Abstract
Subnormal HDL-cholesterol (HDL-C) and apolipoprotein (apo)AI levels are characteristic of familial hypercholesterolemia (FH), reflecting perturbed intravascular metabolism with compositional anomalies in HDL particles, including apoE enrichment. Does LDL-apheresis, which reduces HDL-cholesterol, apoAI, and apoE by adsorption, induce selective changes in HDL subpopulations, with relevance to atheroprotection? Five HDL subpopulations were fractionated from pre- and post-LDL-apheresis plasmas of normotriglyceridemic FH subjects (n = 11) on regular LDL-apheresis (>2 years). Apheresis lowered both plasma apoE (−62%) and apoAI (−16%) levels, with preferential, genotype-independent reduction in apoE. The mass ratio of HDL2:HDL3 was lowered from ∼1:1 to 0.72:1 by apheresis, reflecting selective removal of HDL2 mass (80% of total HDL adsorbed). Pre-LDL-apheresis, HDL2 subpopulations were markedly enriched in apoE, consistent with ∼1 copy of apoE per 4 HDL particles. Large amounts (50-66%) of apoE-HDL were removed by apheresis, preferentially in the HDL2b subfraction (−50%); minor absolute amounts of apoE-HDL were removed from HDL3 subfractions. Furthermore, pre-β1-HDL particle levels were subnormal following removal (−53%) upon apheresis, suggesting that cellular cholesterol efflux may be defective in the immediate postapheresis period. In LDL-receptor (LDL-R) deficiency, LDL-apheresis may enhance flux through the reverse cholesterol transport pathway and equally attenuate potential biglycan-mediated deposition of apoE-HDL in the arterial matrix.
Keywords: high density lipoprotein heterogeneity, HDL proteome, low density lipoprotein receptor mutations, premature atherosclerosis, apolipoprotein E
Until now, premature atherosclerosis and coronary heart disease (CHD) typical of familial hypercholesterolemia (FH) have been primarily equated with marked elevation in circulating levels of atherogenic cholesterol-rich LDL, resulting in attenuated LDL receptor activity due to LDL receptor gene mutations (1). Accumulating evidence suggests, however, that subnormal levels of atheroprotective HDL may equally contribute to accelerated atherogenesis in FH (2–7).
The large Emerging Risk Factor Collaboration study firmly established that circulating HDL-cholesterol (HDL-C) levels less than approximately 1.2 mmol/l (50 mg/dl), which typically correspond to the 50th percentile in population studies, are independently and strongly associated with elevated coronary risk (3). For a one standard deviation decrement in HDL-C, CHD risk increased by 22% (3). Significantly, subnormal levels of both HDL-C and apolipoprotein (apo)AI are characteristic of heterozygous and homozygous FH (8–11); these abnormalities arise on the one hand from cholesteryl ester transfer protein (CETP)-mediated depletion of cholesteryl ester (CE) in FH HDL (6), and on the other from a dual defect in apoAI metabolism involving a decreased production rate concomitant with enhanced degradation (8, 11). Increased catabolism of apoAI may originate from accelerated CETP activity with triglyceride enrichment and renal clearance and equally from the 2-fold-expanded pool size of apoE in FH HDL relative to that in normolipidemic (NL) subjects (8, 12, 13). Under these conditions, apoE-HDL may be efficiently removed by an apoE-dependent receptor pathway in the liver, such as that involving the LDL-receptor-related protein (LRP), thereby enhancing apoAI-HDL catabolism in FH (8, 14–17). ApoE-containing HDL may therefore function to compensate for the defective role of LDL in returning cholesterol to the liver in FH. This compensatory mechanism is insufficient to avoid development of atherosclerosis, as cholesterol-loaded macrophage foam cells are typical of xanthomas and atherosclerotic plaques in FH subjects.
It is well established that HDL particles are heterogeneous in physicochemical properties, structure, metabolism, and atheroprotective function (18–22); indeed, HDL consists of multiple spherical particle subpopulations differing in size and lipid and protein composition (18, 20, 23). Interestingly, apoE is a component of the proteome of all HDL particle subpopulations in normolipidemic subjects, but it is enriched in light, large CE-rich HDL2b and small, dense lipid-poor HDL3c (18). Furthermore, lipid-poor, apoAI-containing, pre-β1-HDL accounts for <10% of total HDL (18, 20, 23). Significantly, pre-β1-HDL not only exhibits high affinity for cellular cholesterol efflux via the ATP-binding cassette class A member 1 (ABCA1)-mediated pathway but also equally appears to represent a marker of the efficiency of plasma HDL formation and of particle remodeling (18, 20, 24).
LDL-apheresis is highly efficacious in acutely reducing atherogenic lipoprotein levels (mainly LDL; up to −80%) in severe FH patients with CHD in whom treatment with diet and lipid-lowering agents fails to attain desirable LDL-cholesterol (LDL-C) levels (25, 26). Such procedures are based on the electrostatic binding and retention of positively charged lipoprotein particles containing apoB100 to a polyanion matrix (27). Equally, however, variable amounts of HDL particles may be removed by this procedure (25, 28, 29); recent evidence suggests that HDL-apoE content may constitute a major determinant of such removal (30, 31). The impact of apheresis on pre-β1-HDL remains indeterminate. In view of the role of apoE-rich HDL and of pre-β1-HDL in the reverse cholesterol transport pathway and of the binding of apoE-HDL to arterial matrix biglycans (32–34), we evaluated the potential impact of particle adsorption and perturbed metabolism during LDL-apheresis on HDL heterogeneity and on potential HDL-mediated atheroprotection on a background of LDL-receptor (LDL-R) deficiency.
METHODS
FH patients, LDL-apheresis, control subjects, and blood samples
Eleven patients (mean age: 35 ± 5 years; body mass index (BMI): 23.5 ± 1.4 kg/m2) displaying severe FH (seven men; four women), who regularly underwent LDL-apheresis once every two weeks in our Hemobiotherapy unit, were included in the study; the minimum period of regular treatment by LDL-apheresis was two years. Such prolonged treatment resulted in a high degree of consistency in the systemic profile of lipid and inflammatory biomarkers at the end of each interim period between apheresis sessions (pre-LDL-apheresis time point) (see Table 1 ). It is noteworthy that the majority of patients underwent apheresis during the morning; their low plasma triglyceride levels are consistent with a fasting state (Table 1). The rationale for treatment by LDL-apheresis was established in accordance with the recommendations of the HEART-UK LDL-apheresis Working Group (35); despite treatment at maximized tolerated doses of lipid-lowering agents, all patients displayed LDL-C > 200 mg/dl. Diagnosis of FH was validated by clinical and biological phenotyping, genetic analyses, and familial histories. Ten patients displayed molecular defects in the LDL-R gene ( Table 2 ). Two patients were homozygous for distinct single-point mutations in the LDL-R gene, three were heterozygous carriers, and six were compound heterozygotes for two distinct (primarily missense) mutations. ApoE genotyping revealed that eight patients were homozygous for apoE3, one for apoE4/E4, one for poE2/apoE3, and one for apoE3/apoE4. All patients were treated with lipid-lowering drugs in combination (atorvastatin/ezetimibe, 80 mg/10 mg daily).
TABLE 1.
Impact of LDL-apheresis on plasma lipid, lipoprotein, apolipoprotein, CRP, and LpPLA2 levels in FH patients
| Parameters (mg/dl) | Controls | Pre LDL-apheresis | Post-LDL-apheresis | P \(pre- versus post-LDL-apheresis) |
| Total cholesterol | 162.5 ± 8.7 | 278.6 ± 24.8 a | 92.1 ± 7.7 a (−67%) | <0.0001 |
| Triglyceride | 62.6 ± 3.6 | 81.8 ± 10.3 | 29.2 ± 4.5 a (−64%) | <0.0001 |
| LDL-C | 96.5 ± 7.9 | 223.3 ± 25.6 a | 53.1 ± 8.9 b (−76%) | <0.0001 |
| ApoB | 76.0 ± 7.3 | 142.8 ± 12.4 a | 36.4 ± 4.3 a (−75%) | <0.0001 |
| HDL-C | 56.6 ± 3.3 | 39.0 ± 3.0 a | 33.2 ± 2.8 a (−15%) | <0.0001 |
| ApoAI | 142.4 ± 6.5 | 113.0 ± 7.1 b | 94.8 ± 6.9 a (−16%) | <0.0001 |
| Lp(a) | 18 (10-45) | 11 (10-49) | 10 (10-15) b | <0.05 |
| TC/HDL-C ratio | 2.9 ± 0.2 | 8.1 ± 1.5 b | 3.2 ± 0.7 (−60%) | <0.0002 |
| ApoB/apoAI ratio | 0.5 ± 0.1 | 1.4 ± 0.2 b | 0.4 ± 0.1 (−68%) | <0.0001 |
| ApoE | 4.1 ± 0.7 | 3.79 ± 0.31 | 1.45 ± 0.13 a (−62%) | <0.0001 |
| LpPLA2 (ng/ml) | 195.7 ± 36.4 | 249.0 ± 29.0 | 87.4 ± 11.7 b (−65%) | <0.0001 |
| hsCRP (mg/l) | 0.4 ± 0.2 | 0.53 ± 0.21 | 0.18 ± 0.04 | NS |
Comparison of lipid profile in FH patients with lipid profile in NL control subjects. Values for lipid and apolipoprotein levels are expressed as means ± SEM (n = 11 for FH, n = 10 for NL). Percentage change on LDL-apheresis is in parentheses. Due to its asymmetric distribution, Lp(a) levels are expressed as median (minimum-maximum).
NS, nonsignificant.
P < 0.001 versus NL.
0.001 < P < 0.01 versus NL.
TABLE 2.
Mutations in the LDL-R gene in FH patients undergoing LDL-apheresis
| Patients (n = 10) | Nucleotide change | Codon change | Type |
| 1 | c.2043 C>A | p.Cys681X | Nonsense |
| 2 | c.2177 C>T | p.Thr726Ile | Missense |
| c.910 G>T | p.Asp304Tyr | Missense | |
| 3 | c.681 C>G | p.Asp227Glu | Missense |
| c.1268 T>C | p.Ile423Thr | Missense | |
| c.829 G>A | p.Glu277Lys | Missense | |
| 4 | c.1705 G>T | p.Asp569Thr | Missense |
| c.2101 C>G | p.Leu401Val | Missense | |
| 5 | c.1975 A>C | p.Thr659Pro | Missense |
| c.259 T>G | p.Trp87Gly | Missense | |
| 6 | c.1069 G>A | p.Glu357Lys | Missense |
| c.530 C>T | p.Ser156Leu | Missense | |
| 7 | c.1019 GC>TG | p.Cys340Leu | Missense |
| 8 | c.(?_191)_(940_?)dup | Frameshift | |
| 9 | c.(?_1846)_(2541_?)del | Frameshift | |
| 10 | c.1359-1 G>A | Splice Site | Frameshift |
The nucleotide change represents the numbering of the mutation in the DNA sequence. The numbering of the codon change includes the 21 amino acids of the signal peptide. For one patient, no searched mutation was found in the LDL-R gene or in the apoB gene. Description of DNA sequence variants is according to nomenclature of Human Genome Variation Society (52).
Three types of adsorption columns were used for LDL-apheresis: the Kaneka® Whole Blood system (n = 4), the Kaneka® Plasma system (Kaneka Corporation, Japan) (n = 1) and the Fresenius Direct Adsorption of Lipoprotein (DALI®) system (n = 6) (Fresenius Medical Care, Germany). To avoid blood coagulation in the column, 3,000-1,1000 units of sodium heparin (PanPharma® 25,000 UI/5 ml) were added; heparin dose was dependent on the long term anticoagulant therapy used in each patient. The blood volume to be treated was a function of the patient's body weight, total blood volume, and LDL-C level preapheresis (26). In our Apheresis Center, the plasma LDL-C target postapheresis is 50 mg/dl or less; consequently, it was necessary to apherese larger plasma volumes in homozygous compared with heterozygous patients. The duration of each LDL-apheresis session depended upon the blood flow rate (50-80 ml/min), the blood volume to treat and attainment of the LDL-C target. To obtain a high level of compliance with satisfactory tolerance, each LDL-apheresis session was <3 h duration. Our apheresis methodology involved expansion of plasma volume by <5%; such minor dilution did not significantly modify our experimental findings and, therefore, a correction factor was not applied.
Ten normolipidemic subjects (lipid profile; Table 1) constituted the control group (one woman and nine men without medication and without chronic disease); mean age was 39 ± 5 years, and mean BMI = 22.1 ± 0.4 kg/m2.
Blood samples were obtained by venipuncture i) before coupling the instrument to the cephalic vein of the patient (pre-LDL-apheresis) and ii) immediately after uncoupling the instrument (post-LDL-apheresis). The samples were collected into sterile EDTA-containing tubes (final concentration 1 mg/ml). Blood samples were equally obtained from healthy, age and sex-matched normolipidemic control subjects after overnight fasting (n = 10; for plasma lipid phenotype, see Table 1). Plasma was immediately separated from blood cells by low-speed centrifugation at 2,500 rpm for 20 min at 4°C and frozen at −80°C for up to one month until analysis. It is noteworthy that no deleterious effects of frozen storage on the physicochemical properties or apoE content of HDL were detected. The study was performed in accordance with the ethical principles set forth in the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Isolation of plasma lipoprotein subfractions
Plasma lipoproteins were isolated by single-step, isopycnic nondenaturing density gradient ultracentrifugation in a Beckman SW41 Ti rotor at 40,000 rpm for 44 h in a Beckman XL70 ultracentrifuge at 15°C by a slight modification of the method of Chapman et al. (36) as previously described (37). After centrifugation, each gradient was fractionated into predefined volumes from the meniscus downwards with an Eppendorf precision pipette into 11 fractions corresponding to VLDL + intermediate density lipoproteins (IDL) (d < 1.019 g/ml); five LDL subfractions (LDL-1, d = 1.019-1.023 g/ml; LDL-2, d = 1.023-1.029 g/ml ; LDL-3, d = 1.029-1.039 g/ml; LDL-4, d = 1.039-1.050 g/ml; and LDL-5, d = 1.050-1.063 g/ml; and five HDL subfractions (HDL2b, d = 1.063-1.091 g/ml; HDL2a, d = 1.091-1.110 g/ml; HDL3a, d = 1.110-1.133 g/ml; HDL3b, d = 1.133-1.156 g/ml, and HDL3c, d = 1.156-1.179 g/ml). Gradient fractions were dialyzed at 4°C in the dark to remove salts as described earlier (37).
Plasma lipid and apolipoprotein profile and chemical analyses of lipoproteins
Plasma levels of total cholesterol (TC), triglycerides (TG), HDL-C, LDL-C, apoAI, apoB, apoE, and lipoprotein(a) [Lp(a)]), and the lipid (phospholipid, triglyceride, free cholesterol, and cholesteryl ester) and protein contents of isolated lipoprotein fractions were quantified as described earlier (19, 36, 38) and expressed as total mass of lipoprotein lipid and protein. Plasma lipoprotein concentration was calculated as the sum of the mass of the individual lipid and protein components for each lipoprotein fraction. Plasma apoE concentrations were determined using an immunoturbidimetric assay (Diasys reagents, calibrators, and controls). HDL-apoE concentrations were determined on supernatants from plasma aliquots after precipitation of apoB-containing lipoproteins with manganese-phosphotungstic acid (39).
Determination of plasma pre-beta1-HDL by ELISA
Pre-β1-HDL concentrations in FH plasma pre- and post-LDL-apheresis were determined using the pre-β1-HDL ELISA kit from Sekisui Medical Co. (Tokyo, Japan) as described by Miyazaki et al. (40).
Proteomic profiling of lipoprotein particle subfractions by SELDI-TOF MS
Sample preparation and HDL capture.
Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry) (SELDI-TOF MS) analysis of pre- and postapheresis plasma samples from FH patients (n = 10) was performed as described previously (41). In brief, plasma aliquots were applied onto separate spots of PS20 chips in duplicate. HDL was captured by addition of 100 µl of a 1:2 diluted aliquot with Tris-buffered saline, pH 7.4, onto single SELDI spots on which anti-apoAI was coupled; samples were then allowed to bind for 2 h at room temperature on a horizontal shaker (600 rpm). The chips were washed three times with TBS for 10 min, followed by rinsing with TBS-Tween (0.005%) for 5 min, with a final wash step with HEPES solution (5 mM). All spots were allowed to dry; subsequently, 1.2 µl sinapinic acid (10 mg/ml) in a solvent containing a mixture of acetonitrile/H2O/trifluoroacetic acid (50/49.9/0.1%) was applied to each spot for solubilisation of bound proteins.
SELDI-TOF analysis and data preprocessing.
Spectra were recorded at a laser intensity of 210 relative units, and the focus mass was set to 28 kDa. Measurement of spectra was performed with approximately 100 shots at 13 positions per SELDI spot. Analysis was carried out with a PBS IIc protein chip reader (Ciphergen Biosystems, Fremont, CA) and an automated data collection protocol (Protein-Chip Software, version 3.1.1.). Data were collected to an upper limit of protein mass of 100,000 kDa. Calibration was performed using a protein calibration chip (Bio-Rad). All spectra were automatically corrected for baseline values and spot-to-spot correction was performed with Ciphergen protein chip software.
Bioinformatics and statistics.
Prior to statistical analysis, all generated spectra were preprocessed as described before (42). In brief, each individual mass-to-charge ratio spectrum was divided into nonoverlapping intervals whose sizes increased proportionately with mass-to-charge ratio values. The size of an interval starting at m/z 3,000 was computed as m·r, with r fixed at 0.5%. The intensity associated with each interval was taken as the sum of the intensities over the interval.
Systemic biomarkers of inflammation
Systemic inflammation was assessed as high-sensitivity C reactive protein (hsCRP) concentration in pre- and postapheresis plasma samples using an immunoturbidimetric test (CRP U-hs, Diasys). In addition, mass concentrations of lipoprotein-associated phospholipase A2 (LpPLA2) were assayed using the immunoturbidimetric PLAC test (43), the kind gift of DiaDexus. A high-sensitivity, biochip ELISA array assay was used for determination of plasma vascular endothelial growth Factor (VEGF) levels (Randox Laboratories, Northern Ireland).
Statistical analysis
The effect of LDL-apheresis on each parameter was determined by comparison of baseline values with those after LDL-apheresis by ANOVA. For comparison of control subjects with FH patients pre- or postapheresis, the nonpaired Student t-test was used. The Bonferroni correction was applied to correct for multiple comparisons when such analyses were performed for HDL-apoE content and apoE:apoAI ratio in the five HDL subfractions. All results are expressed as means ± SEM for normally distributed variables and as median (minimum-maximum) for asymmetrically distributed parameters; distribution normality was assessed using the Kolmogorov-Smirnov test.
RESULTS
Comparison of plasma lipid, apolipoprotein, and inflammatory biomarker levels in FH patients pre-LDL-apheresis with those in NL control subjects
At the end of the interim period of ∼14 days between LDL-apheresis sessions, plasma levels of TC, LDL-C, and apoB were markedly higher (up to 2.3-fold) in our FH patient group relative to control subjects (Table 1). In contrast to the markedly elevated levels of atherogenic particles in FH patients, concentrations of HDL-C and apoAI were subnormal (−32%, P < 0.001 and −21%, P < 0.01, respectively) (Table 1). Consequently, both the TC/HDL-C and apoB/apoAI ratios were significantly higher (2-fold or more) in the FH group. Plasma levels of TG and Lp(a) were similar in the two groups. The level of chronic systemic inflammation in the FH group, measured as plasma concentrations of hsCRP and LpPLA2, did not differ significantly from that in the control group.
The major impact of apheresis on reduction of plasma lipid and apoprotein biomarkers in our FH subjects is entirely consistent with that previously reported by others, and it is largely independent of the nature of the apheresis procedure (25, 28). Despite the interval of ∼14 days between sessions, which is equivalent to 3-4 half-lives of HDL apoAI (8, 11), these observations do not allow us to infer that a steady state was attained either in HDL-C metabolism or in the structure and function of HDL particles at the initiation of each apheresis procedure.
HDL particle profile and apoE distribution in FH patients pre-LDL-apheresis and in control subjects
Subnormal levels of HDL-C in FH subjects were principally accounted for by lower abundance of HDL2b, HDL2a, and HDL3a (range, −26 to −29%) relative to control subjects; HDL3b and HDL3c levels were, however, indistinguishable between the two groups (data not shown).
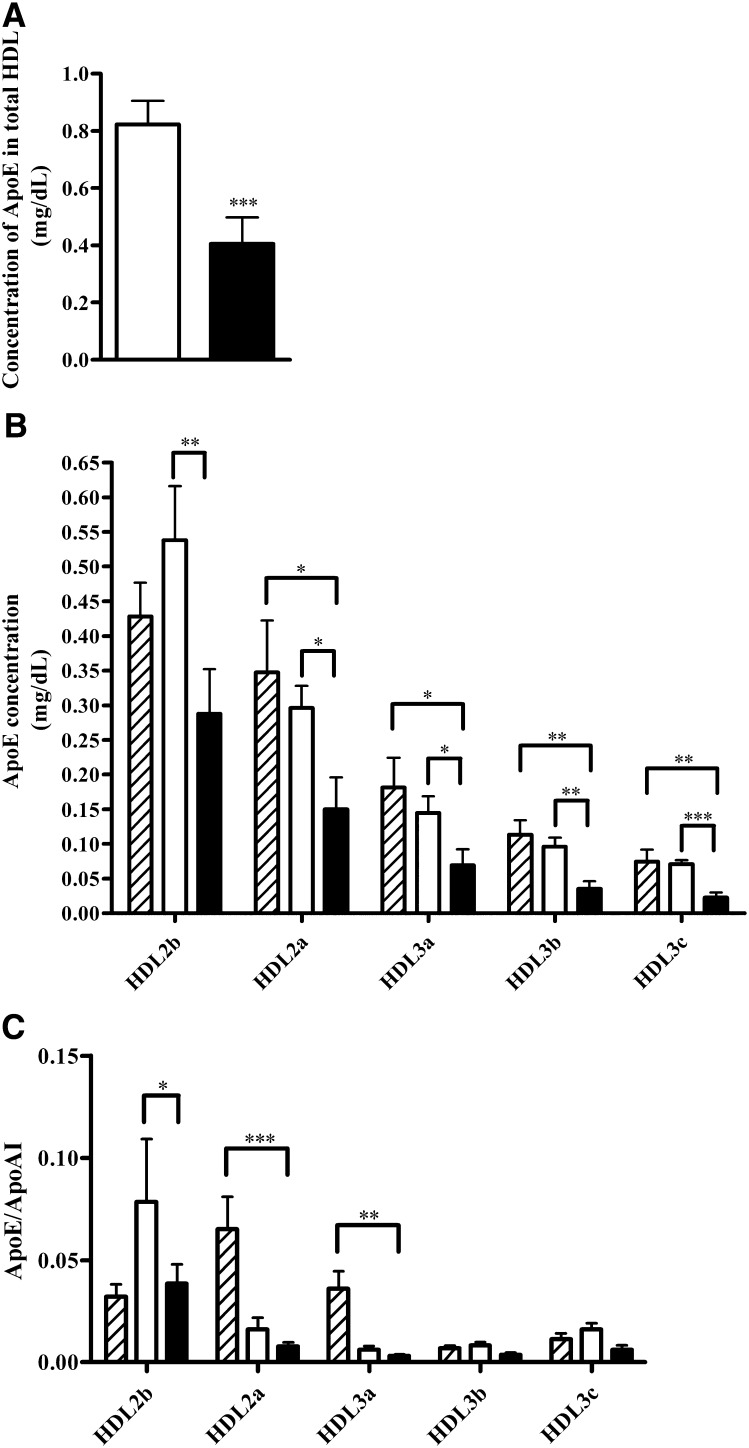
Plasma apoE levels pre-LDL-apheresis (mean, 3.8 ± 0.3 mg/dl) were indistinguishable from those in control subjects; 20% of total plasma apoE was associated with HDL in the FH (pre-LDL-apheresis) group ( Fig. 1A ), of which ∼70% was present in large, CE-rich HDL2 subfractions (Fig. 1B). The density profile of apoE across HDL subfractions in control subjects was indistinguishable from that in FH patients preapheresis (Fig. 1B). The HDL2b subfraction in the FH group preapheresis was distinguished by marked enrichment in apoE, displaying an apoE:apoAI molar ratio of 0.075 and indicative of the presence of 1 copy of apoE per 13 copies of apoAI. This ratio is consistent with the findings of Gibson et al. (12) (fraction III HDL), which observed marked apoE enrichment in the HDL of nontreated FH subjects, with apoE/apoAI molar ratios of 0.11 and 0.03 in FH and control subjects, respectively. In contrast to HDL2b, apoE/apoAI molar ratios were ≤0.01 in HDL2a, HDL3a, HDL3b, and HDL3c subfractions preapheresis.
Fig. 1.
Bar graphs showing (A) apoE concentration in total HDL (n = 11), (B) apoE concentration in each density gradient HDL subfraction (n = 8), and (C) apoE:apoAI ratio in each density gradient HDL subfraction (n = 8) in normolipidemic subjects (dashed bar, n = 6), in FH patients before (open bar) and after (closed bar) LDL-apheresis. Values are means ± SEM. ***P < 0.001, **0.001 < P < 0.01, and *0.01 < P < 0.05 versus before LDL-apheresis.
Impact of LDL-apheresis on plasma lipid, apolipoprotein, and inflammatory biomarker levels in FH patients
Consistent with earlier reports (25, 28, 44), LDL-apheresis significantly lowered total plasma triglyceride, cholesterol, LDL-C, apoB, and Lp(a) levels (−54 to −76%; Table 1). Equally, significant reductions were observed in HDL-C and apoAI levels (−15 and −16%, respectively; P < 0.0001). Finally, the TC:HDL-C ratio was reduced some 2-fold to values less than 5 (range, 1.8-2.9) in 9 of the 11 patients with a 3-fold reduction in apoB:apoAI ratio. By contrast, apheresis effected marked reduction in apoE abundance (−62%; P < 0.0001; Table 1); indeed, removal of HDL-C during apheresis was strongly correlated with baseline (pre-apheresis) apoE content in total HDL (r = −0.71; P < 0.015). Column retention of HDL-C, apoAI, and apoE during apheresis was confirmed by their detection in the regeneration solution following washing of the matrices postapheresis.
Although plasma hsCRP levels pre-LDL-apheresis were within the normal range, LDL-apheresis induced up to a 50% reduction in this biomarker, which is most feasibly explained by retention of CRP on the apheresis column (45). Moreover, whereas baseline levels of LpPLA2 mass in FH patients were at the upper limit of the normal range, apheresis dramatically reduced (up to 3-fold) LpPLA2 (Table 1); this finding is consistent with marked decrease in LDL, the major transport particle for this potentially proinflammatory phospholipase. Finally, apheresis induced reduction (−29%; P < 0.0005) in plasma levels of VEGF, a biomarker of endothelial activation; it is notable that preapheresis levels were similar in FH patients and controls (data not shown).
LDL-apheresis modifies plasma HDL profile and apoE particle abundance in FH patients
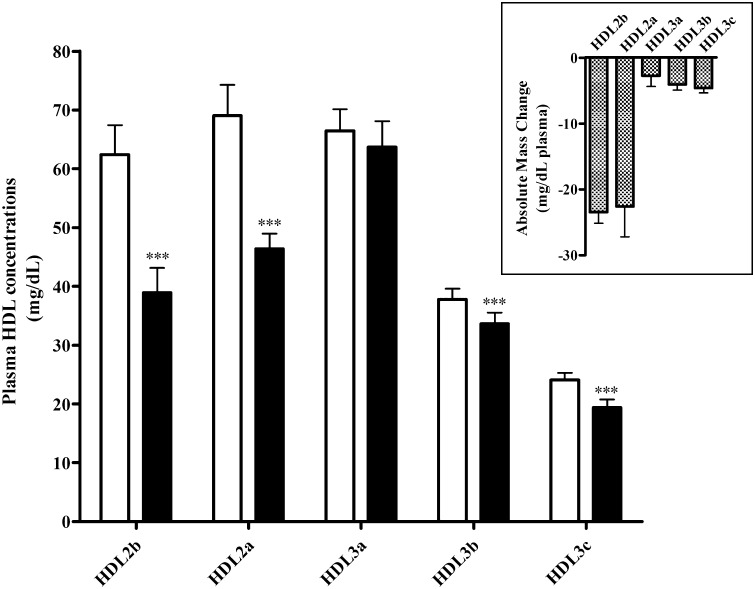
LDL-apheresis induced significant decrease in total mass of HDL (−22%; P < 0.0001; pre, 259.7 ± 13.2 mg/dl versus post, 202.1 ± 10.8 mg/dl, respectively) and to a greater degree than that indicated by HDL-C estimation alone (−15%); reductions of 35% and 9% in levels of both HDL2 (P < 0.0001) and HDL3 mass (P = 0.0001), respectively, accounted for the overall fall in HDL mass. Within HDL2 and HDL3 subpopulations, marked reductions were observed in HDL2b (−37%; P < 0.0001) and HDL2a (−33%; P < 0.001), but only minor decrements occurred in HDL3b (−11%; P < 0.001) and HDL3c (−19%; P = 0.00012) ( Fig. 2 ). Thus, apheresis induced major and preferential reduction in the mass of HDL2b (−23.4 ± 1.7 mg/dl) and HDL2a (−22.6 ± 4.6 mg/dl) in comparison with that of HDL3a to HDL3c (range, −2.7 to −4.6 mg/dl) (Fig. 2, insert).
Fig. 2.
Bar graph showing the plasma concentrations of HDL subspecies in FH patients (n = 11) before (open bar) and after (closed bar) LDL-apheresis. HDL2b (d = 1.063-1.091 g/ml); HDL2a (d = 1.091-1.110 g/ml); HDL3a (d = 1.110-1.133 g/ml); HDL3b (d = 1.133-1.156 g/ml); HDL3c (d = 1.156-1.179 g/ml). Insert: Bar graph showing the reduction in absolute mass concentration HDL subspecies after LDL-apheresis in FH patients (n = 11). Values are means ± SEM. ***P < 0.001, **0.001 < P < 0.01 and *0.01 < P < 0.05 versus before LDL-apheresis.
On a quantitative basis, the dramatic reduction in total apoE content in total HDL (55%; P < 0.001) (Fig. 1A) primarily reflected removal of apoE-rich HDL2b and HDL2a (∼45%, respectively; Fig. 1B); even greater proportions of each of the denser, apoE-containing HDL subfractions, HDL3a, HDL3b, and HDL3c, were removed by apheresis (range, −50 to 66%), although the absolute concentrations of HDL3 particles adsorbed on the column (∼10 mg/dl lipoprotein mass) were substantially less than those of HDL2 (∼45 mg/dl). These apheresis-induced changes in apoE-HDL were equally reflected in the HDL-apoE:apoAI ratio, which diminished by ∼50% to ≤0.01; such ratios are indicative of one apoE copy per 100 apoAI (Fig. 1C).
In our study, FH patients have a pre-LDL-apheresis plasma apoE concentration and a pre-LDL-apheresis distribution of apoE in HDL subfractions similar to normolipidemic subjects (Table 1 and Fig. 1B), despite a HDL-C concentration lower in FH than in controls (Table 1). Post-LDL-apheresis, HDL subfractions were depleted in apoE compared with normolipidemic HDL subfractions (except for HDL2b) from −50% to −71% (Fig. 1B). This observation is consistent with a specific retention of apoE-HDL on the apheresis column. In addition, we observed that the apoE:apoAI ratio (Fig. 1C) was significantly different only for HDL2a and HDL3a between normolipidemic subjects and post-LDL-apheresis subjects (−86% and −82%, respectively).
Effect of LDL-apheresis on pre-beta1-HDL concentrations in FH patients
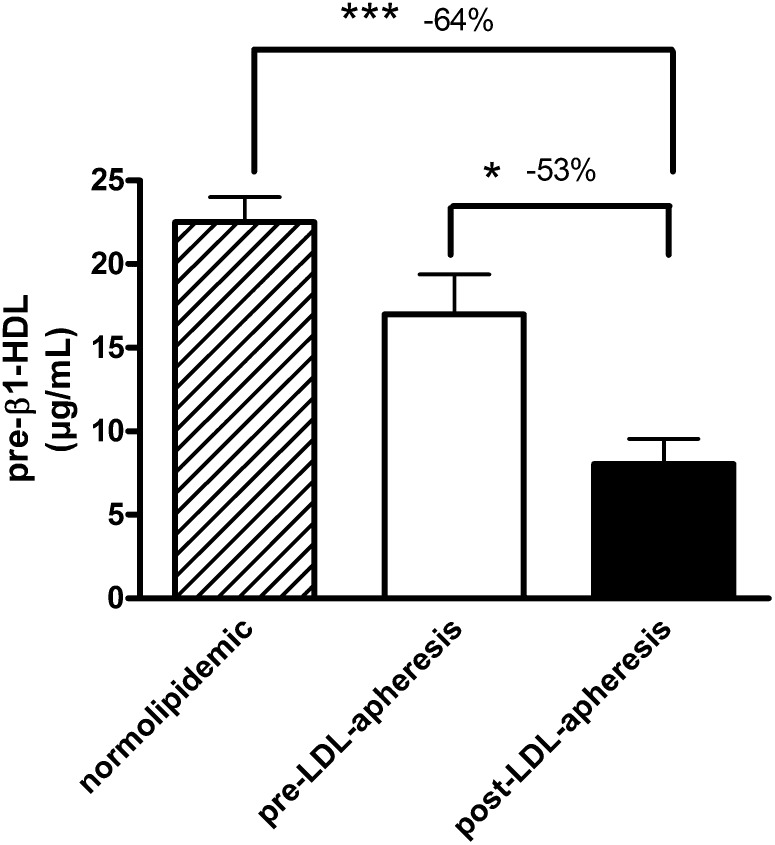
Concentrations of pre-β1-HDL in pre-LDL-apheresis plasma of FH patients who had undergone LDL-apheresis over a period of at least two years were within the normal range (16.99 ± 2.24 µg/ml versus 22.50 ± 1.50 µg/ml, respectively (40)) ( Fig. 3 ). However, LDL-apheresis induced a marked decrease (−53%) in pre-β1-HDL concentrations in FH (16.99 ± 2.24 µg/ml preapheresis versus 8.04 ± 1.52 µg/ml post-LDL-apheresis); indeed, pre-β1-HDL levels became subnormal, some 64% lower than those in normolipidemic controls (Fig. 3). When expressed as percentage total apoAI, LDL-apheresis was found to induce a decrease of −44% in the apoAI content of pre-β particles (1.6 ± 0.2% versus 0.9 ± 0.2% of total apoAI; P < 0.05).
Fig. 3.
Bar graph showing the plasma concentrations of pre-β1-HDL in normolipidemic subjects (dashed bar; n = 25), in FH patients (n = 10) before (open bar) and after (closed bar) LDL-apheresis. Values are means ± SEM. ***P < 0.001, *0.01 < P < 0.05.
Impact of LDL-apheresis on the HDL proteome in FH
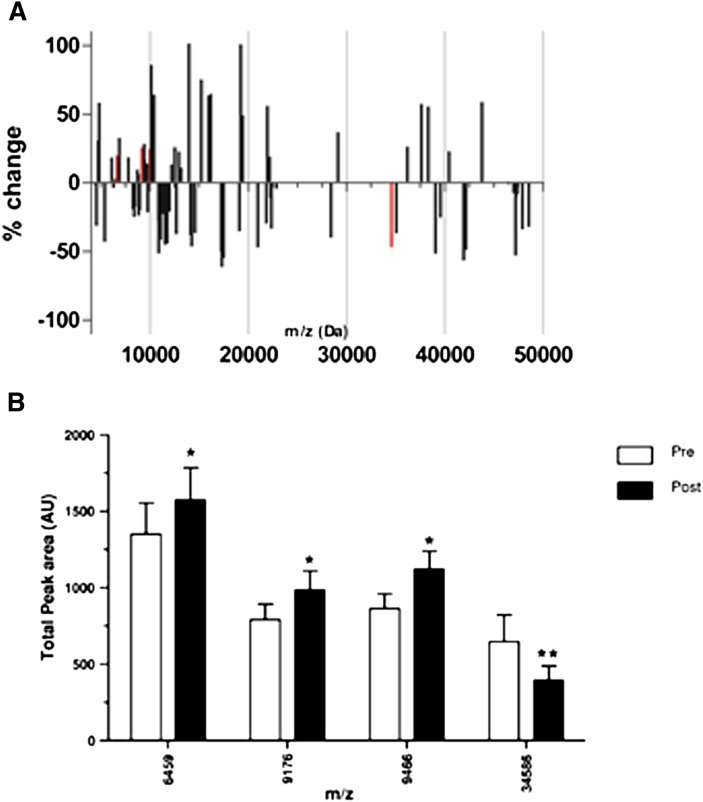
The effects of LDL-apheresis on the HDL proteome in FH, as revealed by HDL protein profiling using the SELDI-TOF MS approach, are summarized in Fig. 4 .
Fig. 4.
Bar graphs demonstrating the changes in HDL proteome upon apheresis. A: Relative changes in the HDL proteome FH patients (n = 10) after apheresis. Bars in red reached a statistical significance change (P < 0.05). B: Absolute levels (peak areas) preapheresis (open bars) and postapheresis (black bars) of the statistically significant changes (Bonferroni corrected). *P < 0.05, **P < 0.001.
The apheresis procedure (Fig. 4A) induced marked modification in the components of the HDL proteome in FH subjects; such changes were detected in both the lower and upper mass ranges. In the lower mass range (up to 20 kDa) of the protein size spectrum, three specific protein mass species were up to 25% more abundant following apheresis (Fig. 4A, red bars) (P < 0.05 for protein species of 6,459, 9,176, and 9,466 kDa); these proteins were closely related in size to apoCI, apoCII, and apoCIII. Few changes were seen in the spectrum of protein components larger than 20 kDa in size. However, a major reduction was observed in the 34,586 m/z component postapheresis, whose size is compatible with that of apoE (Fig. 4B); this finding is consistent with our immunoassay data above.
DISCUSSION
Resolution of HDL particle heterogeneity in FH patients based on physicochemical properties has allowed us to reveal for the first time that i) all five major HDL particle subpopulations contained apoE at baseline preapheresis; ii) the predominant light, large cholesteryl ester-rich HDL2a and HDL2b subfractions contained ∼70% of total HDL apoE, HDL2b displaying both the highest apoE enrichment (apoE:apoAI wt ratio: 0.075:1) and greatest absolute amount of apoE; iii) variable amounts of absolute lipoprotein mass (5-37%) were removed from each HDL subfraction by apheresis, with maximal removal in HDL2b and HDL2a (∼35%), consistent with their degree of apoE enrichment; iv) the percentage of apoE removed from each subfraction was greater than the percentage reduction in absolute lipoprotein mass of the same subfraction; thus, 50-65% of apoE was removed from HDL3a, HDL3b, and HDL3c, whereas absolute reduction in lipoprotein mass by apheresis was <20%; and v) more than 50% of pre-β1-HDL was removed by apheresis, despite small particle size and apparent absence of apoE (unpublished data). Considered together, these data indicate not only that apoE-rich HDL are preferentially removed from all HDL subfractions by apheresis, with the highest selectivity in small, dense HDL3b and HDL3c, but also that pre-β1-HDL is significantly depleted. The mechanism of adsorption of apoE-HDL particles to the column apparently occurs primarily via electrostatic interaction of positively charged clusters of amino acid residues in apoE (46) with negative charges on the polyanionic matrix (25). The mechanisms underlying removal of pre-β1-HDL remain to be defined, however.
It is relevant to points ii and iv above that we have applied a single-step, nondenaturing density gradient ultracentrifugation technology to subfractionate plasma HDL (36); therefore, the question arises whether this technology or any other fractionation method might affect apolipoprotein-defined HDL subfractions. Recently, the distribution of apoE across HDL subfractions isolated from normolipidemic plasmas by high-resolution size-exclusion chromatography was documented (47). By this method, apoE predominated in large- and medium-sized HDL2-like fractions (fractions 19-22; see Ref. 47, Fig. 7 and Table 8), with trace amounts in HDL fractions of smaller size (fractions 23-29). Such an apoE distribution is very similar to that shown in our control subjects in Fig. 1B, in which ∼70% of total apoE in HDL was detected in HDL2 subfractions, and to that which we reported earlier in DGUC-isolated HDL subfractions from control subjects (18). A similar overall profile, in which apoE was primarily associated with large HDL2, was found in the FH patients pre- and postapheresis. We interpret these data to suggest that our DGUC procedure for HDL subfractionation induces only minor alterations in HDL-apoE profile, with the presence of ∼30% of apoE in small, dense HDL3 subfractions. Interestingly, our immunological assay for apoE is significantly more sensitive for detection of low-abundance proteins in HDL than MS/MS technology; therefore, quantitative data in Gordon et al. (see Ref. 47, Fig. 7 and Table 8) may represent an underestimate of the apoE content in small HDL subfractions.
It is relevant to consider that, because of the dramatic reduction in plasma LDL concentration during the apheresis procedure, the relative distribution of apoE among apoB-containing particles may have been perturbed, with the possibility that part of HDL-associated apoE may have redistributed to apoB-containing particles. No evidence for such redistribution was found, as the pre- and postapheresis ratios of apoE:apoB100 in LDL remained low (<0.01:1 wt/wt).
If it is assumed that the major HDL subpopulations contain 3-4 copies of apoAI per particle (19, 48), then on a molar basis, 1 in 4 or fewer HDL2b particles would contain 1 apoE copy preapheresis. Equally, however, a single HDL particle may contain more than 1 apoE copy; in a particle containing 3 copies of apoE, for example, particle frequency in HDL2b would then be 1 per 12. The present studies do not allow us to differentiate between these possibilities or to exclude the possibility that some apoE-HDL may be devoid of apoAI. Nonetheless, the number of apoE copies per HDL particle may be of singular relevance to their binding affinity - and thus degree of retention - by the negatively charged apheresis column (16). Postapheresis, the abundance of apoE in HDL2b was reduced to fewer than 1 in 8 particles. In contrast to HDL2, apoE abundance in HDL3 particle species was <1 per 33 particles; nonetheless, the efficacy of column adsorption of apoE-HDL3 particles was elevated (>50% of total apoE-HDL3 particles).
ApoE is a major determinant of the reduction in HDL-C typical of LDL-apheresis in FH subjects (12, 30, 31). Indeed, apheresis-mediated reduction of baseline apoE levels in FH carriers of the apoE4 allele attained −39% (31), a proportion inferior to that in our patients (−62%) who were primarily E3 homozygotes but in whom baseline plasma apoE levels were some 16-fold lower. The origin of the discrepancy in baseline plasma apoE levels between the studies of Moriarty et al. (31) (62 mg/dl) compared with those of Gibson et al. (12) (nonapheresed FH heterozygotes, 6.9 mg/dl) and our own (Table 2, 3.8 mg/dl) is indeterminate. However, variation in the efficacy of apoE removal by apheresis may be at least partly explained by differences in the procedures used, which primarily involved heparin precipitation in the heparin extracorporeal LDL precipitation (HELP) procedure used by Moriarty et al. (31) in contrast to the polyanion columns used here. Whether differences in the net positive charge of specific apoE isoforms might contribute to differences in the efficacy of apoE removal requires head-to-head comparison of the impact of the different apheresis procedures in the same individuals selected for similar baseline lipid phenotype and apoE genotype.
It is noteworthy that apoE levels in nonapheresed, nonstatin-treated, homozygous or heterozygous FH subjects (13.8 and 6.9 mg/dl respectively; Ref. 12) were superior to those in our apheresed FH group, suggestive of apoE accumulation in the former group. Interestingly, apoE levels in control subjects in the studies of Gibson et al. (12) are similar to those in our apheresed FH group (4.6 versus 3.8 mg/dl, respectively), thereby attesting to the efficacy of regular apheresis.
What are the implications of the apheresis-mediated selective removal of apoE-HDL from all HDL subpopulations in FH, among which large, CE-rich HDL2b particles predominated on a quantitative basis? By allowing increase in the cholesterol load of HDL, apoE can facilitate expansion of the neutral lipid core (primarily CE), favoring a shift in the profile toward larger HDL2-like particles (16). The total flux of cholesterol through HDL in the direct reverse cholesterol transport pathway may, therefore, potentially be increased in FH, compensating at least partially for the deficient removal of LDL-cholesterol. Indeed, uptake of FC and CE in apoE-HDL may occur through both the SR-B1 and LRP receptors in the liver (16). Thus, apheresis may be seen to act concomitantly with these receptor pathways in amplifying removal of cell-derived cholesterol in the form of large apoE-HDL2.
The origin of apoE in the HDL subpopulations of our FH patients is conjectural. Nonetheless, evidence is accumulating to suggest that arterial monocyte-derived macrophage foam cells represent an abundant source of apoE (49); thus, apoE binding of macrophage-derived cholesterol effluxed through the ABCA1 transporter appears to facilitate formation of apoE- and apoAI-containing HDL particles of large size in the microenvironment of the atherosclerotic plaque (49). The entry of such particles into plasma cannot be excluded. Equally, macrophage-derived apoE may leak from plaque tissue into plasma, whereupon it may bind HDL. In addition, apoE associated with TG-rich lipoproteins may be transferred to HDL during lipolysis. Multiple mechanisms may therefore underlie formation of circulating apoE-HDL.
ApoE-HDL is specifically bound and retained in the arterial matrix via high affinity electrostatic binding to extracellular proteoglycans, notably biglycan (32–34). Furthermore, apoE-HDL2 binds more avidly to vascular biglycan than HDL3 in vitro (33, 34). These findings indicate that apoE-HDL may exacerbate cholesterol deposition in the atherosclerotic plaque through binding to biglycan; in this case, selective removal of apoE-HDL2 by apheresis in FH subjects may be atheroprotective.
What are the implications of levels of pre-β1-HDL particles within the normal range preapheresis and their marked reduction by apheresis? In vitro studies have shown that pre-β1-HDL is the primary acceptor of cholesterol in ABCA1-mediated efflux from cultured human macrophages (24, 50). Equally, their circulating concentrations appear to be indicative of the efficiency of the intravascular formation of mature spherical HDL particles via remodeling processes (24, 50). Clearly then, normal preapheresis levels of these particles suggest that cholesterol efflux capacity in FH patients at the end of the intermediate period between apheresis procedures is not markedly defective. By contrast, the dramatic reduction of pre-β1-HDL particles by apheresis suggests that cellular cholesterol efflux may be defective in the period immediately following apheresis and indicative of a partial loss of HDL-mediated atheroprotection.
In conclusion, the bulk of HDL-C loss during LDL-apheresis occurs primarily because of reduction in the CE-rich HDL2b subpopulation (∼45%), from which some 50% of apoE-HDL are removed. By contrast, despite removal of small amounts of HDL3 (<20%), selective removal of apoE-containing particles (up to 66%) occurred. LDL-apheresis clearly removes HDL subpopulations in FH subjects in a differential manner that depends in part on apoE content. We interpret the action of apheresis on apoE-HDL in FH to be primarily atheroprotective in nature, given i) the propensity for apoE-HDL2 (whose plasma residence time may be prolonged as a result of both genetic deficiency in hepatic LDL receptors, and of plasma LDL accumulation) to be bound and retained by arterial matrix biglycans, and ii) the apheresis-mediated reduction in cholesterol load in these large CE-rich particles. By contrast, apheresis-mediated depletion of pre-β1-HDL may induce a transient depletion in cholesterol efflux capacity, an HDL function of immediate clinical relevance (51).
Acknowledgments
The authors are indebted to all patients and nursing staff, and to Dr. J. Lamont (Randox Laboratories) for generously providing biochip arrays for assays of inflammatory biomarkers.
Footnotes
Abbreviations:
- BMI
- body mass index
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- CHD
- coronary heart disease
- hsCRP
- high-sensitivity C reactive protein
- DALI
- direct adsorption of lipoproteins
- FH
- familial hypercholesterolemia
- HDL-C
- HDL-cholesterol
- HELP
- heparin extracorporeal LDL precipitation
- LDL-C
- LDL-cholesterol
- LDL-R
- LDL receptor
- Lp(a)
- lipoprotein(a)
- LpPLA2
- lipoprotein-associated phospholipase A2
- LRP
- LDL receptor-related protein
- NL
- normolipidemic
- SELDI-TOF MS
- surface-enhanced laser desorption/ionization time-of-flight mass spectrometry
- SR-B1
- scavenger receptor class B member 1
- TC
- total cholesterol
- TG
- triglyceride
- VEGF
- vascular endothelial growth factor
This work was supported by Institut National de la Santé et de la Recherche Médicale (INSERM) and by a grant from Fondation de France (to A.K.). A.O. was supported by a doctoral studentship from the Fondation Leducq within the framework of a Transatlantic Network of Excellence in Cardiovascular Research entitled “Immune regulation in atherosclerosis” (to M.J.C.).
REFERENCES
- 1. Goldstein J. L., Hobbs H. H., Brown M. S. 2001. Familial hypercholesterolemia. The Metabolic Basis of Inherited Disease. Scriver C. R., Beaudet A. L., Sly W. S., Valle D., McGraw-Hill, New York: 2863–2913. [Google Scholar]
- 2. Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Bangdiwala S., Tyroler H. A. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79: 8–15. [DOI] [PubMed] [Google Scholar]
- 3. The Emerging Risk Factors Collaboration. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 302: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapman M. J., Ginsberg H. N., Amarenco P., Andreotti F., Borén J., Catapano A. L., Descamps O. S., Fisher E., Kovanen P. T., Kuivenhoven J. A., et al. 2011. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur. Heart J. 32: 1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellanger N., Orsoni A., Julia Z., Fournier N., Frisdal E., Duchene E., Bruckert E., Carrie A., Bonnefont-Rousselot D., Pirault J., et al. 2011. Atheroprotective reverse cholesterol transport pathway is defective in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 31: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 6. Guérin M., Dolphin P. J., Chapman M. J. 1994. Preferential cholesteryl ester acceptors among the LDL subspecies of subjects with familial hypercholesterolemia. Arterioscler. Thromb. 14: 679–685. [DOI] [PubMed] [Google Scholar]
- 7. Miltiadous G., Cariolou M. A., Elisaf M. 2002. HDL-cholesterol levels in patients with molecularly defined familial hypercholesterolemia. Ann. Clin. Lab. Sci. 32: 50–54. [PubMed] [Google Scholar]
- 8. Schaefer J. R., Rader D. J., Ikewaki K., Fairwell T., Zech L. A., Kindt M. R., Davignon J., Gregg R. E., Brewer H. B., Jr 1992. In-vivo metabolism of apolipoprotein AI in a patient with homozygous familial hypercholesterolemia. Arterioscler. Thromb. 12: 843–848. [DOI] [PubMed] [Google Scholar]
- 9. Kajinami K., Mabuchi H., Koizumi J., Takeda R. 1992. Serum apolipoproteins in heterozygous familial hypercholesterolemia. Clin. Chim. Acta. 211: 93–99. [DOI] [PubMed] [Google Scholar]
- 10. Hogue J. C., Lamarche B., Gaudet D., Tremblay A. J., Despres J. P., Bergeron J., Gagne C., Couture P. 2007. Association of heterozygous familial hypercholesterolemia with smaller HDL particle size. Atherosclerosis. 190: 429–435. [DOI] [PubMed] [Google Scholar]
- 11. Frénais R., Ouguerram K., Maugeais C., Marchini J. S., Benlian P., Bard J. M., Magot T., Krempf M. 1999. Apolipoprotein AI kinetics in heterozygous familial hypercholesterolemia: a stable isotope study. J. Lipid Res. 40: 1506–1511. [PubMed] [Google Scholar]
- 12. Gibson J. C., Goldberg R. B., Rubinstein A., Ginsberg H. N., Brown W. V., Baker S., Joffe B. I., Seftel H. C. 1987. Plasma lipoprotein distribution of apolipoprotein E in familial hypercholesterolemia. Atherosclerosis. 7: 401–407. [DOI] [PubMed] [Google Scholar]
- 13. Keidar S., Ostlund R. E., Schonfeld G. 1990. Apolipoprotein-E-rich HDL in patients with homozygous familial hypercholesterolemia. Atherosclerosis. 84: 155–163. [DOI] [PubMed] [Google Scholar]
- 14. Beisiegel U., Weber W., Ihrke G., Herz J., Stanley K. K. 1989. The LDL receptor related protein, LRP, is an apolipoprotein-E-binding protein. Nature. 341: 162–164. [DOI] [PubMed] [Google Scholar]
- 15. Kowal R. C., Herz J., Goldstein J. L., Esser V., Brown M. S. 1989. Low-density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein-E-enriched lipoproteins. Proc. Natl. Acad. Sci. USA. 86: 5810–5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahley R. W., Huang Y., Weisgraber K. H. 2006. Putting cholesterol in its place: apoE and reverse cholesterol transport. J. Clin. Invest. 116: 1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rall S. C., Weisgraber K. H., Innerarity T. L., Mahley R. W. 1982. Structural basis for receptor-binding heterogeneity of apolipoprotein-E from type-III hyperlipoproteinemic subjects. Proc. Natl. Acad. Sci. USA. 79: 4696–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davidson W. S., Silva R. A., Chantepie S., Lagor W. R., Chapman M. J., Kontush A. 2009. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 29: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kontush A., Chantepie S., Chapman M. J. 2003. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 20. Kontush A., Chapman M. J. 2006. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58: 342–374. [DOI] [PubMed] [Google Scholar]
- 21. Lewis G. F., Rader D. J. 2005. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96: 1221–1232. [DOI] [PubMed] [Google Scholar]
- 22. Rye K. A., Bursill C. A., Lambert G., Tabet F., Barter P. J. 2009. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 50(Suppl): S195–S200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fielding C. J., Fielding P. E. 1995. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 36: 211–228. [PubMed] [Google Scholar]
- 24. Sviridov D., Miyazaki O., Theodore K., Hoang A., Fukamachi I., Nestel P. 2002. Delineation of the role of pre-beta1-HDL in cholesterol efflux using isolated pre-beta1-HDL. Arterioscler. Thromb. Vasc. Biol. 22: 1482–1488. [DOI] [PubMed] [Google Scholar]
- 25. Thompson G. R. 2003. LDL-apheresis. Atherosclerosis. 167: 1–13. [DOI] [PubMed] [Google Scholar]
- 26. Thompson G. R., Barbir M., Davies D., Dobral P., Gesinde M., Livingston M., Mandry P., Marais A. D., Matthews S., Neuwirth C., et al. 2010. Efficacy criteria and cholesterol targets for LDL-apheresis. Atherosclerosis. 208: 317–321. [DOI] [PubMed] [Google Scholar]
- 27. Moriarty M. P. 2009. Low-density lipoprotein apheresis. Clinical Lipidology: a Companion to Braunwald's Heart Disease. Ballantyne C. M., editor Saunders Elsevier, Inc, Philadelphia: 363–375. [Google Scholar]
- 28. Franceschini G., Apebe P., Calabresi L., Busnach G., Minetti L., Vaccarino V., Sirtori C. R. 1988. Alterations in the HDL system after rapid plasma cholesterol reduction by LDL-apheresis. Metabolism. 37: 752. [DOI] [PubMed] [Google Scholar]
- 29. Parhofer K. G., Geiss H. C., Schwandt P. 2000. Efficacy of different low-density lipoprotein apheresis methods. Ther. Apher. 4: 382–385. [DOI] [PubMed] [Google Scholar]
- 30. Koizumi J., Inazu A., Fujita H., Takeda M., Uno Y., Kajinami K., Mabuchi H., Takeda R. 1988. Removal of apolipoprotein E-enriched high-density lipoprotein by LDL-apheresis in familial hypercholesterolaemia: a possible activation of the reverse cholesterol transport system. Atherosclerosis. 74: 1. [DOI] [PubMed] [Google Scholar]
- 31. Moriarty P. M., Luyendyk J. P., Gibson C. A., Backes J. M. 2010. Effect of low-density lipoprotein apheresis on plasma levels of apolipoprotein E4. Am. J. Cardiol. 105: 1585–1587. [DOI] [PubMed] [Google Scholar]
- 32. O'Brien K. D., Lewis K., Fischer J. W., Johnson P., Hwang J-Y., Knopp E. A., Kinsella M. G., Barrett P. H. R., Chait A., Wight T. N. 2004. Smooth muscle cell biglycan overexpression results in increased lipoprotein retention on extracellular matrix: implications for the retention of lipoproteins in atherosclerosis. Atherosclerosis. 177: 29. [DOI] [PubMed] [Google Scholar]
- 33. O'Brien K. D., Olin K. L., Alpers C. E., Chiu W., Ferguson M., Hudkins K., Wight T. N., Chait A. 1998. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation. 98: 519–527. [DOI] [PubMed] [Google Scholar]
- 34. Olin K. L., Potter-Perigo S., Barrett P. H. R., Wight T. N., Chait A. 2001. Biglycan, a vascular proteoglycan, binds differently to HDL2 and HDL3: role of apoE. Arterioscler. Thromb. Vasc. Biol. 21: 129–135. [DOI] [PubMed] [Google Scholar]
- 35. Thompson G. R. 2008. Recommendations for the use of LDL-apheresis. Atherosclerosis. 198: 247–255. [DOI] [PubMed] [Google Scholar]
- 36. Chapman M. J., Goldstein S., Lagrange D., Laplaud P. M. 1981. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 22: 339–358. [PubMed] [Google Scholar]
- 37. Guérin M., Bruckert E., Dolphin P. J., Turpin G., Chapman M. J. 1996. Fenofibrate reduces plasma cholesteryl ester transfer from HDL to VLDL and normalizes the atherogenic, dense LDL profile in combined hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 16: 763–772. [DOI] [PubMed] [Google Scholar]
- 38. Hansel B., Giral P., Nobecourt E., Chantepie S., Bruckert E., Chapman M. J., Kontush A. 2004. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 89: 4963–4971. [DOI] [PubMed] [Google Scholar]
- 39. Lopes-Virella M. F., Stone P., Ellis S., Colwell J. A. 1977. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem. 23: 882–884. [PubMed] [Google Scholar]
- 40. Miyazaki O., Kobayashi J., Fukamachi I., Miida T., Bujo H., Saito Y. 2000. A new sandwich enzyme immunoassay for measurement of plasma pre-beta1-HDL levels. J. Lipid Res. 41: 2083–2088. [PubMed] [Google Scholar]
- 41. Levels J., Bleijlevens B., Rezaee F., Aerts J., Meijers J. 2007. SELDI-TOF mass spectrometry of high-density lipoprotein. Proteome Sci. 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geurts P., Fillet M., de Seny D., Meuwis M. A., Malaise M., Merville M. P., Wehenkel L. 2005. Proteomic mass spectra classification using decision tree based ensemble methods. Bioinformatics. 21: 3138–3145. [DOI] [PubMed] [Google Scholar]
- 43. Rawlins M. L., La'ulu S. L., Moon N., Roberts W. L. 2009. Performance characteristics of an immunoturbidimetric assay for lipoprotein-associated phospholipase A2. Clin. Chim. Acta. 406: 66–70. [DOI] [PubMed] [Google Scholar]
- 44. Gordon B. R., Kelsey S. F., Bilheimer D. W., Brown D. C., Dau P. C., Gotto A. M., Illingworth D. R., Jones P. H., Leitman S. F., Prihoda J. S., et al. 1992. Treatment of refractory familial hypercholesterolemia by low-density lipoprotein apheresis using an automated dextran sulfate cellulose adsorption system. Am. J. Cardiol. 70: 1010. [DOI] [PubMed] [Google Scholar]
- 45. Otto C., Geiss H. C., Empen K., Parhofer K. G. 2004. Long-term reduction of C-reactive protein concentration by regular LDL-apheresis. Atherosclerosis. 174: 151–156. [DOI] [PubMed] [Google Scholar]
- 46. Mahley R. W. 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 240: 622–630. [DOI] [PubMed] [Google Scholar]
- 47. Gordon S. M., Deng J., Lu L. J., Davidson W. S. 2010. Proteomic characterization of human plasma high-density lipoprotein fractionated by gel filtration chromatography. J. Proteome Res. 9: 5239–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Silva R. A., Huang R., Morris J., Fang J., Gracheva E. O., Ren G., Kontush A., Jerome W. G., Rye K. A., Davidson W. S. 2008. Structure of apolipoprotein AI in spherical high density lipoproteins of different sizes. Proc. Natl. Acad. Sci. USA. 105: 12176–12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yancey P. G., Yu H., Linton M. F., Fazio S. 2007. A pathway-dependent on apoE, ApoAI, and ABCA1 determines formation of buoyant high-density lipoprotein by macrophage foam cells. Arterioscler. Thromb. Vasc. Biol. 27: 1123–1131. [DOI] [PubMed] [Google Scholar]
- 50. Fielding P. E., Fielding C. J. 2002. Biochemistry in lipids, lipoproteins and membranes. Biochemistry in Lipids, Lipoproteins and Membranes. Vance D. E., Vance J. E., Elsevier, Boston: 527–552. [Google Scholar]
- 51. Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. den Dunnen J.T., Antonarakis S. E. 2000. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum. Mutat. 15: 7–12. [DOI] [PubMed] [Google Scholar]