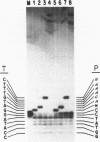
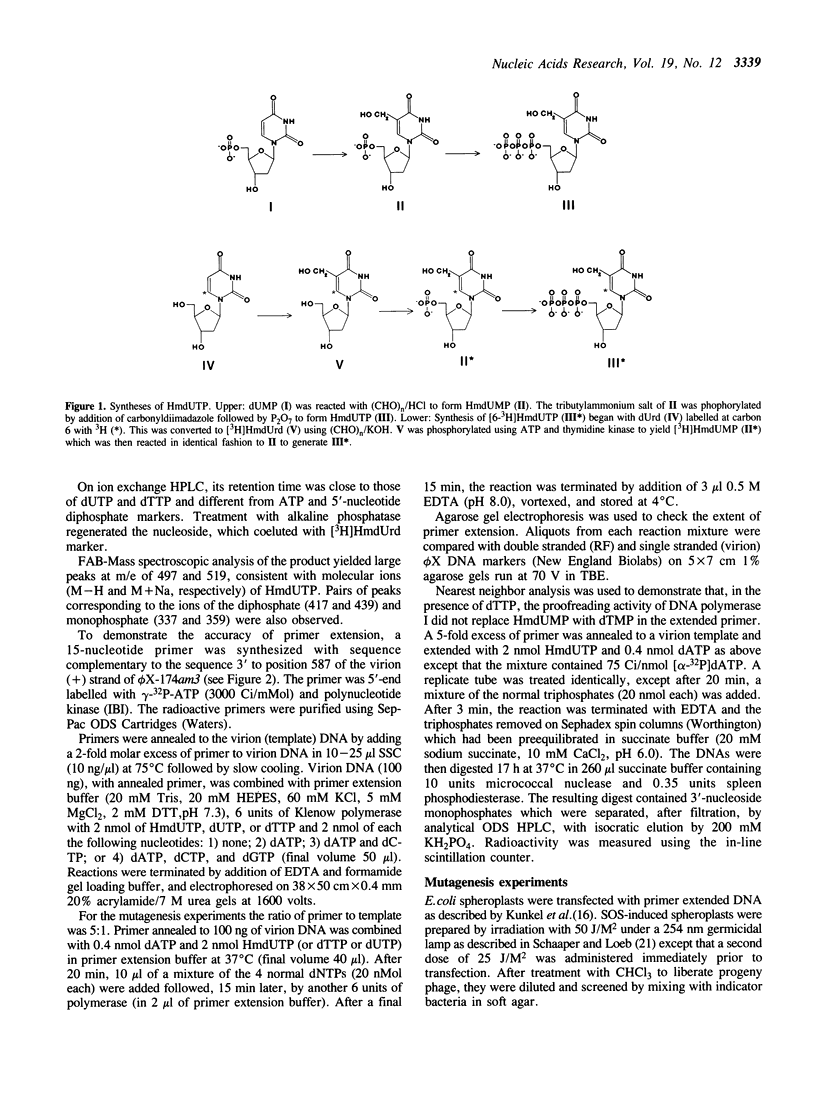
Abstract
5-hydroxymethyluracil (HmUra) is formed in DNA as a product of oxidative attack on the methyl group of Thy. It is removed from DNA by HmUra-DNA glycosylase. To determine whether the replacement of Thy by HmUra is mutagenic, which might explain the repairability of HmUra, a HmUra residue was substituted for Thy in a target (amber) codon by in vitro extension of an oligonucleotide primer annealed to phi X-174am3 virion DNA. This was accomplished by synthesizing HmdUTP and using DNA polymerase to effect primer extension. E. coli spheroplasts were transfected with the HmUra-containing DNA and the yield of revertant phage determined following replication in the bacterial host. Since E. coli do not express HmUra-DNA glycosylase activity, mutagenesis could be assessed in the absence of repair. chi 2c analysis showed that replacing Thy with HmUra did not result in an increase in revertant phage. These data indicate that the oxidation of Thy to HmUra in cellular DNA probably does not result in substantial mutagenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilimoria M. H., Gupta S. V. Comparison of the mutagenic activity of 5-hydroxymethyldeoxyuridine with 5-substituted 2'-deoxyuridine analogs in the Ames Salmonella/microsome test. Mutat Res. 1986 Mar;169(3):123–127. doi: 10.1016/0165-1218(86)90091-1. [DOI] [PubMed] [Google Scholar]
- Boorstein R. J., Chiu L. N., Teebor G. W. Phylogenetic evidence of a role for 5-hydroxymethyluracil-DNA glycosylase in the maintenance of 5-methylcytosine in DNA. Nucleic Acids Res. 1989 Oct 11;17(19):7653–7661. doi: 10.1093/nar/17.19.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorstein R. J., Levy D. D., Teebor G. W. Toxicity of 3-aminobenzamide to Chinese hamster cells containing 5-hydroxymethyluracil in their DNA. Cancer Res. 1987 Aug 15;47(16):4372–4377. [PubMed] [Google Scholar]
- Boorstein R. J., Teebor G. W. Mutagenicity of 5-hydroxymethyl-2'-deoxyuridine to Chinese hamster cells. Cancer Res. 1988 Oct 1;48(19):5466–5470. [PubMed] [Google Scholar]
- Cannon-Carlson S. V., Gokhale H., Teebor G. W. Purification and characterization of 5-hydroxymethyluracil-DNA glycosylase from calf thymus. Its possible role in the maintenance of methylated cytosine residues. J Biol Chem. 1989 Aug 5;264(22):13306–13312. [PubMed] [Google Scholar]
- Frenkel K., Chrzan K. Hydrogen peroxide formation and DNA base modification by tumor promoter-activated polymorphonuclear leukocytes. Carcinogenesis. 1987 Mar;8(3):455–460. doi: 10.1093/carcin/8.3.455. [DOI] [PubMed] [Google Scholar]
- Frenkel K., Cummings A., Solomon J., Cadet J., Steinberg J. J., Teebor G. W. Quantitative determination of the 5-(hydroxymethyl)uracil moiety in the DNA of gamma-irradiated cells. Biochemistry. 1985 Aug 13;24(17):4527–4533. doi: 10.1021/bi00338a007. [DOI] [PubMed] [Google Scholar]
- Grosse F., Krauss G., Knill-Jones J. W., Fersht A. R. Accuracy of DNA polymerase-alpha in copying natural DNA. EMBO J. 1983;2(9):1515–1519. doi: 10.1002/j.1460-2075.1983.tb01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Heinrich M., Krauss G. The fidelity of DNA polymerases during in vitro replication of a template containing 5-bromouracil at a specific site. J Biol Chem. 1989 Jan 5;264(1):119–123. [PubMed] [Google Scholar]
- Hollstein M. C., Brooks P., Linn S., Ames B. N. Hydroxymethyluracil DNA glycosylase in mammalian cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4003–4007. doi: 10.1073/pnas.81.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLEN R. G., SIMON M., MARMUR J. The new occurrence of a new pyrimidine base replacing thymine in a bacteriophage DNA:5-hydroxymethyl uracil. J Mol Biol. 1962 Aug;5:248–250. doi: 10.1016/s0022-2836(62)80087-4. [DOI] [PubMed] [Google Scholar]
- Kahilainen L. I., Bergstrom D. E., Vilpo J. A. 5-Hydroxymethyl-2'-deoxyuridine. Cytotoxicity and DNA incorporation studied by using a novel [2-14C]-derivative with normal and leukemic human hematopoietic cells. Acta Chem Scand B. 1985;39(6):477–484. doi: 10.3891/acta.chem.scand.39b-0477. [DOI] [PubMed] [Google Scholar]
- Kaufman E. R. Biochemical analysis of toxic effects of 5-hydroxymethyl-2'-deoxyuridine in mammalian cells. Somat Cell Mol Genet. 1986 Sep;12(5):501–512. doi: 10.1007/BF01539921. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALEY F. Nucleotide interconversions. VI. The enzymic formation of 5-methyluridylic acid. Arch Biochem Biophys. 1962 Mar;96:550–556. doi: 10.1016/0003-9861(62)90335-1. [DOI] [PubMed] [Google Scholar]
- Preston B. D., Singer B., Loeb L. A. Comparison of the relative mutagenicities of O-alkylthymines site-specifically incorporated into phi X174 DNA. J Biol Chem. 1987 Oct 5;262(28):13821–13827. [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau G. T., Schinazi R. F., Chen M. S., Prusoff W. H. Synthesis and biological activities of 5-(hydroxymethyl, azidomethyl, or aminomethyl)-2'-deoxyuridine and related 5'-substituted analogues. J Med Chem. 1980 Feb;23(2):127–133. doi: 10.1021/jm00176a005. [DOI] [PubMed] [Google Scholar]
- Shirnamé-Moré L., Rossman T. G., Troll W., Teebor G. W., Frenkel K. Genetic effects of 5-hydroxymethyl-2'-deoxyuridine, a product of ionizing radiation. Mutat Res. 1987 Jun;178(2):177–186. doi: 10.1016/0027-5107(87)90267-3. [DOI] [PubMed] [Google Scholar]
- Sowers L. C., Shaw B. R., Veigl M. L., Sedwick W. D. DNA base modification: ionized base pairs and mutagenesis. Mutat Res. 1987 Apr;177(2):201–218. doi: 10.1016/0027-5107(87)90003-0. [DOI] [PubMed] [Google Scholar]
- Teebor G. W., Frenkel K., Goldstein M. S. Ionizing radiation and tritium transmutation both cause formation of 5-hydroxymethyl-2'-deoxyuridine in cellular DNA. Proc Natl Acad Sci U S A. 1984 Jan;81(2):318–321. doi: 10.1073/pnas.81.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A. S., Haeusslein E., Milman G. Purification and characterization of herpes simplex virus (type 1) thymidine kinase produced in Escherichia coli by a high efficiency expression plasmid utilizing a lambda PL promoter and cI857 temperature-sensitive repressor. J Biol Chem. 1983 Oct 10;258(19):11571–11575. [PubMed] [Google Scholar]