Abstract
Cardiolipin (CL) is a unique phospholipid (PL) found in the mitochondria of mammalian cells. CL remodeling is accompanied by turnover of its fatty acid acyl groups. Abnormalities in CL remodeling have been found in Barth's syndrome, diabetes, and obesity. The objective of this study was to determine nonessential fatty acid turnover in CL and phosphatidylethanolamine (PE) in the rat heart in vivo. Sprague-Dawley rats were fed either a regular chow or a high-fat diet for 15 weeks, and consumed 6% deuterium-enriched drinking water as a tracer for 14 days. CL and PE were extracted from cardiac tissue and isolated by TLC. Fatty acids from CL, PE, and plasma were analyzed by GC/MS for deuterium incorporation. Results showed oleate and vaccenate turnover were the highest in CL whereas palmitate and stearate turnover were low. Among the nonessential fatty acids in PE, turnover of stearate and vaccenate were the highest. The high turnover rate in vaccenate was unexpected, because vaccenate previously had no known metabolic or physiologic function. In conclusion, the similarly high turnover rates of both oleate and vaccenate readily suggest that remodeling is an important functional aspect of PL metabolism in CL.
Keywords: cardiolipin remodeling, vaccenate, phosphatidylethanolamine
Cardiolipin (CL) is a polyglycerol phospholipid (PL) that is found almost exclusively in mitochondrial and bacterial lipids. Its molecular composition consists of three glycerols and four acyl groups, forming a dimeric structure with a glycerol linker between two phosphotidyl groups, providing a pair of sn1 and sn2 positions per CL molecule. In mammals, CL fatty acid composition is dominated by linolenic acid on the sn1 positions whereas the sn2 positions are occupied by monounsaturated, di-unsaturated, and polyunsaturated fatty acids (1–4). A symmetrical tetra-acyl CL has also been identified with linolenic acid at the two sn1 and sn2 positions (5, 6). CL is a specific marker of mitochondria where it appears to play multiple roles related to energy transformation, apoptosis, and membrane integrity (7–12).
It has been proposed that synthesis of CL is via conversion of phosphatidic acid into cytidinediphosphate-diacylglycerol (CDP-DAG) in the mitochondria, which then forms phosphatidylglycerolphosphate (PGP). PGP is converted to phosphatidylglycerol which then reacts with another molecule of CDP-DAG in presence of CL synthase to form CL (13–17). In order for CL to achieve its specific acyl composition, synthesized CL undergoes acyl chain remodeling with monolysocardiolipin (MLCL) as an intermediate. There are two proposed mechanisms for remodeling of CL from MLCL. The first proposed pathway incorporates acyl-CoAs in the presence of monolysocardiolipin acyltransferase (MLCL AT) (18–20). Another proposed pathway, referred to as transacylation, has been reported in which acyl groups are directly transferred from phospholipids into MLCL (21–23).
Fatty acids are either incorporated into the nascent CL backbone via CDP-DAG during its biosynthesis or its subsequent remodeling by MLCL AT. Specific composition of acyl groups within the sn1 and sn2 positions may be finalized through the process of transacylation. The fatty acids in CL turn over at different rates during remodeling. The turnover of linoleic acid is higher than that of nonessential fatty acids; and the turnover of either acyl group is higher than the turnover of glycerol phosphate (24). Even though CL is a structural lipid, a critical component of the mitochondria inner membrane, the dynamics of fatty acid turnover in CL suggests that the remodeling process is very active and may play a functional role in the regulation of mitochondrial metabolism.
The turnover of nonessential fatty acids was previously investigated using a bolus injection of 14C-acetate intraperitoneally (24). In that study, 14C-acetate was incorporated into nonessential fatty acids, and the decay of 14C activity in cardiolipin was used as a measure of the nonessential fatty acids turnover. However, that study did not distinguish the turnover of the individual nonessential fatty acids. Recently, deuterium-enriched water (D2O) has been used for the study of de novo lipogenesis (25, 26). The incorporation of deuterium into each of the nonessential fatty acids allows the determination of fraction of new synthesis for that fatty acid over the period of study. In this report, turnover of nonessential saturated acyl groups in comparison to the monounsaturated acyl groups in CL from animals fed a high fat diet and a regular chow diet was studied. D2O was used as the tracer and deuterium incorporation into palmitate, stearate, oleate, and vaccenate in plasma, phosphotidylethanolamine (PE), and CL from the heart was analyzed. The amount of deuterium incorporated reflects the relative turnover rates of these fatty acids, and the turnover rates of cardiac CL fatty acids from animals fed RCD and HFD were compared.
MATERIALS AND METHODS
Materials
Fatty acid methyl ester standards for palmitate, stearate, oleate, vaccenate, and derivatizing agent methanolic hydrochloride (0.5N) were obtained from Supelco. Bovine cardiolipin sodium salt as the CL standard and 1,2-diheptadecanoyl-sn-glycero-3-phosphoethanolamine as the PE standard were obtained from Sigma.
Animals experimental design
Animal handling and experimentation were in accordance with the recommendation of American Veterinary Medical Association and were approved by the Animal Care and Use review committee at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. Six-week old male Sprague-Dawley rats were purchased from Charles River Laboratories, Inc. (Wilmington, MA) and housed individually in temperature and humidity controlled rooms providing exposure to 12 h light and dark cycles. An adaptation period of 2 weeks preceded the initiation of the experiment. The rats were randomly placed into two treatment groups: regular chow diet (RCD) and high-fat diet (HFD) for 15 weeks (n = 5 in each group). The RCD provided 16% energy from fat, 27% from protein, and 56% from carbohydrates. The HFD provided 71% energy from fat, 18% energy from protein, and 11% from carbohydrates (Dyets Inc.). To determine lipogenesis in different treatment groups, a deuterated water method and mass isotopomer distribution analysis was used. The deuterium enrichment protocol was conducted as follows: At the beginning of 13th week of the experiment, the animals were anesthetized and given an intraperitoneal injection of deuterium oxide (D2O) (Cambridge Isotope) equal to 4% of lean body weight determined by DEXA scan. This was followed by 14 days of a 6% D2O in drinking water for both the control and HFD groups. Because the HFD was provided as a liquid diet, D2O was added to the diet to an enrichment of 6% to ensure constant deuterium enrichment in body water. The period of 14 days was chosen to allow de novo lipogenesis to reach steady state (26). At the completion of the study, the rats were weighed and euthanized by an overdose of pentobarbital after an overnight fast. On the day of euthanization, the means and standard deviations of the animal weights were 587 ± 27 g for the RCD group and 627 ± 26 g for the HFD group. The blood was collected from the aorta ventralis and centrifuged (4°C, 3,200 rpm, 10 min), and serum was frozen at −20°C for until analysis. Heart tissue was snap-frozen in liquid nitrogen and stored at −80°C until analysis.
Isolation of fatty acids from CL and PE of the heart
Total lipid extractions from cardiac tissue samples were carried out according to the method reported by Folch et al. (27). Total lipids were fractionated by TLC (Silica 60A, 20 × 20 × 0.5 cm) with a mobile phase system reported by Corcelli et al. (28). CL and PE fractions were identified according to the position of CL and PE standards. Extraction of the PLs from the isolated TLC spots was carried with chloroform:methanol (2:1, 500 µL). Dried PLs were hydrolyzed to isolate the fatty acids according to methods reported by Aveldano and Horrocks (29).
Isolation of fatty acids from serum
Serum samples (50μl) were saponified overnight at 70°C with 100 ml of 30% KOH (w/v) and 100 ml of 200-proof ethanol. The aqueous phase was acidified with HCl, followed by fatty acid extraction with petroleum ether. Samples were dried under a nitrogen stream.
GC/MS analysis
Fatty acid samples were treated with methanolic HCl (0.5N, 500 μL) at 70°C for one h to form its methyl ester derivatives. Dried methyl ester fatty acids were extracted with hexane then analyzed by GC/MS in triplicates. GC/MS setup for the analysis followed the reported protocol of Yee, et al. (30). Palmitate, stearate, oleate, vaccenate, and linoleate were separated on the gas chromatograph with a Bpx70 column (30-m length, 250-μm diameter, 0.25-μm film thickness) from SGE, Inc. (Austin, TX). The GC conditions were as follows: helium flow rate, 1 ml/min; initial oven temperature, 150°C, which was programmed to increase at 3°C/min to 221°C. After a 1 min hold, the temperature gradient was changed to 20°C/min until reaching the final temperature of 235°C. The expected retention times for palmitate, stearate, oleate, vaccenate, and linoleate under these conditions were as follows: 5.79, 8.47, 9.0, 9.14, and 10.14 min, respectively. Mass spectra of fatty acids were acquired using electron impact ionization in both scan and selective ion monitoring (SIM) modes. Palmitate ion clusters monitored in SIM mode for the quantitation of isotopomers of were m/z 269–276 with m+0 at m/z 270. The clusters corresponding to stearate were m/z 298–302 with m+0 at m/z 298. For oleate and vaccenate, the clusters were m/z 263–274, with m+0 at m/z 264 for oleate and vaccenate.
Spectral data analysis
Distribution of the mass isotopomers, deuterium enrichment and fraction of new synthesis were determined from the spectral data using a linear regression approach previously described by Lee et al. (25, 26) that corrects for the contribution of derivatizing agent and 13C natural abundance to the mass isotopomer distribution of the compound of interest. The resulting mass isotopomer distribution was expressed in molar fractions (m0, m1, m2, m3, etc.) corresponding to the fraction of molecules that contain 0, 1, 2, 3, …, mn 2 H substitutions. The sum of all mns is equal to 1. Because the spectra of oleate and vaccenate do not follow a binomial distribution, the average mass approach was used to determine new synthesis (31). The change in average mass due to deuterium incorporation was first calculated. Then the change in average mass was divided by the deuterium enrichment to determine the fraction of new synthesis. Mass shift was calculated as shown below where mn is the molar fraction of the isotopomer containing n number of deuterium atoms:
SigmaPlot 11.0 was used to carry out statistical analysis and prepare data for graphical presentation.
Deuterium incorporation and fraction of new synthesis
The amount of deuterium incorporated into the individual fatty acid is given by the mass shift or delta mass. It is a function of the rate of synthesis, dilution by unlabeled fatty acid from the diet, and the turnover rate of fatty acid in the period of observation. When the turnover rate of the fatty acid (e.g., palmitate) is rapid relative to the period of deuterated water treatment (14 days), the amount of deuterium incorporated is considered to be at steady state, and the fraction of new synthesis approximates the turnover rate (as stated by Lee et al. (26). Fraction of new synthesis in samples obtained on day 14 was calculated based on the previously reported method for each fatty acid. Deuterium enrichment (p) in water was first determined using consecutive mass isotopomer ratio in palmitate (26, 32). Once the enrichment in water was determined for each animal, the delta mass for theoretical (100%) new fraction was given by p×n, where n is the number of exchangeable hydrogen in the fatty acid (e.g., n = 21 in palmitate) (25, 33, 34). The fraction of new synthesis for the period of deuterated water treatment was given by the observed delta mass divided by the theoretical delta mass. An example of the various calculations is provided in the following. In Table 1 , spectral data of unlabeled palmitate and palmitate from experimental samples of an animal on HFD are presented. The spectral data were processed to remove the contribution of 13C giving the corresponding mass isotopomer distributions, which were used to calculate delta mass (Σmn), p, and fractional new synthesis.
TABLE 1.
Sample calculation (triplicate) of plasma D2O enrichment, delta mass and fraction of new synthesis of plasma palmitate from one of the animals on the high fat diet
| m/z 270 | m/z 271 | m/z 272 | m/z 273 | m/z 274 | ||
| blank | 100 | 18.96 | 2.12 | 0.18 | 0.02 | |
| Sample 1 | 100 | 23.09 | 4.84 | 1.31 | 0.35 | |
| Sample 2 | 100 | 23.10 | 4.84 | 1.30 | 0.35 | |
| Sample 3 | 100 | 23.11 | 4.83 | 1.31 | 0.35 | |
| m0 | m1 | m2 | m3 | Σmn or Δmass | ||
| blank | 1 | 0 | 0 | 0 | 0 | |
| Sample 1 | 0.9352 | 0.0386 | 0.0181 | 0.0063 | 0.1011 | |
| Sample 2 | 0.9351 | 0.0387 | 0.0180 | 0.0062 | 0.1012 | |
| Sample 3 | 0.9350 | 0.0388 | 0.0180 | 0.0064 | 0.1014 | |
| Σmn or Δmass | m2/m1 | P a | N | FSR b | ||
| Sample 1 | 0.1011 | 0.4688 | 0.0448 | 21 | 0.1075 | |
| Sample 2 | 0.1012 | 0.4667 | 0.0446 | 21 | 0.1081 | |
| Sample 3 | 0.1014 | 0.4638 | 0.0443 | 21 | 0.1090 |
p/q = (m2/m1)/((N-1)/2) and P = (p/q)/(p/q+1)
FSR = Δmass/(N*p)
RNA extraction and real-time PCR for gene expression studies
Total RNA was extracted from rat heart tissue using Trizol reagent (Invitrogen). cDNA was synthesized by reverse transcription using the Superscript III first strand cDNA synthesis kit (Invitrogen). Real-time PCR was performed in duplicates for each sample in each gene using LightCycle®480 SYBR Green real time PCR kit (Roche) with denaturation 95°C; 15 s, annealing 60°C; 30 s, extension 72°C; 30 s for 40 cycles. The primer sequences used were as follows: Acyl-CoA lysocardiolipin acyltransferase (ALCAT) forward 5′→3′ CAGAAGGAACTGACCTCACAGAAAA, reverse 5′→3′ GTG GTT CTTGGG TGT AATACA TAC TCA, product size 123 bp. Phosphatidylglycerophosphate synthetase (PGPS), forward 5′→3′ AGAGGTGAACGG CTTCTTTGG, reverse 5′→3′ CACTGTAGAACTGTCGCTCAATGTG, 69 bp. Cardiolipin synthase (CLS), forward 5′→3′ TGGATGGATTTATTGCTCGAAA reverse 5′→3′ TGGGACTGGAATAAGATCTGCAT, 134 bp. Glycerol-3-phosphate acyltransferase 1 (GPAT-1), forward 5′→3′TCAGAATACAGCCTTGGCCGATGT reverse 5′→3′ AGCGTTGCGGATCTGAAGAAGGTA, 89 bp. Tubulin forward 5′→3′ GACAATGAGGCCATCTATGACATCT, reverse 5′→3′ CTGAGGGAAGCAGTG ATGGAA, bp 116. Results of each gene of interest were normalized to tubulin. Results are presented as the fold change of the HFD group compared with the RCD group, ± SEM.
RESULTS
PE and CL fatty acid profiles
The fatty acid profiles of PE and CL fractions were determined using GCin scan mode ( Table 2 ). The fatty acid profiles of CL and PE were distinct from each other, confirming that isolation of CL and PE fractions by thin-layer chromatography was achieved. In the total ion chromatogram of the PE fatty acid profile, stearate was observed as the most abundant, with low components of palmitate, oleate, and vaccenate. Linoleate were detected at low levels in the PE fatty acid profile. The CL fatty acid profile was dominated by linoleate with minor components of palmitate, stearate, oleate, and vaccenate identified. The relative distribution of these nonessential fatty acids in CL is consistent with previous reports on the unique fatty acid profile of CL provided in Table 2.
TABLE 2.
CL and PE fatty acid profiles from both diet groups (n = 5 each group) as compared with previously reported values
| Sprague Dawley Rats |
||||
| Fatty acid | RCD | HFD | Wistar Rats a (weaned) | CDF-344 rats b (4 months) |
| Fatty Acids in Phosphatidylethanolamine (% of total) | ||||
| Palmitate | 17.05 ± 1.59 | 13.83 ± 0.94 | not reported | 17.84 |
| Stearate | 71.04 ± 2.66 | 69.20 ± 1.58 | not reported | 60.34 |
| Oleate | 4.86 ± 0.75 | 5.80 ± 4.12 | not reported | 6.03 |
| Vaccenate | 1.55 ± 0.97 | 3.33 ± 4.44 | not reported | n/a |
| Linoleate | 5.51 ± 2.23 | 5.72 ± 1.96 | not reported | 15.79 |
| Fatty Acids in Cardiolipin (% of total) | ||||
| Palmitate | 7.04 ± 3.52 | 7.68 ± 2.03 | 2.02 | 0.75 |
| Stearate | 13.41 ± 7.67 | 12.86 ± 2.63 | 0.50 | 0.80 |
| Oleate | 2.45 ± .055 | 6.39 ± 3.05 | 3.40 | 4.73 |
| Vaccenate | 3.32 ± 0.48 | 4.15 ± 0.58 | 13.98 | not reported |
| Linoleate | 68.90 ± 10.58 | 68.92 ± 6.63 | 80.10 | 93.72 |
Percentages of the five identifiable fatty acids were calculated as the individual peak area ratio to the sum of peak areas of the five fatty acids.
Data obtained from weaned male Wistar rat, quantified by GC equipped with flame ionization detector (FID) detector (1). PE values were not reported.
Data obtained from 4-month-old male CDF-344 rats, quantified by GC equipped with FID detector (4). Vaccenate value was not listed.
Deuterium enrichment and fraction of new synthesis (FNS)
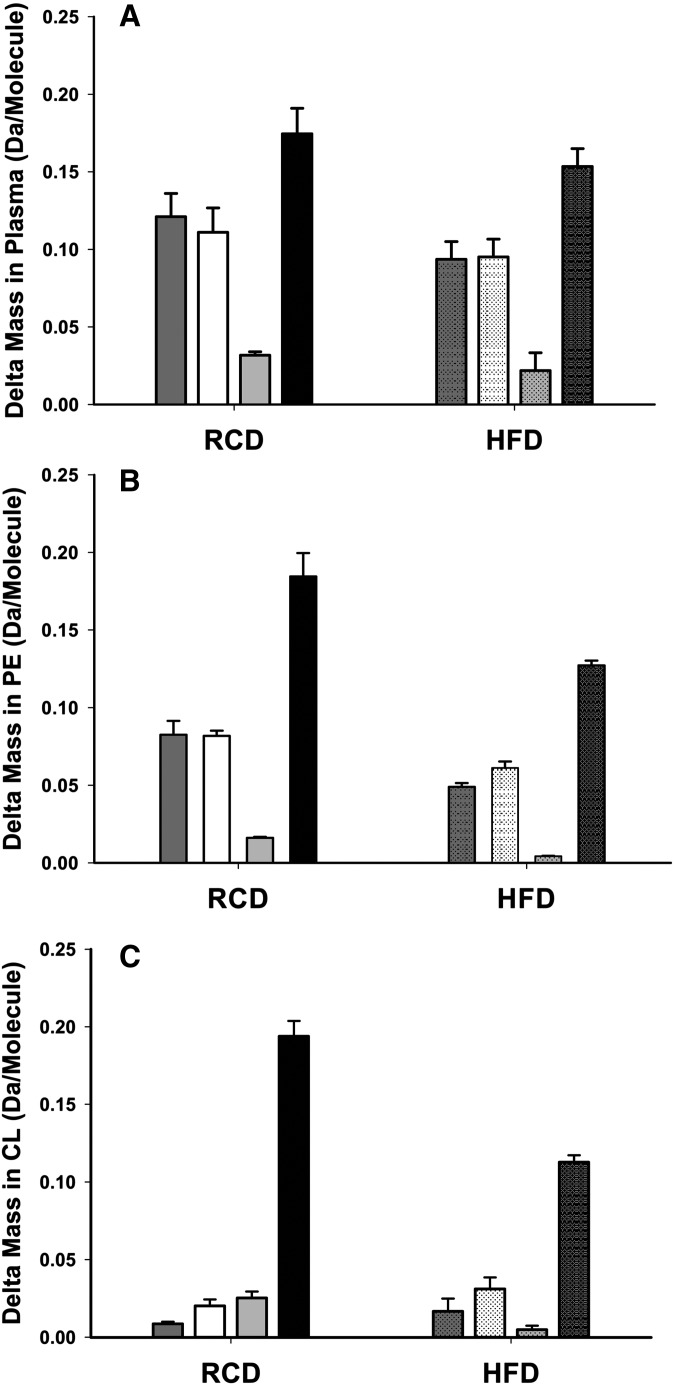
Isotopomers of palmitate (C16:0), stearate (C18:0), oleate (C18:1n-9), and vaccenate (C18:1n-7) were analyzed in SIM mode. Incorporation of deuterium resulted in an increased isotopomer molar fraction of m1, m2, m3, …mn (n = number of deuterium substitution), which was also represented as a increment in the average molecular weight (delta mass) of the fatty acid. The average mass shift of each fatty acid as the result of deuterium incorporation during de novo lipogenesis is presented in Fig. 1 . The amount of deuterium incorporated into the individual fatty acid is a function of the rate of synthesis, dilution by unlabeled fatty acid from the diet, and the turnover rate of fatty acid in the period of observation. When the turnover rate of the fatty acid (e.g., palmitate) is rapid relative to the period of deuterated water treatment (14 days), the amount of deuterium incorporated is considered to be at steady state, and the fraction of new synthesis approximates the turnover rate. 2 During fatty acid synthesis in the liver, palmitate is the first product of de novo lipogenesis, which is the precursor of stearate and oleate. The amount of deuterium incorporated into these fatty acids followed a predicted pattern of precursor/product relationship. The order of deuterium enrichment (delta mass) in these nonessential fatty acids from plasma was palmitate>stearate>oleate (Fig. 1) regardless of dietary fat intake. Vaccenate (C18:1n-7) is the product of chain elongation of palmitoleate which is produced from palmitate by desaturation. The deuterium enrichment in vaccenate was found to be the highest among the nonessential fatty acids suggesting a high turnover rate with less dilution from preformed vaccenate compared with palmitate. Deuterium incorporation into nonessential fatty acids of plasma, PE, and CL in animals fed the HFD was less than those from animals fed on the RCD. The lower enrichment in plasma fatty acids from animals fed HFD is due to the dilution by dietary fat and in part to the suppression of de novo lipogenesis (35). Deuterium incorporation in nonessential fatty acids of phospholipids did not follow the pattern of precursor-product relationship as in plasma fatty acids (Fig. 1), and the changes in delta mass were generally less than those of plasma fatty acids, suggesting the uptake of these fatty acids from plasma during synthesis or remodeling of phospholipids.
Fig. 1.
Average mass shifts of fatty acids in (A) plasma, (B) PE, and (C) CL due to the incorporation of deuterium during de novo synthesis from animals fed a regular chow diet (RCD, solid bars) and animals fed a high-fat diet (HFD, textured bars). The mass shift for palmitate is represented bars in dark gray, stearate in white, oleate in light gray, and vaccenate in black.
The fraction of new synthesis over 14 days ( Table 3 ) was calculated based on the previously reported method for each fatty acid. When the time to sampling (or the duration of D2O treatment) is much longer than the synthesis rate in fraction per day, the condition approaches steady state and the fraction of new synthesis is the fractional contribution of new synthesis to the fatty acid pool at steady state. In the RCD group, de novo lipogenesis (FNS) of palmitate contributed 16.0% to the plasma palmitate pool at steady state. Similarly, the contribution of de novo lipogenesis was 13.0% to the stearate pool, 3.4% to the oleate pool, and 22.4% to the vaccenate pool. The lower enrichment in plasma fatty acids from animals fed the HFD translates into significantly lower FNS in all nonessential fatty acids (Table 3) with FNS of palmitate being 9.6%, stearate 9.0%, oleate 2.4%, and vaccenate 16.0%.
TABLE 3.
FNS of palmitate, stearate, oleate, and vaccenate in plasma, PE, and CL of both diet groups
| FNS Palmitate (%) | FNS Stearate (%) | FNS Oleate (%) | FNS Vaccenate (%) | ||
| Plasma | RCD | 16.02 ± 2.06 | 13.00 ± 1.87 | 3.46 ± 0.25 | 22.35 ± 2.3 |
| HFD | 9.58 ± 1.13 a | 8.95 ± 1.10 a | 2.38 ± 1.25 | 16.00 ± 1.25 a | |
| PE | RCD | 11.08 ± 1.26 | 10.95 ± 0.53 | 2.06 ± 0.11 | 24.74 ± 2.18 |
| HFD | 5.32 ± 0.26 a | 6.65 ± 0.19 a | 0.44 ± 0.02 a | 13.87 ± 0.68 a | |
| PE/Plasma ratio | RCD | 70.83 ± 4.97 c | 90.58 ± 11.76 | 60.09 ± 3.20 c | 113.41 ± 10.10 |
| HFD | 57.94 ± 5.74 c | 78.66 ± 9.95 | 44.30 ± 15.00 c | 88.18 ± 4.95 b | |
| CL | RCD | 1.15 ± 0.17 | 2.22 ± 0.24 | 3.42 ± 0.61 | 26.00 ± 1.60 a |
| HFD | 1.71 ± 0.79 | 2.93 ± 0.40 | 0.55 ± 0.28 | 12.27 ± 0.68 | |
| CL/Plasma ratio | RCD | 7.54 ± 1.14 d | 18.53 ± 3.51 d | 96.43 ± 11.90 | 95.00 ± 6.16 |
| HFD | 18.79 ± 8.43 d | 35.16 ± 7.68 d | 47.22 ± 35.25 | 60.88 ± 4.32 b |
Determination of the FNS was based on deuterium incorporation into each fatty acid and calculated plasma deuterium enrichment. FNS is the molecular fraction of the newly synthesized fatty acid, and is expressed as the mean percent ± SEM. The PE/Plasma and CL/Plasma ratios are also expressed as percent ± the SEM. The boldface type indicates summary of results that are discussed in the text of the manuscript.
FNS is statistically different in animals fed HFD compared with animals fed RCD (P < 0.05).
PE/Plasma and CL/Plasma ratios are statistically different in animals fed HFD compared with animals fed RCD (P < 0.05).
PE/Plasma FNS ratio of palmitate and oleate are statistically different from that of vaccenate (P < 0.05).
CL/Plasma FNS ratio of palmitate and stearate are statistically different from that of vaccenate (P < 0.05).
Assuming plasma fatty acids to be the source of acyl-groups of cardiac phospholipids, FNS of acyl groups in cardiac phospholipids were expected to be less than or equal to those found in plasma fatty acids when the turnover of acyl groups is less (slower) than that of the corresponding plasma fatty acid. This was observed in all nonessential fatty acids in PE and in CL (Table 3). The ratio of FNS in a fatty acid from CL or PE to the FNS of the respective fatty acids in plasma represents relative turnover of these fatty acids in CL and PE by synthesis or remodeling during the 14 day period. These ratios are presented as the PE/plasma and CL/plasma ratios (Table 3). In PE, the remodeling with stearate was very rapid, and its enrichment approached 90.6% of plasma stearate in RCD fed animals. The remodeling of vaccenate was similarly rapid, approaching 100% in 14 days. In contrast, the remodeling with palmitate and oleate in PE were only moderate being 70.8% and 60.1%, respectively. Interestingly, the pattern of remodeling in CL was distinctly different from that of PE. In CL, remodeling with vaccenate was most rapid at 95.0%, comparable to the remodeling of oleate at 96.4%. The remodeling of saturated fatty acids, palmitate and stearate (C16:0 and C18:0) were low at 7.5% and 18.5%, respectively. In animals fed the HFD, remodeling was suppressed in PE and in CL as evident by the suppressed vaccenate turnover rate, suggesting dietary influences on PE and CL synthesis or remodeling.
Gene expression studies by real-time PCR
To investigate whether any of the enzymes involved in cardiolipin synthesis and remodeling were influenced by the dietary change at the level of gene expression, we performed gene expression analysis using reverse transcription of mRNA extracted from the heart and real-time PCR. Gene expression data is listed as fold change of the RCD group ± SEM. Because the turnover of fatty acids in PE and CL are regulated by genes responsible for phosphatidic acid synthesis and acylation and transacylation, the following genes were studied with the corresponding results: ALCAT, HFD group fold-change is 0.85 ± 0.27 that of the RCD group; PGPS, 1.02 ± 0.28-fold-change; CLS, 1.10 ± 0.18-fold-change; GPAT1, 0.91 ± 0.38-fold-change.
DISCUSSION
Cardiolipin is a unique phospholipid found in the mitochondrial membrane. It is composed of two phosphatidyl groups joined by a glycerol bridge. CL interacts with membrane proteins enabling numerous mitochondrial functions such as oxidative phosphorylation, transport of ATP/ADP, and apoptosis (36). The molecular species of CL vary from tissue to tissue (37). The fatty acid composition of CL depends on the synthesis of phosphatidic acid, and the acylation and deacylation or transacylation cycle (remodeling), which is affected by the nutritional and physiological state. Disorders of CL remodeling have been reported in many clinical conditions as in low fat diet, aging, obesity, diabetes, heart failure, and in a rare genetic disease (Barth syndrome) (1, 4, 38–41).
The turnover 3 of essential and nonessential fatty acids of cardiolipin in rat liver was previously studied by Landriscina et al. (24) using 1-14C linoleate and 14C-acetate. The isotopes were administered as separate intraperitoneal boluses, animals were sacrificed at predetermined intervals, and radioactivity in liver cardiolipin was determined. The decay curve of 14C-linoleate specific activity was used to determine the half-life (turnover) of linoleate in CL, and the decay curve of 14C radioactivity in 14C-acetate experiment, the turnover of nonessential fatty acids. The radioactivity in individual fatty acids was not determined, but the half-life of linoleate as well as nonessential fatty acids was found to be about 2 1/2 days. The turnover of nonessential fatty acids was found to be slightly slower than the turnover of these fatty acids in other phospholipids. The turnover of glycerol in CL was also studied using 2- 3 H-glycerol, and the half-life of the tritium ( 3 H) decay curve of liver CL was found to be much longer than those for 14C-linoleate or 14C-acetate. The disparate turnover rate between acyl groups and glycerol in CL suggests an active process of acylation and deacylation or remodeling instead of new synthesis. However, the Landriscina study has two limitations. First, the radioactivity decay curves can be influenced by the synthesis and/or turnover of these fatty acids in the plasma pool, and second, the different nonessential fatty acids were not separated. Therefore, no specific information regarding the turnover of nonessential fatty acids could be estimated from the study.
In the current study, we used deuterium supplied as D2O to label the individual nonessential fatty acids. The FNS of these fatty acids in plasma, PE, and CL were determined from the mass shift and deuterium enrichment in water. Even though the exact turnover could not be determined from a single time point of FNS (see footnote 1), the relative contribution from newly synthesized fatty acids to the various pools reflects the turnover rate over the time interval, and the relative contribution of the individual fatty acids from plasma to PE and CL remodeling can be compared (Table 3). In PE, we found that the relative turnover (PE/Plasma ratio) of stearate and vaccenate were the highest. In contrast, in CL, the turnover (CL/Plasma ratio) of oleate and vaccenate were the highest, and palmitate and stearate were the lowest (or minimal). The high turnover rate of oleate and vaccenate in CL, and stearate and vaccenate in PE, suggest possible substrate specificity of PL acylation and transacylation enzymes. Therefore, PL remodeling is probably an important functional aspect of PL metabolism in general. The turnover of nonessential fatty acids in PE and CL from animals fed the HFD was lower than those in lipids from animals fed the RCD. Such findings suggest possible downregulation of the acylation and reacylation processes by HFD or the expansion of these fatty acid pools in PE and CL.
Fatty acid turnover in CL can occur during its biosynthesis or remodeling. Acylation is the primary process in phosphatidic acid synthesis during new synthesis. Remodeling of CL is known to occur via deacylation-acylation in the sn2 position. The deacylation step has been proposed to be mediated by phospholipase A2, resulting in formation of MLCL, which is reacylated by two proposed enzymatic reactions converting MLCL back to CL. In the first acylation reaction, acylation occurs via MLCL AT, which utilizes acyl-CoA as a substrate (18–20). Studies of MLCL AT enzyme have shown higher incorporation of linoleate-CoA and oleate-CoA than palmitate-CoA into CL. The activity of MLCL AT toward vaccenate has not been investigated. Decrease in enzyme activities toward the CoAs was also reported in presence of PE, lyso-PE, phosphotidylcholine (PC), and lyso-PC suggesting substrate competition at both acyl-donor and acyl-acceptor levels for MLCL AT. The second reaction for CL remodeling occurs via the transfer of acyl group of PC and PE into MLCL (21–23). The transacylation was linoleoyl-specific for a PC donor in comparison to oleyl and arachidonoyl based on a rat liver study by Xu et al. (21). The specificity is reduced for acyl-transfer from PE although preference toward linoleoyl was maintained.
The low fraction of new synthesis of palmitate and stearate in CL (Fig. 1 and Table 3) suggests that the two fatty acids were probably introduced to CL during its synthesis but not significantly involved in regeneration of MLCL into CL. These results were expected because MLCL acylation was reported to incorporate linoleoyl-CoA and oleyl-CoA more than palmitate-CoA as substrates of MLCL AT (18), whereas the transacylation approach was specific toward linoleoyl transfer (21). An unexpected result was observed in the acylation of CL by vaccenate, a fatty acid that has been identified in CL previously (1–3). Although we have not elucidated the mechanism of vaccenate incorporation into CL, the high FNS of vaccenate over the FNS of palmitate and stearate in CL can best be explained by vaccenate being involved in remodeling of preexisting CL. Since the fraction of new synthesis of oleate, vaccenate and stearate of cardiac PE are similar to those in plasma fatty acids (Table 3), it is likely that cardiac phospholipids derive their fatty acids from the plasma pool. Therefore, the deuterated vaccenate must have originated from plasma vaccenate, and was incorporated into CL either directly as vaccenate-CoA by MLCL AT or indirectly from transacylation of phospholipids such as PE and/or PC containing the deuterated vaccenate. Oleate has also been reported as a substrate for acylation of MLCL in CL remodeling with comparable activity of MLCL AT toward oleate and linoleate (18). The high CL/Plasma ratios indicating high turnover for oleate and vaccenate support the possibility that oleate and vaccenate are both substrates for the MLCL AT enzyme.
The fatty acid profile of CL is known to be comprised mostly of linoleate in contrast to that of PE, which contains a high concentration of stearate. The molecular species of CL are diverse in the combination of saturated and unsaturated fatty acids of different chain lengths in the four acyl groups. “Shotgun” lipidomics studies have yielded information on CL molecular species and features of remodeling (42, 43). For example, two major molecular species have been found in CL of heart, liver, and muscle tissues. The major molecular species are: 18:2, 18:2, 18:2, 18:2- CL, and 18:2, 18:2, 18:2, 18:1- CL in the approximate ratio of 5 to 2 accounting for over half of the CL content in these tissues (44). Therefore, the turnover of C18:1 (oleate or vaccenate) can substantially alter the ratio of these two molecular species. The distinction between the two C18:1, oleate (C18:1n-9) and vaccenate (C18:1n-7), was often not made in previous reports (38, 39, 44–46). This may be due to their identical molecular weights and the difficulty in the separation of the two C18:1 species by chromatographic methods. In one report that did differentiate between oleate and vaccenate, there were slight differences in the reported levels of fatty acids (Table 2). These differences in vaccenate levels in CL could be attributed to the differences in the animal species, age, diet treatment, and experimental procedure such as method of sample preparation and detection.
The role of vaccenate in metabolism and physiology has yet to be fully explored. Utilizing tracer-based metabolomics, we found rapid incorporation of the stable isotope tracer into CL vaccenate. The high deuterium enrichment in vaccenate as compared with those of palmitate or stearate confirms its participation as one of the substrates for MLCL acylation during CL remodeling. Our analysis showed that the vaccenate enrichment from de novo synthesis in plasma, PE, and CL was greater compared with palmitate and stearate. The concentration of vaccenate has been known to be low in lipid species except in bacterial lipids such as cardiolipin. Although both oleate and vaccenate are 18-carbon monounsaturated fatty acids, vaccenate is produced through the 16-carbon desaturation pathway. (Palmitate is desaturated into palmitoleate, which undergoes subsequent chain elongation to form vaccenate) (30). Therefore, unlike oleate, vaccenate synthesis is not dependent on that of stearate, as observed in the higher FNS vaccenate compared with FNS stearate and FNS oleate. The high synthesis rate of vaccenate suggests rapid conversion of palmitoleate into vaccenate, with a possible rate-limiting step in the desaturation of palmitate into palmitoleate. Such a high rate of turnover suggests vaccenate to be a metabolically active substrate, and the synthesis of palmitoleate and its conversion to vaccenate to be an important regulatory mechanism in mitochondrial metabolism. Although HFD affected vaccenate turnover, it did not affect enzymes involved in cardiolipin synthesis and remodeling at the level of gene expression. However, it remains possible these metabolic processes are affected by HFD at multiple levels posttranscriptionally. Nonetheless, our present finding that vaccenate turnover was affected by HFD suggests that vaccenate may play an important role in the regulation of mitochondrial function by regulating the composition of CL species through CL remodeling.
Footnotes
- CDP-DAG
- cytidinediphosphate-diacylglycerol
- CL
- cardiolipin
- D2O
- deuterium-enriched water
- FNS
- fraction of new synthesis
- HFD
- high-fat diet
- MLCL
- monolysocardiolipin
- MLCL AT
- monolysocardiolipin acyltransferase
- PC
- .
- PE
- phosphatidylethanolamine
- PGP
- phosphatidylglycerolphosphate
- PL
- phospholipid
- RCD
- regular chow diet
- SIM
- selective ion monitoring
The approach to steady state is a multi-exponential function dependingon the number of pools. It is often approximated by that of asingle exponential. For that reason, a single measurement of the FNSalong the exponential curve underestimates the true turnover rate(26).
The turnover rate or replacement rate refers to the contribution ofa metabolic process such as de novo lipogenesis to the maintenance ofa steady state level of a substrate. At steady state, the FNS is the turnoverrate. For example, the FNS of plasma palmitate is 16%, meaning that16% of the palmitate is derived from de novo lipogenesis. The remainderof palmitate is either from a dietary source or from lipolysis of fat.
This study is supported by the Biomedical Mass Spectrometry Laboratory of the General Clinical Research Center (GCRC) at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (PHS M01-RR00425; UCLA CTSI 1UL1-RR033176) and the Metabolomics Core Laboratory of the UCLA Center of Excellence in Pancreatic Diseases (P01 AT003960). J.Z. and M.A.T. are supported by California Institute of Regenerative Medicine (CIRM) awards TG2-01169 and RB1-01397. J.K.Y. is supported by K23 DK083241 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Clinical Scholar Award from the Pediatric Endocrine Society.
REFERENCES
- 1. Wolff R. L., Entressangles B. 1991. Compositional changes of fatty acids in the 1(1”)-and 2(2”)-positions of cardiolipin from liver, heart, and kidney mitochondria of rats fed a low-fat diet. Biochim. Biophys. Acta. 1082: 136–142. [DOI] [PubMed] [Google Scholar]
- 2. Wolff R. L., Combe N. A., Entressangles B., Sebedio J. L., Grandgirard A. 1993. Preferential incorporation of dietary cis-9,cis-12,trans-15 18:3 acid into rat cardiolipins. Biochim. Biophys. Acta. 1168: 285–291. [DOI] [PubMed] [Google Scholar]
- 3. Wolff R. L., Combe N. A., Entressangles B. 1985. Positional distribution of fatty acids in cardiolipin of mitochondria from 21-day-old rats. Lipids. 20: 908–914. [DOI] [PubMed] [Google Scholar]
- 4. Lee H. J., Mayette J., Rapoport S. I., Bazinet R. P. 2006. Selective remodeling of cardiolipin fatty acids in the aged rat heart. Lipids Health Dis. 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schlame M., Ren M., Xu Y., Greenberg M. L., Haller I. 2005. Molecular symmetry in mitochondrial cardiolipins. Chem. Phys. Lipids. 138: 38–49. [DOI] [PubMed] [Google Scholar]
- 6. Sparagna G. C., Chicco A. J., Murphy R. C., Bristow M. R., Johnson C. A., Rees M. L., Maxey M. L., McCune S. A., Moore R. L. 2007. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid Res. 48: 1559–1570. [DOI] [PubMed] [Google Scholar]
- 7. Esposti M. D., Cristea I. M., Gaskell S. J., Nakao Y., Dive C. 2003. Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death Differ. 10: 1300–1309. [DOI] [PubMed] [Google Scholar]
- 8. Schlame M., Rua D., Greenberg M. L. 2000. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 39: 257–288. [DOI] [PubMed] [Google Scholar]
- 9. McMillin J. B., Dowhan W. 2002. Cardiolipin and apoptosis. Biochim. Biophys. Acta. 1585: 97–107. [DOI] [PubMed] [Google Scholar]
- 10. Hoch F. L. 1992. Cardiolipins and biomembrane function. Biochim. Biophys. Acta. 1113: 71–133. [DOI] [PubMed] [Google Scholar]
- 11. Hatch G. M. 2004. Cell biology of cardiac mitochondrial phospholipids. Biochem. Cell Biol. 82: 99–112. [DOI] [PubMed] [Google Scholar]
- 12. Houtkooper R. H., Vaz F. M. 2008. Cardiolipin, the heart of mitochondrial metabolism. Cell. Mol. Life Sci. 65: 2493–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanacev N. Z., Davidson J. B., Stuhne-Sekalec L., Domazet Z. 1972. The mechanism of the biosynthesis of cardiolipin in mitochondria. Biochem. Biophys. Res. Commun. 47: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 14. Hostetler K. Y., van den Bosch H., van Deenen L. L. 1972. The mechanism of cardiolipin biosynthesis in liver mitochondria. Biochim. Biophys. Acta. 260: 507–513. [DOI] [PubMed] [Google Scholar]
- 15. Sparagna G. C., Lesnefsky E. J. 2009. Cardiolipin remodeling in the heart. J. Cardiovasc. Pharmacol. 53: 290–301. [DOI] [PubMed] [Google Scholar]
- 16. Schlame M. 2008. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid Res. 49: 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Houtkooper R. H., Akbari H., van Lenthe H., Kulik W., Wanders R. J., Frentzen M., Vaz F. M. 2006. Identification and characterization of human cardiolipin synthase. FEBS Lett. 580: 3059–3064. [DOI] [PubMed] [Google Scholar]
- 18. Ma B. J., Taylor W. A., Dolinsky V. W., Hatch G. M. 1999. Acylation of monolysocardiolipin in rat heart. J. Lipid Res. 40: 1837–1845. [PubMed] [Google Scholar]
- 19. Taylor W. A., Hatch G. M. 2003. Purification and characterization of monolysocardiolipin acyltransferase from pig liver mitochondria. J. Biol. Chem. 278: 12716–12721. [DOI] [PubMed] [Google Scholar]
- 20. Taylor W. A., Hatch G. M. 2009. Identification of the human mitochondrial linoleoyl-coenzyme A monolysocardiolipin acyltransferase (MLCL AT-1). J. Biol. Chem. 284: 30360–30371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu Y., Kelley R. I., Blanck T. J., Schlame M. 2003. Remodeling of cardiolipin by phospholipid transacylation. J. Biol. Chem. 278: 51380–51385. [DOI] [PubMed] [Google Scholar]
- 22. Xu Y., Malhotra A., Ren M., Schlame M. 2006. The enzymatic function of tafazzin. J. Biol. Chem. 281: 39217–39224. [DOI] [PubMed] [Google Scholar]
- 23. Malhotra A., Xu Y., Ren M., Schlame M. 2009. Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochim. Biophys. Acta. 1791: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Landriscina C., Megli F. M., Quagliariello E. 1976. Turnover of fatty acids in rat liver cardiolipin: comparison with other mitochondrial phospholipids. Lipids. 11: 61–66. [DOI] [PubMed] [Google Scholar]
- 25. Lee W. N., Bassilian S., Guo Z., Schoeller D., Edmond J., Bergner E. A., Byerley L. O. 1994. Measurement of fractional lipid synthesis using deuterated water (2H2O) and mass isotopomer analysis. Am. J. Physiol. 266: E372–E383. [DOI] [PubMed] [Google Scholar]
- 26. Lee W. N., Bassilian S., Ajie H. O., Schoeller D. A., Edmond J., Bergner E. A., Byerley L. O. 1994. In vivo measurement of fatty acids and cholesterol synthesis using D2O and mass isotopomer analysis. Am. J. Physiol. 266: E699–E708. [DOI] [PubMed] [Google Scholar]
- 27. Folch J., Lees M., Stanley G. H. S. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 28. Corcelli A., Saponetti M. S., Zaccagnino P., Lopalco P., Mastrodonato M., Liquori G. E., Lorusso M. 2010. Mitochondria isolated in nearly isotonic KCl buffer: focus on cardiolipin and organelle morphology. Biochim. Biophys. Acta. 1798: 681–687. [DOI] [PubMed] [Google Scholar]
- 29. Aveldano M. I., Honocks L. A. 1982. Quantitative release of fatty acids from lipids by a simple hydrolysis procedure. J. Lipid Res. 24: 1101–1105. [PubMed] [Google Scholar]
- 30. Yee J. K., Mao C. S., Hummel H. S., Lim S., Sugano S., Rehan V. K., Xiao G., Lee W-N. P. 2008. Compartmentalization of stearoyl-coenzyme A desaturase 1 activity in HepG2 cells. J. Lipid Res. 49: 2124–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blom K., Dybowsky C., Munson B., Gates B., Hasselbring L. 1987. Mass spectral analysis of isotopically labeled compounds: average mass approach. Anal. Chem. 59: 1372–1374. [Google Scholar]
- 32. Lee W. N., Byerley L. O., Bergner E. A., Edmond J. 1991. Mass isotopomer analysis: theoretical and practical considerations. Biol. Mass Spectrom. 20: 451–458. [DOI] [PubMed] [Google Scholar]
- 33. Ajie H. O., Connor M. J., Lee W. N., Bassilian S., Bergner E. A., Byerley L. O. 1995. In vivo study of the biosynthesis of long-chain fatty acids using deuterated water. Am. J. Physiol. 269: E247–E252. [DOI] [PubMed] [Google Scholar]
- 34. Lee W. N., Bergner E. A., Guo Z. K. 1992. Mass isotopomer pattern and precursor-product relationship. Biol. Mass Spectrom. 21: 114–122. [DOI] [PubMed] [Google Scholar]
- 35. Lee W. N., Bassilian S., Lim S., Boros L. G. 2000. Loss of regulation of lipogenesis in the Zucker diabetic (ZDF) rat. Am. J. Physiol. Endocrinol. Metab. 279: E425–E432. [DOI] [PubMed] [Google Scholar]
- 36. Schlame M., Rustow B. 1990. Lysocardiolipin formation and reacylation in isolated rat liver mitochondria. Biochem. J. 272: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Houtkooper R. H., Turkenburg M., Poll-The B. T., Karall D., Perez-Cerda C., Morrone A., Malvagia S., Wanders R. J., Kulik W., Vaz F. M. 2009. The enigmatic role of tafazzin in cardiolipin metabolism. Biochim. Biophys. Acta. 1788: 2003–2014. [DOI] [PubMed] [Google Scholar]
- 38. Han X., Yang J., Yang K., Zhao Z., Abendschein D. R., Gross R. W. 2007. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry. 46: 6417–6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valianpour F., Wanders R. J., Barth P. G., Overmars H., van Gennip A. H. 2002. Quantitative and compositional study of cardiolipin in platelets by electrospray ionization mass spectrometry: application for the identification of Barth syndrome patients. Clin. Chem. 48: 1390–1397. [PubMed] [Google Scholar]
- 40. Li J., Romestaing C., Han X., Li Y., Hao X., Wu Y., Sun C., Liu X., Jefferson L. S., Xiong J., et al. 2010. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 12: 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saini-Chohan H. K., Holmes M. G., Chicco A. J., Taylor W. A., Moore R. L., McCune S. A., Hickson-Bick D. L., Hatch G. M., Sparagna G. C. 2009. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J. Lipid Res. 50: 1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han X., Yang J., Cheng H., Yang K., Abendschein D. R., Gross R. W. 2005. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry. 44: 16684–16694. [DOI] [PubMed] [Google Scholar]
- 43. Zhang L., Bell R. J. A., Kiebish M. A., Seyfried T. N., Han X., Gross R. W., Chuang J. H. 2011. A mathematical model for the determination of steady-state cardiolipin remodeling mechanisms using lipidomic data. PLoS ONE. 6: e21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han X., Yang K., Yang J., Cheng H., Gross R. W. 2006. Shotgun lipidomics of cardiolipin molecular species in lipid extracts of biological samples. J. Lipid Res. 47: 864–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Minkler P. E., Hoppel C. L. 2010. Separation and characterization of cardiolipin molecular species by reverse-phase ion pair high-performance liquid chromatography-mass spectrometry. J. Lipid Res. 51: 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang H. Y., Jackson S. N., Woods A. S. 2007. Direct MALDI-MS analysis of cardiolipin from rat organs sections. J. Am. Soc. Mass Spectrom. 18: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]