
- SREBP
- sterol regulatory-element binding protein
- StAR
- steroidogenic acute regulatory protein
- StarD
- START domain protein
- START
- StAR-related lipid transfer
- TSPO
- mitochondrial transporter protein
- VDAC
- voltage-dependent anion channel
This work was supported by National Institutes of Health Grants DK-37922 (to W.L.M.) and HD-57876 (to H.S.B.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1. Miller W. L., Auchus R. J. 2011. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32: 81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jefcoate C. R., McNamara B. C., Artemenko I., Yamazaki T. 1992. Regulation of cholesterol movement to mitochondrial cytochrome P450scc in steroid hormone synthesis. J. Steroid Biochem. Mol. Biol. 43: 751–767. [DOI] [PubMed] [Google Scholar]
- 3. Spector A. A., Yorek M. A. 1985. Membrane lipid composition and cellular function. J. Lipid Res. 26: 1015–1035. [PubMed] [Google Scholar]
- 4. van Meer G., Voelker D. R., Feigelson G. W. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Porter F. D., Herman G. E. 2011. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 52: 6–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haberland M. E., Reynolds J. A. 1973. Self-association of cholesterol in aqueous solution. Proc. Natl. Acad. Sci. USA. 70: 2313–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lange Y., Steck T. L. 2008. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog. Lipid Res. 47: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikonen E. 2008. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9: 125–138. [DOI] [PubMed] [Google Scholar]
- 9. Chang T. Y., Chang C. C. Y., Ohgami N., Yamauch Y. 2006. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22: 129–157. [DOI] [PubMed] [Google Scholar]
- 10. Goldstein J. L., DeBose-Boyd R. A., Brown M. S. 2006. Protein sensors for membrane sterols. Cell. 124: 35–46. [DOI] [PubMed] [Google Scholar]
- 11. Steck T. L., Lange Y. 2010. Cell cholesterol homeostasis: mediation by active cholesterol. Trends Cell Biol. 20: 680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang J., Ferguson G. W. 1999. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 76: 2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mesmin B., Maxfield F. R. 2009. Intracellular sterol dynamics. Biochim. Biophys. Acta. 1791: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Radhakrishnan A., McConnell H. M. 1999. Condensed complexes of cholesterol and phospholipids. Biophys. J. 77: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Birner R., Nebauer R., Schneiter R., Daum G. 2001. Roles of phosphatidylethanoloamine and several biosynthetic pathways in Saccharomyces cerevisiae. Mol. Biol. Cell. 12: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maxfield F. R., Wüstner D. 2002. Intracellular cholesterol transport. J. Clin. Invest. 110: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simons K., Ikonen E. 1997. Functional rafts in cell membrane. Nature. 387: 569–572. [DOI] [PubMed] [Google Scholar]
- 18. Simons K., Ikonen E. 2000. How cells handle cholesterol. Science. 290: 1721–1726. [DOI] [PubMed] [Google Scholar]
- 19. Maxfield F. R., Tabas I. 2005. Role of cholesterol and lipid organization in disease. Nature. 438: 612–621. [DOI] [PubMed] [Google Scholar]
- 20. Simons K., Gerl M. J. 2010. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 11: 688–699. [DOI] [PubMed] [Google Scholar]
- 21. Lev S. 2010. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell Biol. 11: 739–750. [DOI] [PubMed] [Google Scholar]
- 22. Prinz W. A. 2010. Lipid trafficking sans vesicles: where, why, how? Cell. 143: 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simons K., Vaz W. L. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33: 269–295. [DOI] [PubMed] [Google Scholar]
- 24. García-Sáez A. J., Chiantia S., Schwille P. 2007. Effect of line tension on the lateral organization of lipid membranes. J. Biol. Chem. 282: 33537–33544. [DOI] [PubMed] [Google Scholar]
- 25. Levental I., Grzybek M., Simons K. 2010. Greasing their way: lipid modicications determine protein association with membrane rafts. Biochemistry. 49: 6305–6316. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki K. G., Fujiwara T. K., Sanematsu F., Iino R., Edidin M., Kusumi A. 2007. GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J. Cell Biol. 177: 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goswami D., Gowrishankar K., Bilgrami S., Ghosh S., Raghupathy R., Chadd R., Vishwakarma R., Rao M., Mayor S. 2008. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 135: 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boesze-Battaglia K., Schimmel R. J. 1997. Cell membrane lipid composition and distribution: implications for cell function and lessors learned from photoreceptors and platelets. J. Exp. Biol. 200: 2927–2936. [DOI] [PubMed] [Google Scholar]
- 29. Rigotti A., Miettinen H. E., Krieger M. 2003. The role of the high-density lipoprotein receptor SR-B1 in the lipid metabolism of endocrine and other tissues. Endocr. Rev. 24: 357–387. [DOI] [PubMed] [Google Scholar]
- 30. Lange Y., Ye J., Rigney M., Steck T. L. 1999. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J. Lipid Res. 40: 2264–2270. [PubMed] [Google Scholar]
- 31. Liscum L., Munn N. J. 1999. Intracelllular cholesterol transport. Biochim. Biophys. Acta. 1438: 19–37. [DOI] [PubMed] [Google Scholar]
- 32. Mukherjee S., Ghosh R. N., Maxfield E. R. 1997. Endocytosis. Physiol. Rev. 77: 759–803. [DOI] [PubMed] [Google Scholar]
- 33. Hao M., Lin S. X., Karylowski O. J., Wüstner D., McGraw T. E., Maxfield F. R. 2002. Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J. Biol. Chem. 277: 609–617. [DOI] [PubMed] [Google Scholar]
- 34. Brügger B., Sandhoff R., Wegehingel S., Gorgas K., Malsam J., Helms J. B., Lehmann W. D., Nickel W., Wieland F. T. 2000. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J. Cell Biol. 151: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klemm R. W., Ejsing C. S., Surma M. A., Kaiser H. J., Gerl M. J., Sampaio J. L., de Robillard Q., Ferguson C., Proszynski T., Shevchenko A., et al. 2009. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J. Cell Biol. 185: 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daum G. 1985. Lipids of mitochondria. Biochim. Biophys. Acta. 822: 1–42. [DOI] [PubMed] [Google Scholar]
- 37. Ardail D., Lerme F., Louisot P. 1991. Involvement of contact sites in phosphatidylserine import into liver mitochondria. J. Biol. Chem. 266: 7978–7981. [PubMed] [Google Scholar]
- 38. Ardail D., Privat J. P., Egret-Charlier M., Levrat C., Lerme F., Louisot P. 1990. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 265: 18797–18802. [PubMed] [Google Scholar]
- 39. Emoto K., Kuge O., Nishijima M., Umeda M. 1999. Isolation of a Chinese hamster ovary cell mutant defective in intramitochondrial transport of phosphatidylserine. Proc. Natl. Acad. Sci. USA. 96: 12400–12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vance J. E. 1990. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 265: 7248–7256. [PubMed] [Google Scholar]
- 41. Tontonoz P., Kim J. B., Graves R. A., Spiegelman B. M. 1993. A novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol. 13: 4753–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osborne T. F. 2000. Sterol regulatory element binding protens (SREBPs): key regulators of nutritional homeostasis. J. Biol. Chem. 275: 32379–32382. [DOI] [PubMed] [Google Scholar]
- 43. Shimomura I., Bashmakov Y., Horton J. D. 1999. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 274: 30028–30032. [DOI] [PubMed] [Google Scholar]
- 44. Shimomura I., Shimano H., Horton J. D., Goldstein J. L., Brown M. S. 1997. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J. Clin. Invest. 99: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horton J. D., Goldstein J. L., Brown M. S. 2002. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brown M. S., Goldstein J. L. 1999. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. USA. 96: 11041–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hughes A. L., Todd B. L., Espenshade P. J. 2005. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell. 120: 831–842. [DOI] [PubMed] [Google Scholar]
- 48. Rawson R. B., DeBose-Boyd R., Goldstein J. L., Brown M. S. 1999. Failure to cleave sterol regulatory element-binding proteins (SREBPs) causes cholesterol auxotrophy in Chinese hamster ovary cells with genetic absence of SREBP cleavage-activating protein. J. Biol. Chem. 274: 28549–28556. [DOI] [PubMed] [Google Scholar]
- 49. Motamed M., Zhang Y., Wang M. L., Seemann J., Kwan H. J., Goldstein J. L., Brown M. S. 2011. Identification of luminal loop of scap as the sterol sensor that maintains cholesterol homeostatis. J. Biol. Chem. 286: 18002–18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Porstmann T., Griffiths B., Chung Y. L., Delpuech O., Griffiths J. R., Downward J., Schulze A. 2005. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 24: 6465–6481. [DOI] [PubMed] [Google Scholar]
- 51. Vander Haar E., Lee S. I., Bandhakavi S., Griffin T. J., Kim D. H. 2007. Insulin signalling to mTOR by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9: 316–323. [DOI] [PubMed] [Google Scholar]
- 52. Najafi-Shoushtari S. H., Kristo F., Li Y., Shioda T., Cohen D. E., Gerszten R. E., Näär A. M. 2010. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 328: 1566–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rayner K. J., Suárez Y., Dávalo A., Parathath S., Fitzgerald M. L., Tamehiro N., Fisher E. A., Moore K. J., Fernández-Hernando C. 2010. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 328: 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Attie A. D. 2007. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem. Sci. 32: 172–179. [DOI] [PubMed] [Google Scholar]
- 55. Brown M. S., Ye J., Goldstein J. L. 2010. HDL miR-ed down by SREBP introns. Science. 328: 1495–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reaven G. M. 2005. Why Syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab. 1: 9–14. [DOI] [PubMed] [Google Scholar]
- 57. Mason J. I., Rainey W. E. 1987. Steroidogenesis in the human fetal adrenal: a role for cholesterol synthesized de novo. J. Clin. Endocrinol. Metab. 64: 140–147. [DOI] [PubMed] [Google Scholar]
- 58. Gwynne J. T., Strauss J. F., III 1982. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr. Rev. 3: 299–329. [DOI] [PubMed] [Google Scholar]
- 59. Gill S., Stevenson J., Kristiana I., Brown A. J. 2011. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab. 13: 260–273. [DOI] [PubMed] [Google Scholar]
- 60. Chaffin C. L., Dissen G. A., Stouffer R. L. 2000. Hormonal regulation of steroidogenic enzyme expression in granulosa cells during the periovulatory interval in monkeys. Mol. Hum. Reprod. 6: 11–18. [DOI] [PubMed] [Google Scholar]
- 61. Grummer R. R., Carroll D. J. 1988. A review of lipoprotein cholesterol metabolism: importance to ovarian function. J. Anim. Sci. 66: 3160–3173. [DOI] [PubMed] [Google Scholar]
- 62. LaVoie H. A., Benoit A. M., Garmey J. C., Dailey R. A., Wright D. J., Veldhuis J. D. 1997. Coordinate development expression of genes regulating sterol economy and cholesterol side-chain cleavage in the porcine ovary. Biol. Reprod. 57: 402–407. [DOI] [PubMed] [Google Scholar]
- 63. Soumano K., Price C. A. 1997. Ovarian follicular steroidogenic acute regulatory protein, low density lipoprotein receptor, and cytochrome P450 side chain cleavage messenger ribonucleic acids in cattle undergoing superovulation. Biol. Reprod. 56: 516–522. [DOI] [PubMed] [Google Scholar]
- 64. Illingworth D. R., Kenney T. A., Orwoll E. S. 1982. Adrenal function in heterozygous and homozygous hypobetalipoproteinemia. J. Clin. Endocrinol. Metab. 54: 27–33. [DOI] [PubMed] [Google Scholar]
- 65. Dobs A. S., Schrott H., Davidson M. H., Bays H., Stein E. A., Kush D., Wu M., Mitchel Y., Illingworth R. D. 2000. Effects of high-dose simvastatin on adrenal and gonadal steroidogenesis in men with hypercholesterolemia. Metabolism. 49: 1234–1238. [DOI] [PubMed] [Google Scholar]
- 66. Laue L., Hoeg J. M., Barnes K., Loriaux D. L., Chrousos G. P. 1987. The effect of mevinolin on steroidogenesis in patients with defects in the low density lipoprotein receptor pathway. J. Clin. Endocrinol. Metab. 64: 531–535. [DOI] [PubMed] [Google Scholar]
- 67. Connelly M. A., Williams D. L. 2003. SR-BI and cholesterol uptake intosteroidogenic cells. Trends Endocrinol. Metab. 14: 467–472. [DOI] [PubMed] [Google Scholar]
- 68. Liu J., Heikkila P., Meng Q. H., Kahri A. I., Tikkanen M. J., Voutilainen R. 2000. Expression of low and high density lipoprotein receptor genes in human adrenals. Eur. J. Endocrinol. 142: 677–682. [DOI] [PubMed] [Google Scholar]
- 69. Vergeer M., Korporaal S. J. A., Franssen R., Meurs I., Out R., Hovingh G. K., Hoekstra M., Sierts J. A., Dallinga-Thie G. M., Motazacker M. M., et al. 2011. Genetic variant of the scavenger receptor BI in humans. N. Engl. J. Med. 364: 136–145. [DOI] [PubMed] [Google Scholar]
- 70. Brown M. S., Kovanen P. T., Goldstein J. L. 1979. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog. Horm. Res. 35: 215–257. [DOI] [PubMed] [Google Scholar]
- 71. Anderson R. A., Sando G. N. 1991. Cloning and expression of cDNA encoding human lysosomal acid lipase/cholesterol ester hydrolase. J. Biol. Chem. 266: 22479–22484. [PubMed] [Google Scholar]
- 72. Roussel A., Canaan S., Egloff M. P., Riviere M., Dupuis L., Verger R., Cambillau C. 1999. Crystal structure of human gastric lipase and model of lysosomal acid lipase, two lipolytic enzymes of medical interest. J. Biol. Chem. 274: 16995–17002. [DOI] [PubMed] [Google Scholar]
- 73. Henson M. C., Greene S. J., Reggio B. C., Shi W., Swan K. F. 1997. Effect of reduced lipoprotein-cholesterol availability on placental progesterone biosynthesis in the baboon. Endocrinology. 138: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 74. Holst L. S., Langin D., Mulder H., Laurell H., Grober J., Bergh A., Mohrenweiser H. W., Edgren G., Holm C. 1996. Molecular cloning, genomic organization, and expression of a testicular isoform of hormone-sensitive lipase. Genomics. 35: 441–447. [DOI] [PubMed] [Google Scholar]
- 75. Kraemer F. B., Shen W. J. 2002. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 43: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 76. Kraemer F. B., Tavangar K., Hoffman A. R. 1991. Developmental regulation of hormone-sensitive lipase mRNA in the rat: changes in steroidogenic tissue. J. Lipid Res. 32: 1303–1310. [PubMed] [Google Scholar]
- 77. Holst L. S., Hoffman A. M., Mulder H., Sundler F., Holm C., Bergh A., Fredrickson G. 1994. Localization of hormone-sensitive lipase to rat Sertoli cells and its expresion in developing and degenrating testes. FEBS Lett. 355: 125–130. [DOI] [PubMed] [Google Scholar]
- 78. Golos T. G., Soto E. A., Tureck R. W., Strauss J. F., III 1985. Human chorionic gonadotropin and 8-bromo-adenosine 3′5′-monophosphate stimulate [125I] low density lipoprotein uptake and metabolism by luteinized human granulosa cells in culture. J. Clin. Endocrinol. Metab. 61: 633–638. [DOI] [PubMed] [Google Scholar]
- 79. Golos T. G., Strauss J. F., III 1988. 8-bromo adenosine cyclic 3′5′ phosphate rapidly increases 3-hydroxy-3-methylglutaryl coenzyme A reductase mRNA in human granulosa cells: Role of cellular sterol balance in controlling the response to tropic stimulation. Biochemistry. 27: 3503–3506. [DOI] [PubMed] [Google Scholar]
- 80. Klansek J. J., Warner G. J., Johnson W. J., Glick J. M. 1996. Compartmental isolation of cholesterol participating in the cytoplasmic cholesteryl ester cycle in Chinese hamster ovary 25-RA cells. J. Biol. Chem. 271: 4923–4929. [DOI] [PubMed] [Google Scholar]
- 81. Okazaki H., Igarashi M., Nishi M., Sekiya M., Tajima M., Takase S., Takanashi M., Ohta K., Tamura Y., Okazaki S., et al. 2008. Identification of neutral cholesterol ester hydrolase, a key enzyme removing cholesterol from macrophages. J. Biol. Chem. 283: 33357–33364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ohta K., Sekiya M., Uozaki H., Igarashi M., Takase S., Kumagai M., Takanashi M., Takeuchi Y., Izumida Y., Kubota M., et al. 2011. Abrogation of neutral cholesterol ester hydrolytic activity causes adrenal enlargement. Biochem. Biophys. Res. Commun. 404: 254–260. [DOI] [PubMed] [Google Scholar]
- 83. Klima H., Ullrich K., Aslanidis C., Fehringer P., Lackner K. J., Schmitz G. 1993. A splice junction mutation causes deletion of a 72-base exon from the mRNA for lysosomal acid lipase in a patient with cholesteryl ester storage disease. J. Clin. Invest. 92: 2713–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Anderson R. A., Byrum R. S., Coates P. M., Sando G. N. 1994. Mutations at the lysosomal acid cholesteryl ester hydrolase gene locus in Wolman disease. Proc. Natl. Acad. Sci. USA. 91: 2718–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pagani F., Pariyarath R., Garcia R., Stuani C., Burlina A. B., Ruotolo G., Rabusin M., Baralle F. E. 1998. New lysosomal acid lipase gene mutants explain the phenotype of Wolman disease and cholesteryl ester storage disease. J. Lipid Res. 39: 1382–1388. [PubMed] [Google Scholar]
- 86. Lohse P., Maas S., Lohse P., Sewell A. C., van Diggelen O. P., Seidel D. 1999. Molecular defects underlying Wolman disease appear to be more heterogeneous than those resulting in cholesteryl ester storage disease. J. Lipid Res. 40: 221–228. [PubMed] [Google Scholar]
- 87. Zschenker O., Jung N., Rethmeier J., Trautwein S., Hertel S., Zeigler M., Ameis D. 2001. Characterization of lysosomal acid lipase mutations in the signal peptide and mature polypeptide region causing Wolman disease. J. Lipid Res. 42: 1033–1040. [PubMed] [Google Scholar]
- 88. Pisciotta L., Fresa R., Bellocchio A., Pino E., Guido V., Cantafora A., DiRocco M., Calandra S., Bertolini S. 2009. Cholesteryl ester storage disease (CESD) due to novel mutations in the LIPA gene. Mol. Genet. Metab. 97: 143–148. [DOI] [PubMed] [Google Scholar]
- 89. Perry R., Kecha O., Paquette J., Huot C., Van Vliet G., Deal C. 2005. Primary adrenal insufficiency in children: twenty years experience at the Sainte-Justine Hospital, Montreal. J. Clin. Endocrinol. Metab. 90: 3243–3250. [DOI] [PubMed] [Google Scholar]
- 90. Stein J., Garty B., Dror Y., Fenig E., Zeigler M., Yaniv I. 2007. Successful treatment of Wolman disease by unrelated umbilical cord blood transplantation. Eur. J. Pediatr. 166: 663–666. [DOI] [PubMed] [Google Scholar]
- 91. Tolar J., Petryk A., Khan K., Bjoraker K. J., Jessurun J., Dolan M., Kivisto T., Charnas L., Shapiro E. G., Orchard P. J. 2009. Long-term metabolic, endocrine and neuropsychological outcome of hematopoetic cell transplantation for Wolman disease. Bone Marrow Transplant. 43: 21–27. [DOI] [PubMed] [Google Scholar]
- 92. Gramatges M. M., Dvorak C. C., Regula D. P., Enns G. M., Weinberg K., Agarwal R. 2009. Pathological evidence of Wolman's disease following hematopoetic stem cell transplantation despite correction of lysosomal acid lipase activity. Bone Marrow Transplant. 44: 449–450. [DOI] [PubMed] [Google Scholar]
- 93. Osuga J., Ishibashi S., Oka T., Yagyu H., Tozawa R., Fujimoto A., Shionoiri F., Yahagi N., Kraemer F. B., Tsutsumi O., et al. 2000. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc. Natl. Acad. Sci. USA. 97: 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kraemer F. B., Shen W. J., Harada K., Patel S., Osuga J. L., Ishibashi S., Azhar S. 2004. Hormone-sensitive lipase is required for HDL cholestyryl ester-supported adrenal steroidogenesis. Mol. Endocrinol. 18: 549–557. [DOI] [PubMed] [Google Scholar]
- 95. Reid P. C., Sugii S., Chang T. Y. 2003. Trafficking defects in endogenously synthesized cholesterol in fibroblasts, macrophages, hepatocytes and glial cells from Niemann-Pick type C1 mice. J. Lipid Res. 44: 1010–1019. [DOI] [PubMed] [Google Scholar]
- 96. Chang T. Y., Reid P. C., Sugii S., Ohgami N., Cruz J. C., Chang C. C. Y. 2005. Niemann-Pick type C disease and intracellular cholesterol trafficking. J. Biol. Chem. 280: 20917–20920. [DOI] [PubMed] [Google Scholar]
- 97. Vanier M. T., Millat G. 2003. Niemann-Pick disease type C. Clin. Genet. 64: 269–281. [DOI] [PubMed] [Google Scholar]
- 98. Shulman L. M., David N. J., Weiner W. J. 1995. Psychosis as the initial manifestation of adult-onset Niemann-Pick disease type C. Neurology. 45: 1739–1743. [DOI] [PubMed] [Google Scholar]
- 99. Porter F. D., Scherrer D. E., Lanier M. H., Langmade S. J., Molugu V., Gale S. E., Olzeski D., Sidhu R., Dietzen D. J., Fu R., et al. 2010. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jiang X., Sidhu R., Porter F. D., Yanjanin N. M., Speak A. O., te Vruchte D. T., Platt F. M., Fujiwara H., Scherrer D. E., Zhang J., et al. 2011. A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J. Lipid Res. 52: 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pentchev P. G., Comly M. E., Kruth H. S., Patel S., Proestel M., Weintroub H. 1986. The cholesterol storage disorder of the mutant BALB/c mouse: a primary genetic lesion closely linked to defective esterification of exogenously derived cholesterol and its relationship to human type C Niemann-Pick disease. J. Biol. Chem. 261: 2772–2777. [PubMed] [Google Scholar]
- 102. Akaboshi S., Yano T., Miyawaki S., Ohno K., Takeshita K. A. 1997. A C57BL/KsJ mouse model of Niemann-Pick disease (spm) belongs to the same complementation group as the major childhood type of Niemann-Pick disease type C. Hum. Genet. 99: 350–353. [DOI] [PubMed] [Google Scholar]
- 103. Schedin S., Sindelar P. J., Pentchev P., Brunk U., Dallner G. 1997. Peroxisomal impairment in Niemann-Pick type C disease. J. Biol. Chem. 272: 6245–6251. [DOI] [PubMed] [Google Scholar]
- 104. Sakai Y., Miyawaki S., Shimizu A., Ohno K., Watanabe T. 1991. A molecular genetic linkage map of mouse chromosome 18, including spm, Grl-1, Fim-2/c-fms, and Mbp. Biochem. Genet. 29: 103–113. [DOI] [PubMed] [Google Scholar]
- 105. Carstea E. D., Polymeropoulos M. H., Parker C. C., Detera-Wadleigh S. D., O'Neill R. R., Patterson M. C., Goldin E., Xiao H., Straub R. E., Vanier M. T., et al. 1993. Linkage of Niemann-Pick disease type C to human chromosome 18. Proc. Natl. Acad. Sci. USA. 90: 2002–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Carstea E. D., Morris J. A., Coleman K. G., Loftus S. K., Zhang D., Cummings C., Gu J., Rosenfeld M. A., Pavan W. J., Krizman D. B., et al. 1997. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 277: 228–231. [DOI] [PubMed] [Google Scholar]
- 107. Naureckiene S., Sleat D., Lackland H., Fensom A., Vanier M. T., Wattiaux R., Jadot M., Lobel P. 2000. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 290: 2298–2301. [DOI] [PubMed] [Google Scholar]
- 108. Infante R. E., Abi-Mosleh L., Radhakrishnan A., Dale J. D., Brown M. S., Goldstein J. L. 2008. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J. Biol. Chem. 283: 1052–1063. [DOI] [PubMed] [Google Scholar]
- 109. Friedland C. J., Liou H. L., Lobel P., Stock A. M. 2003. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc. Natl. Acad. Sci. USA. 100: 2512–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ko D. C., Binkley J., Sidow A., Scott M. P. 2003. The integrity of a cholesterol-binding pocket in Niemann-Pick C2 protein is necessary to control lysosome cholesterol levels. Proc. Natl. Acad. Sci. USA. 100: 2518–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gruber A., Mancek M., Wagner H., Kirschning C. J., Jerala R. 2004. Structural model of MD-2 and functional role of its basic amino acid clusters involved in cellular lipopolysaccharide recognition. J. Biol. Chem. 279: 28475–28482. [DOI] [PubMed] [Google Scholar]
- 112. Infante R. E., Radhakrishnan A., Abi-Mosleh L., Kinch L. N., Wang M. L., Grishin N. V., Goldstein J. L., Brown M. S. 2008. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J. Biol. Chem. 283: 1064–1075. [DOI] [PubMed] [Google Scholar]
- 113.Deleted in proof.
- 114. Xu S., Benoff B., Liou H. L., Lobel P., Stock A. M. 2007. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J. Biol. Chem. 282: 23525–23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kwon H. J., Abi-Mosleh L., Wang M. L., Deisenhofer J., Goldstein J. L., Brown M. S., Infante R. E. 2009. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 137: 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cheruku S. R., Xu Z., Dutia R., Lobel P., Storch J. 2006. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J. Biol. Chem. 281: 31594–31604. [DOI] [PubMed] [Google Scholar]
- 117. Infante R. E., Wang M. L., Radhakrishnan A., Kwon H. J., Brown M. S., Goldstein J. L. 2008. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl. Acad. Sci. USA. 105: 15287–15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang M. L., Motamed M., Infante R. E., Abi-Mosleh L., Kwon H. J., Brown M. S., Goldstein J. L. 2010. Identification of surface residues on Niemann-Pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes. Cell Metab. 12: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Platt F. M., Neises G. R., Dwek R. A., Butters T. D. 1994. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J. Biol. Chem. 269: 8362–8365. [PubMed] [Google Scholar]
- 120. Patterson M. C., Vecchio D., Prady H., Abel L., Wraith J. E. 2007. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 6: 765–772. [DOI] [PubMed] [Google Scholar]
- 121. Griffin L. D., Gong W., Verot L., Mellon S. H. 2004. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 10: 704–711. [DOI] [PubMed] [Google Scholar]
- 122. Ahmad I., Lope-Piedrafita S., Bi X., Hicks C., Yao Y., Yu C., Chaitkin E., Howison C. M., Weberg L., Trouard T. P., et al. 2005. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann-Pick C mice. J. Neurosci. Res. 82: 811–821. [DOI] [PubMed] [Google Scholar]
- 123. Langmade S. J., Gale S. E., Frolov A., Mohri I., Suzuki K., Mellon S. H., Walkley S. U., Covey D. F., Schaffer J. E., Ory D. S. 2006. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc. Natl. Acad. Sci. USA. 103: 13807–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zampieri S., Mellon S. H., Butters T. D., Nevyjel M., Covey D. F., Bembi B., Dardis A. 2009. Oxidative stress in NPC1 deficient cells: protective effect of allopregnanolone. J. Cell. Mol. Med. 13: 3786–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liu B., Turley S. D., Burns D. K., Miller A. M., Repa J. J., Dietschy J. M. 2009. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse. Proc. Natl. Acad. Sci. USA. 106: 2377–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ramirez C. M., Li B. L., Taylor A. M., Repa J. J., Burns D. K., Weinberg A. G., Turley S. D., Dietschy J. M. 2010. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearlyevery organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr. Res. 68: 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rosenbaum A. I., Zhang G., Warren J. D., Maxfield F. R. 2010. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc. Natl. Acad. Sci. USA. 107: 5477–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pipalia N. H., Cosner C. C., Huang A., Chaterjee A., Bourbon P., Farley N., Helquist P., Wiest O., Maxfield F. R. 2011. Histone deacetylase treatment dramatically reduces cholesterol accumulation in Niemann-Pick type C1 mutant human fibrobrlasts. Proc. Natl. Acad. Sci. USA. 108: 5620–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tomasetto C., Regnier C., Moog-Lutz C., Mattei M. G., Chenard M. P., Lidereau R., Basset P., Rio M. C. 1995. Identification of four novel human genes amplified and overexpressed in breast carcinoma and localized to the q11-q21.3 region of chromosome 17. Genomics. 28: 367–376. [DOI] [PubMed] [Google Scholar]
- 130. Zhang M., Liu P., Dwyer N. K., Christenson L. K., Fujimoto T., Martinez F., Comly M., Hanover J. A., Blanchette-Mackie E. J., Strauss III J. F. 2002. MLN64 mediates mobilization of lysosomal cholesterol to steroidogenic mitochondria. J. Biol. Chem. 277: 33300–33310. [DOI] [PubMed] [Google Scholar]
- 131. Alpy F., Stoeckel M. E., Dierich A., Escola J. M., Wendling C., Chenard M. P., Vanier M. T., Gruenberg J., Tomasetto C., Rio M. C. 2001. The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein. J. Biol. Chem. 276: 4261–4269. [DOI] [PubMed] [Google Scholar]
- 132. Alpy F., Boulay A., Moog-Lutz C., Andarawewa K. L., Degot S., Stoll I., Rio M. C., Tomasetto C. 2003. Metastatic lymph node 64 (MLN64), a gene overexpressed in breast cancers, is regulated by Sp/KLF transcription factors. Oncogene. 22: 3770–3780. [DOI] [PubMed] [Google Scholar]
- 133. Alpy F., Wendling C., Rio M. C., Tomasetto C. 2002. MENTHO, a MLN64 homologue devoid of the START domain. J. Biol. Chem. 277: 50780–50787. [DOI] [PubMed] [Google Scholar]
- 134. Moog-Lutz C., Tomasetto C., Régnier C. H., Wendling C., Lutz Y., Muller D., Chenard M. P., Basset P., Rio M. C. 1997. MLN64 exhibits homology with the steroidogenic acute regulatory protein (StAR) and is over-expressed in human breast carcinomas. Int. J. Cancer. 71: 183–191. [DOI] [PubMed] [Google Scholar]
- 135. Watari H., Arakane F., Moog-Lutz C., Callen C. B., Tomasetto C., Gerton G. L., Rio M. C., Baker M. E., Strauss J. F., III 1997. MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc. Natl. Acad. Sci. USA. 94: 8462–8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Stocco D. M., Clark B. J. 1996. Regulation of the acute production of steroids in steroidogenic cells. Endocr. Rev. 17: 221–244. [DOI] [PubMed] [Google Scholar]
- 137. Alpy F., Tomasetto C. 2005. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J. Cell Sci. 118: 2791–2801. [DOI] [PubMed] [Google Scholar]
- 138. Ponting C. P., Aravind L. 1999. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem. Sci. 24: 130–132. [DOI] [PubMed] [Google Scholar]
- 139. Iyer L. M., Koonin E. V., Aravind L. 2001. Adaptations of the helix-grip fold for ligand binding and catalysis in the START domain superfamily. Proteins. 43: 134–144. [DOI] [PubMed] [Google Scholar]
- 140. Alpy F., Tomasetto C. 2006. MLN64 and MENTHO, two mediators of endosomal cholesterol transport. Biochem. Soc. Trans. 34: 343–345. [DOI] [PubMed] [Google Scholar]
- 141. Sugawara T., Holt J. A., Driscoll D., Strauss J. F., III, Lin D., Miller W. L., Patterson D., Clancy K. P., Hart I. M., Clark B. J., et al. 1995. Human steroidogenic acute regulatory protein (StAR): functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and an expressed pseudogene to chromosome 13. Proc. Natl. Acad. Sci. USA. 92: 4778–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sugawara T., Lin D., Holt J. A., Martin K. O., Javitt N. B., Miller W. L., Strauss J. F., III 1995. The structure of the human steroidogenic acute regulatory (StAR) protein gene: StAR stimulates mitochondrial cholesterol 27-hydroxylase activity. Biochemistry. 34: 12506–12512. [DOI] [PubMed] [Google Scholar]
- 143. Bose H. S., Whittal R. M., Huang M. C., Baldwin M. A., Miller W. L. 2000. N-218 MLN64, a protein with StAR-like steroidogenic activity, is folded and cleaved similarly to StAR. Biochemistry. 39: 11722–11731. [DOI] [PubMed] [Google Scholar]
- 144. Bose H. S., Lingappa V. R., Miller W. L. 2002. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 417: 87–91. [DOI] [PubMed] [Google Scholar]
- 145. Olvera-Sanchez S., Espinosa-Garcia M. T., Monreal J., Flores-Herrera O., Martinez F. 2011. Mitochondrial heat shock protein participates in placental steroidogenesis. Placenta. 32: 222–229. [DOI] [PubMed] [Google Scholar]
- 146. Li B., Vachali P., Frederick J. M., Bernstein P. S. 2011. Identification of StARD3 as a lutein-binding protein in the macula densa of the primate retina. Biochemistry. 50: 2541–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kishida T., Kostetskii I., Zhang Z., Martinez F., Liu P., Walkley S. U., Dwyer N. K., Blanchette-Mackie E. J., Radice G. L., Strauss J. F., III 2004. Targeted mutation of the MLN64 START domain causes only modest alterations in cellular sterol metabolism. J. Biol. Chem. 279: 19276–19285. [DOI] [PubMed] [Google Scholar]
- 148. Charman M., Barry K. E., Osborne N., Karten B. 2010. MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein. J. Lipid Res. 51: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Goodman J. M. 2008. The gregarious lipid droplet. J. Biol. Chem. 283: 28005–28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Stemberger B. H., Walsh R. M., Paton S. 1984. Morphometric evaluation of lipid droplet associations with secretory vesicles, mitochondria and other components in the lactating cells. Cell Tissue Res. 236: 471–475. [DOI] [PubMed] [Google Scholar]
- 151. Zehmer J. K., Huang Y., Peng G., Pu J., Anderson R. G., Liu P. 2009. A role of lipid droplet in intermembrane lipid traffic. Proteomics. 9: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Murphy S., Martin S., Patron R. G. 2009. Lipid droplet-organelle interactions; sharing the facts. Biochim. Biophys. Acta. 1791: 441–447. [DOI] [PubMed] [Google Scholar]
- 153. Boström P., Andersson L., Rutberg M., Perman J., Lidberg U., Johansson B. R., Fernandez-Rodriguez J., Ericson J., Nilsson T., Borén J., et al. 2007. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat. Cell Biol. 9: 1286–1293. [DOI] [PubMed] [Google Scholar]
- 154. Steegmaier M., Oorschot V., Klumperman J., Scheller R. H. 2000. Syntaxin 17 is abundant in steroidogenic cells and implicated in smooth endoplasmic reticlum membrane dynamics. Mol. Biol. Cell. 11: 2719–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Grant N. J., Hepp R., Krause W., Aunis D., Oehme P., Langley K. 1999. Differential expression of SNAP-25 isoforms and SNAp-23 in the adrenal gland. J. Neurochem. 72: 363–372. [DOI] [PubMed] [Google Scholar]
- 156. Grosse J., Bulling A., Brucker C., Berg U., Amsterdam A., Mayerhofer A., Gratzl M. 2000. Synaptosome-associated protein of 25 kilodaltons in oocytes and steroid-producing cells of rat and human ovary: molecular analysis and regulation of gonadotropins. Biol. Reprod. 63: 643–650. [DOI] [PubMed] [Google Scholar]
- 157. Jo M., Gieske M. C., Payne C. E., Wheeler-Price S. E., Gieske J. B., Ignatius I. V., Curry T. E., Ko C. 2004. Development and application of a rat ovarian gene expression database. Endocrinology. 145: 5384–5396. [DOI] [PubMed] [Google Scholar]
- 158. Shimada M., Yanai Y., Okazaki T., Yamashita Y., Sriman V., Wilson W. C., Richards J. C. 2007. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol. Endocrinol. 21: 2487–2502. [DOI] [PubMed] [Google Scholar]
- 159. Vahouny G. V., Chanderbhan R., Noland B. J., Irwin D., Dennis P., Lambeth J. D., Scallen T. J. 1983. Sterol carrier protein 2: identification of adrenal sterol carrier protein 2 and site of action for mitochondrial cholesterol utilization. J. Biol. Chem. 258: 11731–11737. [PubMed] [Google Scholar]
- 160. Seedorf U., Ellinghaus P., Nofer J. R. 2000. Sterol carrier protein-2. Biochim. Biophys. Acta. 1486: 45–54. [DOI] [PubMed] [Google Scholar]
- 161. Gallegos A. M., Atshaves B. P., Storey S. M., Starodub O., Petrescu A. D., Huang H., McIntosh A. L., Martin G. G., Chao H., Kier A. B., et al. 2001. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog. Lipid Res. 40: 498–563. [DOI] [PubMed] [Google Scholar]
- 162. Choinowski T., Hauser H., Piontek K. 2000. Structure of sterol carrier protein 2 at 1.8 Å resolution reveals a hydrophobic tunnel suitable for lipid binding. Biochemistry. 39: 1897–1902. [DOI] [PubMed] [Google Scholar]
- 163. García F. L., Szyperski T., Dyer J. H., Choinowski T., Seedorf U., Hauser H., Wüthrich K. 2000. NMR structure of the sterol carrier protein-2: implications for the biological role. J. Mol. Biol. 295: 595–603. [DOI] [PubMed] [Google Scholar]
- 164. Seedorf U., Raabe M., Ellinghaus P., Kannenberg F., Fobker M., Engel T., Denis S., Wouters F., Wirtz K. W. A., Wanders R. J. A., et al. 1998. Defective peroxisomal catabolism of branched fatty acyl coenzymeA in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes Dev. 12: 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Fuchs M., Hafer A., Munch C., Kannenberg F., Teichmann S., Scheibner J., Stange E. F., Seedorf U. 2001. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J. Biol. Chem. 276: 48058–48065. [DOI] [PubMed] [Google Scholar]
- 166. Soccio R. E., Adams R. M., Romanowski M. J., Sehayek E., Burley S. K., Breslow J. L. 2002. The cholesterol-regulated StarD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6. Proc. Natl. Acad. Sci. USA. 99: 6943–6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Soccio R. E., Breslow J. L. 2003. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J. Biol. Chem. 278: 22183–22186. [DOI] [PubMed] [Google Scholar]
- 168. Soccio R. E., Adams R. M., Maxwell K. N., Breslow J. L. 2005. Differential gene regulation of StarD4 and StarD5 cholesterol transfer proteins. Activation of StarD4 by sterol regulatory element-binding protein-2 and StarD5 by endoplasmic reticulum stress. J. Biol. Chem. 280: 19410–19418. [DOI] [PubMed] [Google Scholar]
- 169. Bose H. S., Whittal R. M., Ran Y., Bose M., Baker B. Y., Miller W. L. 2008. StAR-like activity and molten globule behavior of StARD6, a male germ-line protein. Biochemistry. 47: 2277–2288. [DOI] [PubMed] [Google Scholar]
- 170. Dawson P. A., Ridgway N. D., Slaughter C. A., Brown M. S., Goldstein J. L. 1989. Cloning and expression of oxysterol-binding protein, an oligomer with potential leucine zipper. J. Biol. Chem. 264: 16798–16803. [PubMed] [Google Scholar]
- 171. Olkkonen V. M., Levine T. P. 2004. Oxysterol binding proteins: in more than one place at one time? Biochem. Cell Biol. 82: 87–98. [DOI] [PubMed] [Google Scholar]
- 172. Beh C. T., Cool L., Philips J., Rine J. 2001. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics. 157: 1117–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Beh C. T., Rine J. 2004. A role for yeast oxysterol-binding protein homologs in endocytosis in the maintenance of intracellular sterol-lipid distribution. J. Cell Sci. 117: 2983–2996. [DOI] [PubMed] [Google Scholar]
- 174. Yang H. 2006. Nonvesicular sterol transport: two protein families and a sterol sensor? Trends Cell Biol. 16: 427–432. [DOI] [PubMed] [Google Scholar]
- 175. Roderick S. L., Chan W. W., Agate D. S., Olsen L. R., Vetting M. W., Rajashankar K. R., Cohen D. E. 2002. Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nat. Struct. Biol. 9: 507–511. [DOI] [PubMed] [Google Scholar]
- 176. Rodriguez-Agudo D., Ren S., Hylemon P. B., Redford K., Natarajan R., Del Castillo A., Gil G., Pandak W. M. 2005. Human StARD5, a cytosolic StAR-related binding protein. J. Lipid Res. 46: 1615–1623. [DOI] [PubMed] [Google Scholar]
- 177. Rodriguez-Agudo D., Ren S., Wong E., Marques D., Redford K., Gil G., Hylemon P. B., Pandak W. M. 2008. Intracellular transporter StARD4 binds cholesterol and increases cholesteryl ester formation. J. Lipid Res. 49: 1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Horibata Y., Sugimoto H. 2010. StarD7 mediates the intracellular trafficking of phosphatidylcholine to mitochondria. J. Biol. Chem. 285: 7358–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Rena V., Flores-Martín J., Angeletti S., Panzetta-Dutari G. M., Genti-Raimondi S. 2011. StarD7 gene expression in trophoblast cells: contribution of SF-1 and Wnt-catenin signaling. Mol. Endocrinol. 25: 1364–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Rodriguez-Agudo D., Calderon-Dominguez M., Ren S., Marquez D., Redford K., Medina-Torres M. A., Hylemon P., Gil G., Pandak W. M. 2011. Subcellular localization and regulation of StarD4 protein in macrophages and fibroblasts. Biochim. Biophys. Acta. 1811: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Arakane F., Sugawara T., Nishino H., Liu Z., Holt J. A., Pain D., Stocco D. M., Miller W. L., Strauss J. F., III 1996. Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial targeting sequence: Implications for the mechanism of StAR action. Proc. Natl. Acad. Sci. USA. 93: 13731–13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Riegelhaupt J. J., Waase M. P., Garbarino J., Cruz D. E., Breslow J. L. 2010. Targeted disruption of steroidogenic acute regulatory protein D4 leads to modest weight reduction and minor alterations in lipid metabolism. J. Lipid Res. 51: 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ferguson J. J. 1963. Protein synthesis and adrenocorticotropin responsiveness. J. Biol. Chem. 238: 2754–2759. [PubMed] [Google Scholar]
- 184. Garren L. D., Ney R. L., Davis W. W. 1965. Studies on the role of protein synthesis in the regulation of corticosterone production by ACTH in vivo. Proc. Natl. Acad. Sci. USA. 53: 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Davis W. W., Garren L. D. 1968. On the mechanism of action of adrenocorticotropic hormone. The inhibitory site of cycloheximide in the pathway of steroid biosynthesis. J. Biol. Chem. 243: 5153–5157. [PubMed] [Google Scholar]
- 186. Voutilainen R., Miller W. L. 1987. Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain cleavage enzyme, P450scc, in human steroidogenic tissues. Proc. Natl. Acad. Sci. USA. 84: 1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mesiano S., Mellon S. H., Jaffe R. B. 1993. Mitogenic action, regulation, and localization of insulin-like growth factors in the human fetal adrenal gland. J. Clin. Endocrinol. Metab. 76: 968–976. [DOI] [PubMed] [Google Scholar]
- 188. Mesiano S., Mellon S. H., Gospodarowicz D., Di Blasio A. M., Jaffe R. B. 1991. Basic fibroblast growth factor expression is regulated by corticotropin in the human fetal adrenal: a model for adrenal growth regulation. Proc. Natl. Acad. Sci. USA. 88: 5428–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Coulter C. L., Read L. C., Carr B. R. 1996. A role for epidermal growth factor in the morphological and functional maturation of the adrenal gland in the fetal rhesus monkey in vivo. J. Clin. Endocrinol. Metab. 81: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 190. Moore C. C. D., Brentano S. T., Miller W. L. 1990. Human P450scc gene transcription is induced by cyclic AMP and repressed by 12-O-tetradecanolyphorbol-13-acetate and A23187 by independent cis-elements. Mol. Cell. Biol. 10: 6013–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Moore C. C. D., Hum D. W., Miller W. L. 1992. Identification of positive and negative placental-specific basal elements, a transcriptional repressor, and a cAMP response element in the human gene for P450scc. Mol. Endocrinol. 6: 2045–2058. [DOI] [PubMed] [Google Scholar]
- 192. Arakane F., King S. R., Du Y., Kallen C. B., Walsh L. P., Watari H., Stocco D. M., Strauss J. F., III 1997. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J. Biol. Chem. 272: 32656–32662. [DOI] [PubMed] [Google Scholar]
- 193. Jo Y., King S. R., Khan S. A., Stocco D. M. 2005. Involvement of protein kinase C and cyclic adenosine 3′,5′-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol. Reprod. 73: 244–255. [DOI] [PubMed] [Google Scholar]
- 194. Stocco D. M., Wang X. J., Jo Y., Manna P. R. 2005. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol. Endocrinol. 19: 2647–2659. [DOI] [PubMed] [Google Scholar]
- 195. Pon L. A., Orme-Johnson N. R. 1986. Acute stimulation of steroidogenesis in corpus luteum and adrenal cortex by peptide hormones. J. Biol. Chem. 261: 6594–6599. [PubMed] [Google Scholar]
- 196. Pon L. A., Hartigan J. A., Orme-Johnson N. R. 1986. Acute ACTH regulation of adrenal corticosteroid biosynthesis: rapid accumulation of a phosphoprotein. J. Biol. Chem. 261: 13309–13316. [PubMed] [Google Scholar]
- 197. Epstein L. F., Orme-Johnson N. R. 1991. Regulation of steroid hormone biosynthesis: identification of precursors of a phosphoprotein targeted to the mitochondrion in stimulated rat adrenal cortex cells. J. Biol. Chem. 266: 19739–19745. [PubMed] [Google Scholar]
- 198. Stocco D. M., Sodeman T. C. 1991. The 30 kDa mitochondrial protein induced by hormone stimulation in MA-10 mouse Leydig tumor cells are processed from larger precursors. J. Biol. Chem. 266: 19731–19738. [PubMed] [Google Scholar]
- 199. Clark B. J., Wells J., King S. R., Stocco D. M. 1994. The purification, cloning and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J. Biol. Chem. 269: 28314–28322. [PubMed] [Google Scholar]
- 200. Lin D., Sugawara T., Strauss J. F., III, Clark B. J., Stocco D. M., Saenger P., Rogol A., Miller W. L. 1995. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 267: 1828–1831. [DOI] [PubMed] [Google Scholar]
- 201. Bose H. S., Sugawara T., Strauss J. F., III, Miller W. L. 1996. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N. Engl. J. Med. 335: 1870–1878. [DOI] [PubMed] [Google Scholar]
- 202. Harikrishna J. A., Black S. M., Szklarz G. D., Miller W. L. 1993. Construction and function of fusion enzymes of the human cytochrome P450scc system. DNA Cell Biol. 12: 371–379. [DOI] [PubMed] [Google Scholar]
- 203. Black S. M., Harikrishna J. A., Szklarz G. D., Miller W. L. 1994. The mitochondrial environment is required for activity of the cholesterol side-chain cleavage enzyme, cytochrome P450scc. Proc. Natl. Acad. Sci. USA. 91: 7247–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Pilon N., Daneau I., Brisson C., Ethier J-F., Lussier J. G., Silversides D. W. 1997. Porcine and bovine steroidogenic acute regulatory protein (StAR) gene expression during gestation. Endocrinology. 138: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 205. Miller W. L. 2007. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim. Biophys. Acta. 1771: 663–676. [DOI] [PubMed] [Google Scholar]
- 206. Miller W. L. 2007. StAR search--what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol. Endocrinol. 21: 589–601. [DOI] [PubMed] [Google Scholar]
- 207. Tsujishita Y., Hurley J. H. 2000. Structure and lipid transport mechanism of a StAR-related domain. Nat. Struct. Biol. 7: 408–414. [DOI] [PubMed] [Google Scholar]
- 208. Romanowski M. J., Soccio R. E., Breslow J. L., Burley S. K. 2002. Crystal structure of the Mus musculus cholesterol-regulated START protein 4 (StarD4) containing a StAR-related lipid transfer domain. Proc. Natl. Acad. Sci. USA. 99: 6949–6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mathieu A. P., Fleury A., Duchame L., Lavigme P., LeHoux J. G. 2002. Insights into steroidogenic acute regulatory protein (StAR)-dependent cholesterol transfer in mitochondria: evidence from molecular modeling and structure-based thermodynamics supporting the existence of partially unfolded states of StAR. J. Mol. Endocrinol. 29: 327–345. [DOI] [PubMed] [Google Scholar]
- 210. Yaworsky D. C., Baker B. Y., Bose H. S., Best K. B., Jensen L. B., Bell J. D., Baldwin M. A., Miller W. L. 2005. pH-dependent interactions of the carboxyl-terminal helix of steroidogenic acute regulatory protein with synthetic membranes. J. Biol. Chem. 280: 2045–2054. [DOI] [PubMed] [Google Scholar]
- 211. Murcia M., Faraldo-Gomez J. D., Maxfield F. R., Roux B. 2006. Modelling the structure of the StART domains of MLN64 and StAR proteins in complex with cholesterol. J. Lipid Res. 47: 2614–2630. [DOI] [PubMed] [Google Scholar]
- 212. Thorsell A. G., Lee W. H., Persson C., Siponen M. I., Nilsson M., Busam R. D., Kotenyova T., Schüler H., Lethio L. 2011. Comparative structural analysis of lipid binding START domains. PLoS ONE. 6: e19521–e19532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Baker B. Y., Yaworsky D. C., Miller W. L. 2005. A pH-dependent molten globule transition is required for activity of the steroidogenic acute regulatory protein, StAR. J. Biol. Chem. 280: 41753–41760. [DOI] [PubMed] [Google Scholar]
- 214. Bose H. S., Whittal R. M., Baldwin M. A., Miller W. L. 1999. The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. Proc. Natl. Acad. Sci. USA. 96: 7250–7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Bose H. S., Whittal R. M., Bose M., Debnath D. 2009. Hydrophopbic core of the steroidogenic acute regulatory protein for cholesterol transport. Biochemistry. 48: 1198–1209. [DOI] [PubMed] [Google Scholar]
- 216. Bose H. S., Whittal R. M., Debnath D., Bose M. 2009. Steroidogenic acute regulatory protein has a more open conformation than the independently folded domains. Biochemistry. 48: 11630–11639. [DOI] [PubMed] [Google Scholar]
- 217. Christensen K., Bose H. S., Harris F. M., Miller W. L., Bell J. D. 2001. Binding of StAR to synthetic membranes suggests an active molten globule. J. Biol. Chem. 276: 17044–17051. [DOI] [PubMed] [Google Scholar]
- 218. Song M., Shao K., Mujeeb A., James T. L., Miller W. L. 2001. Molten globule structure and membrane binding of the N-terminal protease-resistant domain (63–193) of the steroidogenic acute regulatory protein (StAR). Biochem. J. 356: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Tuckey R. C., Headlam M. J., Bose H. S., Miller W. L. 2002. Transfer of cholesterol between phospholipid vesicles mediated by the steroidogenic acute regulatory protein (StAR). J. Biol. Chem. 277: 47123–47128. [DOI] [PubMed] [Google Scholar]
- 220. Bose H. S., Sato S., Aisenberg J., Shalev S. A., Matsuo N., Miller W. L. 2000. Mutations in the steroidogenic acute regulatory protein (StAR) in six patients with congenital lipoid adrenal hyperplasia. J. Clin. Endocrinol. Metab. 85: 3636–3639. [DOI] [PubMed] [Google Scholar]
- 221. Baker B. Y., Epand R. F., Epand R. M., Miller W. L. 2007. Cholesterol binding does not predict activity of the steroidogenic acute regulatory protein. J. Biol. Chem. 282: 10223–10232. [DOI] [PubMed] [Google Scholar]
- 222. Bose M., Whittal R. M., Miller W. L., Bose H. S. 2008. Steroidogenic activity of StAR requires contact with mitochondrial VDAC1 and phosphate carrier protein. J. Biol. Chem. 283: 8837–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Besman M. J., Yanagibashi K., Lee T. D., Kawamura M., Hall P. F., Shively J.E. 1989. Identification of des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent adrenal steroidogenesis-stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor. Proc. Natl. Acad. Sci. USA. 86: 4897–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Papadopoulos V., Baraldi M., Guilatte T. R., Knudsen T. B., Lacapere J. L., Lindermann P., Nonenberg M. D., Nutt D., Weizman A., Zhang M. R., et al. 2006. Translocator protein (18 kDa): new nomenclature for the peripheral type benzodiazapine type receptors-based on its structure and molecular function. Trends Pharmacol. Sci. 27: 402–409. [DOI] [PubMed] [Google Scholar]
- 225. Papadopoulos V. 1993. Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: biological role in steroidogenic cell function. Endocr. Rev. 14: 222–240. [DOI] [PubMed] [Google Scholar]
- 226. Hauet T., Yao Z. X., Bose H. S., Wall C. T., Han Z., Li W., Hales D. B., Miller W. L., Culty M., Papadopoulos V. 2005. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into Leydig cell mitochondria. Mol. Endocrinol. 19: 540–554. [DOI] [PubMed] [Google Scholar]
- 227. Liu J., Rone M. B., Papadopoulos V. 2006. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J. Biol. Chem. 281: 38879–38893. [DOI] [PubMed] [Google Scholar]
- 228. Li H., Yao Z., Degenhardt B., Teper G., Papadopoulos V. 2001. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc. Natl. Acad. Sci. USA. 98: 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Krueger K. E., Papadopoulos V. 1992. Mitochondrial benzodiazapine receptors and the regulation of steroid biosynthesis. Annu. Rev. Pharmacol. Toxicol. 32: 211–237. [DOI] [PubMed] [Google Scholar]
- 230. Gavish M., Bachman R., Shoukrun Y., Katz Y., Veenman L., Weisinger G., Weizman A. 1999. Engine of the peripheral benzodiazepine receptor. Pharmacol. Rev. 51: 629–650. [PubMed] [Google Scholar]
- 231. Yanagibashi K., Ohno Y., Nakamichi N., Matsui T., Hayashida K., Takamura M., Yamada K., Kawamura M. 1989. Peripheral-type benzodiapine receptors are involved in the regulation of cholesterol side-chain cleavage in adrenal mitochondria. J. Biochem. 106: 1026–1029. [DOI] [PubMed] [Google Scholar]
- 232. Midzak A., Akula N., Lecanu L., Papadopoulos V. 2011. Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. J. Biol. Chem. 286: 9875–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. McEnery M. W., Snowman A. M., Trifiletti R. R., Snyder S. H. 1992. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA. 89: 3170–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Rone M. B., Fan J., Papadopoulos V. 2009. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim. Biophys. Acta. 1791: 646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Rone M. B., Liu J., Blonder J., Ye X., Veenstra T. D., Young J. C., Papadopoulos V. 2009. Targeting and insertion of the cholesterol-binding translocator protein into the outer mitochondrial membrane. Biochemistry. 48: 6909–6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Bayrhuber M., Meins T., Habeck M., Becker S., Giller K., Villinger S., Vonrhein C., Griesinger C., Zweckstetter M., Zeth K. 2008. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA. 105: 15370–15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Hiller S., Garces R. G., Malia T. J., Orekhov V. Y., Colombini M., Wagner G. 2008. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 321: 1206–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Feliciello A., Gottesman M. E., Avvedimento E. V. 2005. cAMP-PKA signalling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell. Signal. 17: 279–287. [DOI] [PubMed] [Google Scholar]
- 239. Dyson M. T., Jones J. K., Kowalewski M. P., Manna P. R., Alonso M., Gottesman M. E., Stocco D. M. 2008. Mitochondrial A-kinase anchoring protein 121 binds type II protein kinase A and enhances steroidogenic acute regulatory protein-mediated steroidogenesis in mouse MA-10 Leydig tumor cells. Biol. Reprod. 78: 267–277. [DOI] [PubMed] [Google Scholar]
- 240. Dyson M. T., Kowalewski M. P., Manna P. R., Stocco D. M. 2009. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol. Cell. Endocrinol. 300: 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Shen W. J., Patel S., Natu V., Hong R., Wang J., Azhar S., Kraemer F. B. 2003. Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein. J. Biol. Chem. 278: 43870–43876. [DOI] [PubMed] [Google Scholar]
- 242. Artemenko I. P., Zhao D., Hales D. B., Hales K. H., Jefcoate C. R. 2001. Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J. Biol. Chem. 276: 46583–46596. [DOI] [PubMed] [Google Scholar]
- 243. Sandison A. T. 1955. A form of lipoidosis of the adrenal cortex in an infant. Arch. Dis. Child. 30: 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Prader A., Siebenmann R. E. 1957. [Adrenal insufficiency in congenital lipoid hyperplasia of the adrenals] Helv. Paediatr. Acta. 12: 569–595 [in German]. [PubMed] [Google Scholar]
- 245. Kirkland R. T., Kirkland J. L., Johnson C. M., Horning M. G., Librik L., Clayton G. W. 1973. Congenital lipoid adrenal hyperplasia in an eight-year-old phenotypic female. J. Clin. Endocrinol. Metab. 36: 488–496. [DOI] [PubMed] [Google Scholar]
- 246. Hauffa B. P., Miller W. L., Grumbach M. M., Conte F. A., Kaplan S. L. 1985. Congenital adrenal hyperplasia due to deficient cholesterol side-chain cleavage activity (20,22 desmolase) in a patient treated for 18 years. Clin. Endocrinol. (Oxf.). 23: 481–493. [DOI] [PubMed] [Google Scholar]
- 247. Camacho A. M., Kowarski A., Migeon C. J., Brough A. 1968. Congenital adrenal hyperplasia due to a deficiency of one of the enzymes involved in the biosynthesis of pregnenolone. J. Clin. Endocrinol. Metab. 28: 153–161. [DOI] [PubMed] [Google Scholar]
- 248. Degenhart H. J., Visser K. H. A., Boon H., O'Doherty N. J. D. 1972. Evidence for deficiency of 20α cholesterol hydroxylase activity in adrenal tissue of a patient with lipoid adrenal hyperplasia. Acta Endocrinol. (Copenh.). 71: 512–518. [DOI] [PubMed] [Google Scholar]
- 249. Koizumi S., Kyoya S., Miyawaki T., Kidani H., Funabashi T., Nakashima H., Nakanuma Y., Ohta G., Itagaki E., Katagiri M. 1977. Cholesterol side-chain cleavage enzyme activity and cytochrome P450 content in adrenal mitochondria of a patient with congenital lipoid adrenal hyperplasia (Prader disease). Clin. Chim. Acta. 77: 301–306. [DOI] [PubMed] [Google Scholar]
- 250. Matteson K. J., Chung B., Urdea M. S., Miller W. L. 1986. Study of cholesterol side chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes. Endocrinology. 118: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 251. Lin D., Gitelman S. E., Saenger P., Miller W. L. 1991. Normal genes for the cholesterol side chain cleavage enzyme, P450scc, in congenital lipoid adrenal hyperplasia. J. Clin. Invest. 88: 1955–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Saenger P., Klonari Z., Black S. M., Compagnone N., Mellon S. H., Fleischer A., Abrams C. A. L., Shackleton C. H. L., Miller W. L. 1995. Prenatal diagnosis of congenital lipoid adrenal hyperplasia. J. Clin. Endocrinol. Metab. 80: 200–205. [DOI] [PubMed] [Google Scholar]
- 253. Lin D., Chang Y. J., Strauss J. F., III, Miller W. L. 1993. The human peripheral benzodiazepine receptor gene. Cloning and characterization of alternative splicing in normal tissues and in a patient with congenital lipoid adrenal hyperplasia. Genomics. 18: 643–650. [DOI] [PubMed] [Google Scholar]
- 254. Miller W. L. 1997. Congenital lipoid adrenal hyperplasia: the human gene knockout of the steroidogenic acute regulatory protein. J. Mol. Endocrinol. 19: 227–240. [DOI] [PubMed] [Google Scholar]
- 255. Miller W. L., Strauss J. F., III 1999. Molecular pathology and mechanism of action of the steroidogenic acute regulartory protein, StAR. J. Steroid Biochem. Mol. Biol. 69: 131–141. [DOI] [PubMed] [Google Scholar]
- 256. Tee M. K., Lin D., Sugawara T., Holt J. A., Guiguen Y., Buckingham B., Strauss J. F., III, Miller W. L. 1995. T→A transversion 11 bp from a splice acceptor site in the gene for steroidogenic acute regulatory protein causes congenital lipoid adrenal hyperplasia. Hum. Mol. Genet. 4: 2299–2305. [DOI] [PubMed] [Google Scholar]
- 257. Bose H. S., Pescovitz O. H., Miller W. L. 1997. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia caused by a homozygous frame-shift mutation in the steroidogenic acute regulatory protein. J. Clin. Endocrinol. Metab. 82: 1511–1515. [DOI] [PubMed] [Google Scholar]
- 258. Fujieda K., Tajima T., Nakae J., Sageshima S., Tachibana K., Suwa S., Sugawara T., Strauss J. F., III 1997. Spontaneous puberty in 46,XX subjects with congenital lipoid adrenal hyperplasia. J. Clin. Invest. 99: 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Caron K. M., Soo S-C., Wetsel W. C., Stocco D. M., Clark B. J., Parker K. L. 1997. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc. Natl. Acad. Sci. USA. 94: 11540–11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Hasegawa T., Zhao L., Caron K. M., Majdic G., Suzuki T., Shizawa S., Sasano H., Parker K. L. 2000. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol. Endocrinol. 14: 1462–1471. [DOI] [PubMed] [Google Scholar]
- 261. Chen X., Baker B. Y., Abduljabber M. A., Miller W. L. 2005. A genetic isolate of congenital lipoid adrenal hyperplasia with atypical clinical findings. J. Clin. Endocrinol. Metab. 90: 835–840. [DOI] [PubMed] [Google Scholar]
- 262. Voutilainen R., Miller W. L. 1986. Developmental expression of genes for the steroidogenic enzymes P450scc (20,22 desmolase), P450c17 (17α-hydroxylase/17,20 lyase) and P450c21 (21-hydroxylase) in the human fetus. J. Clin. Endocrinol. Metab. 63: 1145–1150. [DOI] [PubMed] [Google Scholar]
- 263. Khoury K., Barbar E., Ainmelk Y., Ouellet A., Lehoux J. G. 2009. Gonadal function, first cases of pregnancy, and child delivery in a woman with lipoid congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 94: 1333–1337. [DOI] [PubMed] [Google Scholar]
- 264. Sertedaki A., Pantos K., Vrettou C., Kokkali G., Christoforidou C., Kanavakis E., Dacou-Voutetakis C. 2009. Conception and pregnancy outcome in a patient with 11-bp deletion of the steroidogenic acute regulatory protein gene. Fertil. Steril. 91: 934.e15–18. [DOI] [PubMed] [Google Scholar]
- 265. Nakae J., Tajima T., Sugawara T., Arakane F., Hanaki K., Hotsubo T., Igarashi N., Igarashi Y., Ishii T., Koda N., et al. 1997. Analysis of the steroidogenic acute regulatory protein (StAR) gene in Japanese patients with congenital lipoid adrenal hyperplasia. Hum. Mol. Genet. 6: 571–576. [DOI] [PubMed] [Google Scholar]
- 266. Yoo H. W., Kim G. H. 1998. Molecular and clinical characterization of Korean patients with congenital lipoid adrenal hyperplasia. J. Pediatr. Endocrinol. Metab. 11: 707–711. [DOI] [PubMed] [Google Scholar]
- 267. Kim J. M., Choi J. H., Lee J. H., Kim G. H., Lee B. H., Kim H. S., Shin J. H., Shin C. H., Kim C. J., Yu J., et al. 2011. High allele frequency of the p.Q258X mutation and identification of a novel mis-splicing mutation in the StAR gene in Korean patients with congenital lipoid adrenal hyperplasia. Eur. J. Endocrinol. 165: 771–778. [DOI] [PubMed] [Google Scholar]
- 268. Flück C. E., Maret A., Mallet D., Portrat-Doyan S., Achermann J. C., Leheup B., Theintz G. E., Mullis P. E., Morel Y. 2005. A novel mutation L260P of the steroidogenic acute regulatory protein in three unrelated pateints of Swiss ancestry with congenital lipoid adrenal hyperplasia. J. Clin. Endocrinol. Metab. 90: 5304–5308. [DOI] [PubMed] [Google Scholar]
- 269. Baker B. Y., Lin L., Kim C. J., Raza J., Smith C. P., Miller W. L., Achermann J. C. 2006. Non-classic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J. Clin. Endocrinol. Metab. 91: 4781–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Metherell L. A., Naville D., Halaby G., Begeot M., Huebner A., Nürnberg G., Nürnberg P., Green J., Tomlinson J. W., Krone K. P., et al. 2009. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J. Clin. Endocrinol. Metab. 94: 3865–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Gonzalez F. J. 1988. The molecular biology of cytochrome P450s. Pharmacol. Rev. 40: 243–288. [PubMed] [Google Scholar]
- 272. Tijet N., Helvig C., Feyereisen R. 2001. The cytochrome P450 gene superfamily in Drosophila melanogaster: annotation, intron-exon organization and phylogeny. Gene. 262: 189–198. [DOI] [PubMed] [Google Scholar]
- 273. Nelson D. R., Zeldin D. C., Hoffman S. M. G., Maltais L. J., Wain H. M., Nebert D. W. 2004. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 14: 1–18. [DOI] [PubMed] [Google Scholar]
- 274. Solish S. B., Picado-Leonard J., Morel Y., Kuhn R. W., Mohandas T. K., Hanukoglu I., Miller W. L. 1988. Human adrenodoxin reductase: Two mRNAs encoded by a single gene of chromosome 17cen → q25 are expressed in steroidogenic tissues. Proc. Natl. Acad. Sci. USA. 85: 7104–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Picado-Leonard J., Voutilainen R., Kao L., Chung B., Strauss J. F., III, Miller W. L. 1988. Human adrenodoxin: cloning of three cDNAs and cycloheximide enhancement in JEG-3 cells. J. Biol. Chem. 263: 3240–3244. [PubMed] [Google Scholar]
- 276. Pawlak K. J., Prasad M., Thomas J. L., Whittal R. M., Bose H. S. 2011. Inner mitochondrial translocase Tim50 interacts with 3beta-hydroxysteroid dehydrogenase type-2 to regulate adrenal and gonadal steroidogenesis. J. Biol. Chem. Epub ahead of print. September 19, 2011; doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Stone D., Hechter O. 1955. Studies on ACTH action in perfused bovine adrenals: aspects of progesterone as an intermediary in cortico-steroidogenesis. Arch. Biochem. Biophys. 54: 121–128. [DOI] [PubMed] [Google Scholar]
- 278. Halkerston I. D. K., Eickhorn J., Hechter O. 1961. A requirement for reduced triphosphopyridine nucleotide for cholesterol side-chain cleavage by mitochondrial fractions of bovine adrenal cortex. J. Biol. Chem. 236: 374–380. [PubMed] [Google Scholar]
- 279. Koritz S. B., Kumar A. M. 1970. On the mechanism of action of adrenocorticotropic hormone - the stimulation of the activity of enzymes involved in pregnenolone synthesis. J. Biol. Chem. 245: 152–159. [PubMed] [Google Scholar]
- 280. Hall P. F. 1986. Cytochromes P450 and the regulation of steroid syntheses. Steroids. 48: 131–196. [DOI] [PubMed] [Google Scholar]
- 281. Tuckey R. C., Cameron K. J. 1993. Side-chain specificities of human and bovine cytochromes P-450scc. Eur. J. Biochem. 217: 209–215. [DOI] [PubMed] [Google Scholar]
- 282. Toaff M. E., Schleyer H., Strauss J. F., III 1982. Metabolism of 25-hydroxycholesterol by rat luteal mitochondria and dispersed cells. Endocrinology. 111: 1785–1790. [DOI] [PubMed] [Google Scholar]
- 283. Kuwada M., Kitajima R., Suzuki H., Horie S. 1991. Purification and properties of cytochrome P-450(SCC) from pig testis mitochondria. Biochem. Biophys. Res. Commun. 176: 1501–1508. [DOI] [PubMed] [Google Scholar]
- 284. Miller W. L. 1988. Molecular biology of steroid hormone synthesis. Endocr. Rev. 9: 295–318. [DOI] [PubMed] [Google Scholar]
- 285. Simpson E. R. 1979. Cholesterol side-chain cleavage, cytochrome P450, and the control of steroidogenesis. Mol. Cell. Endocrinol. 13: 213–227. [DOI] [PubMed] [Google Scholar]
- 286. Mast N., Annalora A. J., Lodowski D. T., Palczewski K., Stout C. D., Pikuleva I. A. 2011. Structural basis for three-step sequential catalysis by the cholesterol side chain cleavage enzyme CYP11A1. J. Biol. Chem. 286: 5607–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Strushkevich N., MacKenzie F., Cherkesova T., Grabovec I., Usanov S., Park H. W. 2011. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc. Natl. Acad. Sci. USA. 108: 10139–10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Chung B. C., Matteson K. J., Voutilainen R., Mohandas T. K., Miller W. L. 1986. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc. Natl. Acad. Sci. USA. 83: 8962–8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Morohashi K., Sogawa K., Omura T., Fujii-Kuriyama Y. 1987. Gene structure of human cytochrome P-450(scc), cholesterol desmolase. J. Biochem. 101: 879–887. [DOI] [PubMed] [Google Scholar]
- 290. Yang X., Iwamoto K., Wang M., Artwohl J., Mason J. I., Pang S. 1993. Inherited congenital adrenal hyperplasia in the rabbit is caused by a deletion in the gene encoding cytochrome P450 cholesterol side-chain cleavage enzyme. Endocrinology. 132: 1977–1982. [DOI] [PubMed] [Google Scholar]
- 291. Hu D., Cao K., Peterson-Wakeman R., Wang R. 2002. Altered profile of gene expression in rat hearts induced by nicotine consumption. Biochem. Biophys. Res. Commun. 297: 729–736. [DOI] [PubMed] [Google Scholar]
- 292. Tajima T., Fujieda K., Kouda N., Nakae J., Miller W. L. 2001. Heterozygous mutation in the cholesterol side chain cleavage enzyme (P450scc) gene in a patient with 46,XY sex reversal and adrenal insufficency. J. Clin. Endocrinol. Metab. 86: 3820–3825. [DOI] [PubMed] [Google Scholar]
- 293. Kim C. J., Lin L., Huang N., Quigley C. A., AvRuskin T. W., Achermann J. C., Miller W. L. 2008. Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J. Clin. Endocrinol. Metab. 93: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Katsumata N., Ohtake M., Hojo T., Ogawa E., Hara T., Sato N., Tanaka T. 2002. Compound heterozygous mutations in the cholesterol side-chain cleavage enzyme gene (CYP11A) cause congenital adrenal insufficiency in humans. J. Clin. Endocrinol. Metab. 87: 3808–3813. [DOI] [PubMed] [Google Scholar]
- 295. Parker K. L., Schimmer B. P. 1997. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr. Rev. 18: 361–377. [DOI] [PubMed] [Google Scholar]
- 296. Morohashi K. 1999. Gonadal and extragonadal functions of Ad4BP/SF-1: developmental aspects. Trends Endocrinol. Metab. 10: 169–173. [DOI] [PubMed] [Google Scholar]
- 297. Huang N., Miller W. L. 2005. LBP proteins modulate SF1-independent expression of P450scc in human placental JEG-3 cells. Mol. Endocrinol. 19: 409–420. [DOI] [PubMed] [Google Scholar]
- 298. Huang N., Miller W. L. 2000. Cloning of factors related to HIV-inducible LBP proteins that regulate steroidogenic factor-1-independent human placental transcription of the cholesterol side-chain cleavage enzyme, P450scc. J. Biol. Chem. 275: 2852–2858. [DOI] [PubMed] [Google Scholar]
- 299. Henderson Y. C., Frederick M. J., Wang M. T., Hollier L. M., Clayman G. L. 2008. LBP-1b, LBP-9, and LBP-32/MGR detected in syncytiotrophoblasts from first-trimester human placental tissues and their transcriptional regulation. DNA Cell Biol. 27: 71–79. [DOI] [PubMed] [Google Scholar]
- 300. Henderson Y. C., Frederick M. J., Jayakmar Choi A. Y., Wang M. T., Kang Y., Evans R., Spring P. M., Uesugi M., Clayman G. L. 2007. Human LBP 32/MGR is a suppressor of P450scc in human choriocarcinoma cell line JEG-3. Placenta. 28: 152–160. [DOI] [PubMed] [Google Scholar]
- 301. Gizard F., Lavallée B., DeWitte F., Hum D. W. 2001. A novel zinc finger protein TReP-132 interacts with CBP/p300 to regulate human CYP11A1 gene expression. J. Biol. Chem. 276: 33881–33892. [DOI] [PubMed] [Google Scholar]
- 302. Gizard F., Lavallee B., DeWitte F., Teissier E., Staels B., Hum D. W. 2002. The transcriptional regulating protein of 132 kDa (TReP-132) enhances P450scc gene transcription through interaction with steroidogenic factor-1 in human adrenal cells. J. Biol. Chem. 277: 39144–39155. [DOI] [PubMed] [Google Scholar]
- 303. Tuckey R. C., Holland J. W. 1989. Comparison of pregnenolone synthesis by cytochrome P-450scc in mitochondria from porcine corpora lutea and granulosa cells of follicles. J. Biol. Chem. 264: 5704–5709. [PubMed] [Google Scholar]
- 304. Freeman M. R., Dobritsa A., Gaines P., Segraves W. A., Carlson J. R. 1999. The dare gene: steroid hormone production, olfactory behaviour, and neural degradation in drosophila. Development. 126: 4591–4602. [DOI] [PubMed] [Google Scholar]
- 305. Lin D., Shi Y., Miller W. L. 1990. Cloning and sequence of the human adrenodoxin reductase gene. Proc. Natl. Acad. Sci. USA. 87: 8516–8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Sparkes R. S., Klisak I., Miller W. L. 1991. Regional mapping of genes encoding human steroidogenic enzymes: P450scc to 15q23-q24, adrenodoxin to 11q22; adrenodoxin reductase to 17q24-q25; and P450c17 to 10q24-q25. DNA Cell Biol. 10: 359–365. [DOI] [PubMed] [Google Scholar]
- 307. Brandt M. E., Vickery L. E. 1992. Expression and characterization of human mitochondrial ferredoxin reductase in Escherichia coli. Arch. Biochem. Biophys. 294: 735–740. [DOI] [PubMed] [Google Scholar]
- 308. Schneider M., Tanaka-Nozaki M., Kato S., Blomeke B. 2010. Influence of 5-fluorouracil on ferredoxin reductase mRNA splice variants in colorectal carcinomas. Oncology Lett. 1: 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Brentano S. T., Black S. M., Lin D., Miller W. L. 1992. cAMP post-transcriptionally diminishes the abundance of adrenodoxin reductase mRNA. Proc. Natl. Acad. Sci. USA. 89: 4099–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Ziegler G. A., Vonrheim C., Hanukoglu I., Schulz G. E. 1999. The structure of adrenodoxin reductase of mitochondrial P450 systems: electron transfer for steroid biosynthesis. J. Mol. Biol. 289: 981–990. [DOI] [PubMed] [Google Scholar]
- 311. Vickery L. E. 1997. Molecular recognition and electron transfer in mitochondrial steroid hydroxylase systems. Steroids. 62: 124–127. [DOI] [PubMed] [Google Scholar]
- 312. Brandt M. E., Vickery L. E. 1993. Charge pair interactions stabilizing ferredoxin-ferredoxin reductase complexes: identification by complementary site-specific mutations. J. Biol. Chem. 268: 17126–17130. [PubMed] [Google Scholar]
- 313. Chang C-Y., Wu D-A., Lai C. C., Miller W. L., Chung B. 1988. Cloning and structure of the human adrenodoxin gene. DNA. 7: 609–615. [DOI] [PubMed] [Google Scholar]
- 314. Hanukoglu I., Suh B. S., Himmelhoch S., Amsterdam A. 1990. Induction and mitochondrial localization of cytochrome P450scc system enzymes in normal and transformed ovarian granulosa cells. J. Cell Biol. 111: 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Sheftel A. D., Stehling O., Pierik A. J., Elsässer H. P., Mühlenhoff U., Webert H., Hobler A., Hannemann F., Bernhard R., Lill R. 2010. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc. Natl. Acad. Sci. USA. 107: 11775–11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Müller A., Müller J. J., Müller Y. A., Uhlmann H., Bernhardt R., Heinemann U. 1998. New aspects of electron transfer revealed by the crystal structure of a truncated bovine adrenodoxin, Adx(4–108). Structure. 6: 269–280. [DOI] [PubMed] [Google Scholar]
- 317. Coghlan V. M., Vickery L. E. 1991. Site-specific mutations in human ferrodoxin that affect binding to ferrodoxin reductase and cytochrome P450scc. J. Biol. Chem. 266: 18606–18612. [PubMed] [Google Scholar]
- 318. Wada A., Waterman M. R. 1992. Identification by site-directed mutagenesis of two lysine residues in cholesterol side chain cleavage cytochrome P450 that are essential for adrenodoxin binding. J. Biol. Chem. 267: 22877–22882. [PubMed] [Google Scholar]
- 319. Cao P. R., Bülow H., Dumas B., Bernhardt R. 2000. Construction and characterization of a catalytic fusion protein system: P-450(11beta)-adrenodoxin reductase-adrenodoxin. Biochim. Biophys. Acta. 1476: 253–264. [DOI] [PubMed] [Google Scholar]
- 320. Miller W. L. 1995. Mitochondrial specificity of the early steps in steroidogenesis. J. Steroid Biochem. Mol. Biol. 55: 607–616. [DOI] [PubMed] [Google Scholar]
- 321. Hiort O., Holterhus P. M., Wesrner R., Marschke C., Hoppe U., Riepe F. G., Achermann J. C., Struve D. 2005. Homozygous disruption of P450 side-chanin cleavage (CYP11A1) is associated with prematurity, complete 46,XY sex reversal, and severe adrenal failure. J. Clin. Endocrinol. Metab. 90: 538–541. [DOI] [PubMed] [Google Scholar]
- 322. al Kandari H., Katsumata N., Alexander S., Rasoul M. A. 2006. Homozygous mutation of P450 side-chain cleavage enzyme gene (CYP11A1) in 46,XY patient with adrenal insufficiency, complete sex reversal, and agenesis of corpus callosum. J. Clin. Endocrinol. Metab. 91: 2821–2826. [DOI] [PubMed] [Google Scholar]
- 323. Sahakitrungruang T., Tee M. K., Blackett P. R., Miller W. L. 2011. Partial defect in the cholesterol side-chain cleavage enzyme P450scc (CYP11A1) resembling nonclassic congenital lipoid adrenal hyperplasia. J. Clin. Endocrinol. Metab. 96: 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Rubtsov P., Karmanov M., Sverdlova P., Spirin P., Tiulpakov A. 2009. A novel homozygous mutation in CYP11A1 gene is associated with late onset adrenal insufficiency and hypospadias in a 46 XY patient. J. Clin. Endocrinol. Metab. 94: 936–939. [DOI] [PubMed] [Google Scholar]