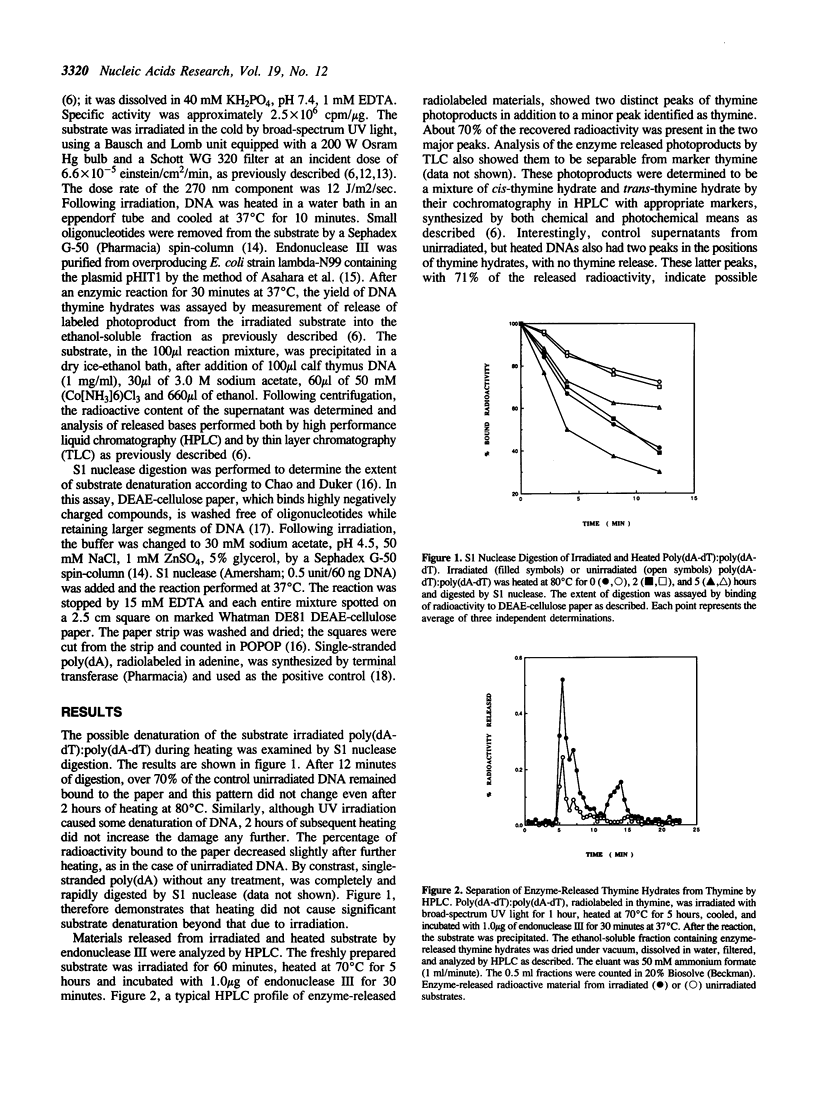
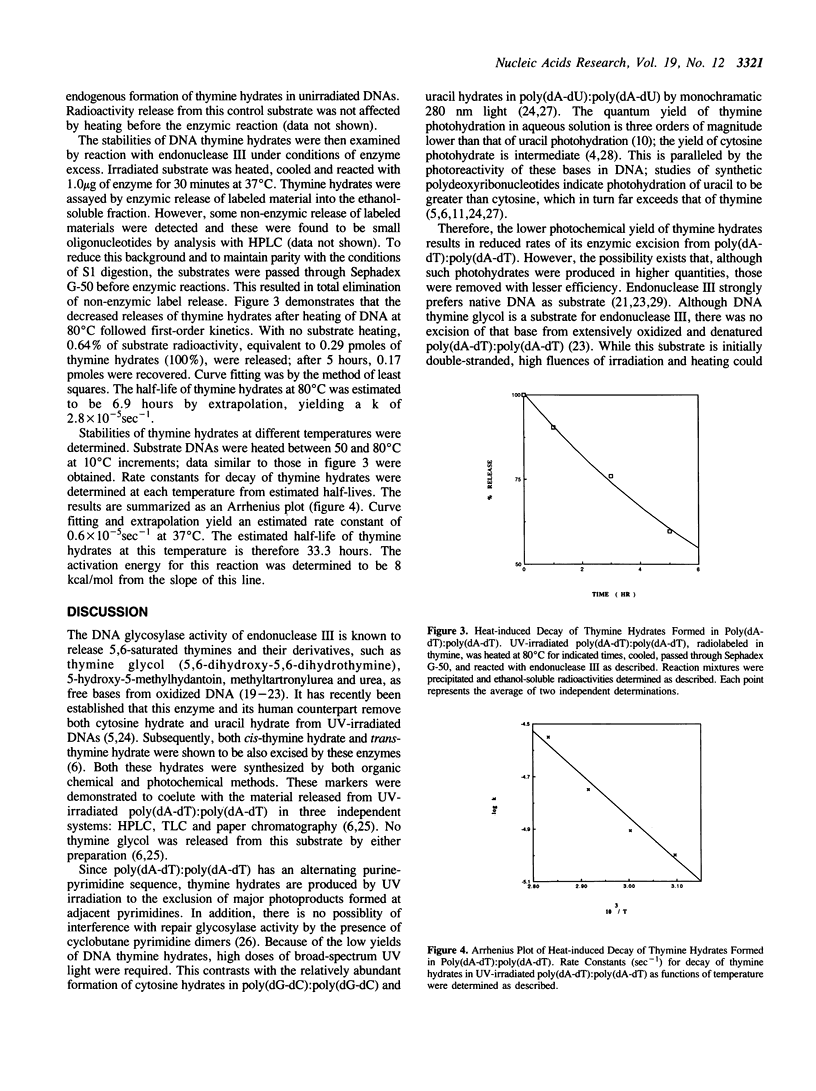
Abstract
Pyrimidine hydrates are products of ultraviolet irradiation of DNA. We have already demonstrated the formation of both cis-thymine hydrate and trans-thymine hydrate (6-hydroxy-5,6-dihydrothymine) in irradiated poly(dA-dT):poly(dA-dT). These are released from DNA as free bases by bacterial or human glycosylases. Thymine hydrate stabilities were studied in irradiated DNA substrates using purified E. coli endonuclease III as a reagent for their removal. After irradiation, substrate poly(dA-dT):poly(dA-dT), radiolabeled in thymine, was incubated at 50, 60, 70 or 80 degrees C, cooled, and then reacted with the enzyme under standard conditions. Thymine hydrates were assayed by enzymic release of labeled material into the ethanol-soluble fraction. Their identities were confirmed by high performance liquid chromatography. The decay of thymine hydrates in heated DNA followed first-order kinetics with a k = 2.8 x 10(-5)/sec at 80 degrees C. These hydrates were also detected in lesser quantities in the unirradiated, control substrate. Extrapolation from an Arrhenius plot yields an estimated half-life of 33.3 hours at 37 degrees C for DNA thymine hydrates. Such stability, together with their formation in unirradiated DNA, suggest thymine hydrates to be formed under physiological conditions and to be sufficiently stable in DNA to be potentially genotoxic. This necessitates their constant removal from DNA by the excision-repair system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asahara H., Wistort P. M., Bank J. F., Bakerian R. H., Cunningham R. P. Purification and characterization of Escherichia coli endonuclease III from the cloned nth gene. Biochemistry. 1989 May 16;28(10):4444–4449. doi: 10.1021/bi00436a048. [DOI] [PubMed] [Google Scholar]
- Billen D. Spontaneous DNA damage and its significance for the "negligible dose" controversy in radiation protection. Radiat Res. 1990 Nov;124(2):242–245. [PubMed] [Google Scholar]
- Boorstein R. J., Hilbert T. P., Cadet J., Cunningham R. P., Teebor G. W. UV-induced pyrimidine hydrates in DNA are repaired by bacterial and mammalian DNA glycosylase activities. Biochemistry. 1989 Jul 25;28(15):6164–6170. doi: 10.1021/bi00441a007. [DOI] [PubMed] [Google Scholar]
- Boorstein R. J., Hilbert T. P., Cunningham R. P., Teebor G. W. Formation and stability of repairable pyrimidine photohydrates in DNA. Biochemistry. 1990 Nov 20;29(46):10455–10460. doi: 10.1021/bi00498a004. [DOI] [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. DNA glycosylase activities for thymine residues damaged by ring saturation, fragmentation, or ring contraction are functions of endonuclease III in Escherichia coli. J Biol Chem. 1984 May 10;259(9):5543–5548. [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. Thymine lesions produced by ionizing radiation in double-stranded DNA. Biochemistry. 1985 Jul 16;24(15):4018–4022. doi: 10.1021/bi00336a032. [DOI] [PubMed] [Google Scholar]
- Breimer L. H. Urea--DNA glycosylase in mammalian cells. Biochemistry. 1983 Aug 30;22(18):4192–4197. doi: 10.1021/bi00287a005. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Chao T. L., Duker N. J. Effects of 5-azacytosine in DNA on enzymic uracil excision. Mutat Res. 1984 Jun-Jul;140(2-3):93–98. doi: 10.1016/0165-7992(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Cunningham R. P. The enzymology of apurinic/apyrimidinic endonucleases. Mutat Res. 1990 Sep-Nov;236(2-3):173–201. doi: 10.1016/0921-8777(90)90004-o. [DOI] [PubMed] [Google Scholar]
- Duker N. J., Chao T. L., Resnick E. M. Rates of heat-induced DNA purine alterations in synthetic polydeoxyribonucleotides. Chem Biol Interact. 1986 Jun;58(3):241–251. doi: 10.1016/s0009-2797(86)80101-6. [DOI] [PubMed] [Google Scholar]
- Duker N. J., Davies W. A., Hart D. M. Alteration of uracil-DNA glycosylase activity by uracil dimers in DNA. Photochem Photobiol. 1981 Aug;34(2):191–195. [PubMed] [Google Scholar]
- Duker N. J., Jensen D. E., Hart D. M., Fishbein D. E. Perturbations of enzymic uracil excision due to purine damage in DNA. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4878–4882. doi: 10.1073/pnas.79.16.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duker N. J., Jensen D. E., Hart D. M. Modifications of circular DNA by photoalkylation. Radiat Res. 1985 Jul;103(1):114–121. [PubMed] [Google Scholar]
- Ganguly T., Duker N. J. Differential cell cycle modulation of human DNA glycosylases against oxidized pyrimidines. Mutat Res. 1990 Mar;235(2):137–146. doi: 10.1016/0921-8777(90)90067-f. [DOI] [PubMed] [Google Scholar]
- Ganguly T., Weems K. M., Duker N. J. Ultraviolet-induced thymine hydrates in DNA are excised by bacterial and human DNA glycosylase activities. Biochemistry. 1990 Aug 7;29(31):7222–7228. doi: 10.1021/bi00483a009. [DOI] [PubMed] [Google Scholar]
- Higgins S. A., Frenkel K., Cummings A., Teebor G. W. Definitive characterization of human thymine glycol N-glycosylase activity. Biochemistry. 1987 Mar 24;26(6):1683–1688. doi: 10.1021/bi00380a029. [DOI] [PubMed] [Google Scholar]
- Katcher H. L., Wallace S. S. Characterization of the Escherichia coli X-ray endonuclease, endonuclease III. Biochemistry. 1983 Aug 16;22(17):4071–4081. doi: 10.1021/bi00286a013. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Teebor G. W., Boorstein R. J., Cadet J. The repairability of oxidative free radical mediated damage to DNA: a review. Int J Radiat Biol. 1988 Aug;54(2):131–150. doi: 10.1080/09553008814551591. [DOI] [PubMed] [Google Scholar]
- Wallace S. S. AP endonucleases and DNA glycosylases that recognize oxidative DNA damage. Environ Mol Mutagen. 1988;12(4):431–477. doi: 10.1002/em.2860120411. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Gallagher P. E., Brent T. P., Duker N. J. Cytosine photoproduct-DNA glycosylase in Escherichia coli and cultured human cells. Biochemistry. 1989 Feb 21;28(4):1488–1492. doi: 10.1021/bi00430a010. [DOI] [PubMed] [Google Scholar]


