Abstract
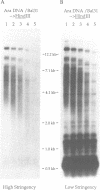
We describe the structure of an Arabidopsis thaliana genomic clone containing two classes of repetitive DNA elements derived from the centromere region of chromosome 1. One class is comprised of tandem arrays of a highly reiterated repeat containing degenerate telomere sequence motifs. Adjacent to these telomere-similar repeats we found a dispersed repetitive element reiterated approximately five times in the A. thaliana genome. The nucleotide sequence of the dispersed repeat is unusual, being extremely AT-rich and composed of numerous, overlapping repeat motifs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Gosden J. R., Cross S. H., Cranston G., Rout D., Sugawara N., Szostak J. W., Fantes P. A., Hastie N. D. Telomeric repeat from T. thermophila cross hybridizes with human telomeres. Nature. 1988 Apr 14;332(6165):656–659. doi: 10.1038/332656a0. [DOI] [PubMed] [Google Scholar]
- Boseley P. G., Moss T., Birnstiel M. L. 5'-Labeling and poly(dA) tailing. Methods Enzymol. 1980;65(1):478–494. [PubMed] [Google Scholar]
- Cheng C. L., Dewdney J., Nam H. G., den Boer B. G., Goodman H. M. A new locus (NIA 1) in Arabidopsis thaliana encoding nitrate reductase. EMBO J. 1988 Nov;7(11):3309–3314. doi: 10.1002/j.1460-2075.1988.tb03201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Amstutz H., Fishel B., Carbon J. Analysis of centromeric DNA in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8253–8257. doi: 10.1073/pnas.83.21.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Baum M. P. Functional analysis of a centromere from fission yeast: a role for centromere-specific repeated DNA sequences. Mol Cell Biol. 1990 May;10(5):1863–1872. doi: 10.1128/mcb.10.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange T., Kooter J. M., Michels P. A., Borst P. Telomere conversion in trypanosomes. Nucleic Acids Res. 1983 Dec 10;11(23):8149–8165. doi: 10.1093/nar/11.23.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore E., Pace T., Ponzi M., Picci L., Frontali C. Organization of subtelomeric repeats in Plasmodium berghei. Mol Cell Biol. 1990 May;10(5):2423–2427. doi: 10.1128/mcb.10.5.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel B., Amstutz H., Baum M., Carbon J., Clarke L. Structural organization and functional analysis of centromeric DNA in the fission yeast Schizosaccharomyces pombe. Mol Cell Biol. 1988 Feb;8(2):754–763. doi: 10.1128/mcb.8.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry K., Salser W. Nucleotide sequences of HS-alpha satellite DNA from kangaroo rat Dipodomys ordii and characterization of similar sequences in other rodents. Cell. 1977 Dec;12(4):1069–1084. doi: 10.1016/0092-8674(77)90170-2. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist G. P., Dancis B. Telomere replication, kinetochore organizers, and satellite DNA evolution. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4566–4570. doi: 10.1073/pnas.76.9.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Green P., Abrahamson J., Barlow A., Daly M. J., Lincoln S. E., Newberg L. A., Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987 Oct;1(2):174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Levinson A., Silver D., Seed B. Minimal size plasmids containing an M13 origin for production of single-strand transducing particles. J Mol Appl Genet. 1984;2(6):507–517. [PubMed] [Google Scholar]
- Meyne J., Baker R. J., Hobart H. H., Hsu T. C., Ryder O. A., Ward O. G., Wiley J. E., Wurster-Hill D. H., Yates T. L., Moyzis R. K. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma. 1990 Apr;99(1):3–10. doi: 10.1007/BF01737283. [DOI] [PubMed] [Google Scholar]
- Nakaseko Y., Adachi Y., Funahashi S., Niwa O., Yanagida M. Chromosome walking shows a highly homologous repetitive sequence present in all the centromere regions of fission yeast. EMBO J. 1986 May;5(5):1011–1021. doi: 10.1002/j.1460-2075.1986.tb04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaseko Y., Kinoshita N., Yanagida M. A novel sequence common to the centromere regions of Schizosaccharomyces pombe chromosomes. Nucleic Acids Res. 1987 Jun 25;15(12):4705–4715. doi: 10.1093/nar/15.12.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H. G., Giraudat J., Den Boer B., Moonan F., Loos WDB., Hauge B. M., Goodman H. M. Restriction Fragment Length Polymorphism Linkage Map of Arabidopsis thaliana. Plant Cell. 1989 Jul;1(7):699–705. doi: 10.1105/tpc.1.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N. E., Martin F. B., Ausubel F. M. Specialized binary vector for plant transformation: expression of the Arabidopsis thaliana AHAS gene in Nicotiana tabacum. Nucleic Acids Res. 1988 Nov 25;16(22):10765–10782. doi: 10.1093/nar/16.22.10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T., Ponzi M., Dore E., Frontali C. Telomeric motifs are present in a highly repetitive element in the Plasmodium berghei genome. Mol Biochem Parasitol. 1987 Jun;24(2):193–202. doi: 10.1016/0166-6851(87)90106-x. [DOI] [PubMed] [Google Scholar]
- Richards E. J., Ausubel F. M. Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell. 1988 Apr 8;53(1):127–136. doi: 10.1016/0092-8674(88)90494-1. [DOI] [PubMed] [Google Scholar]
- Simoens C. R., Gielen J., Van Montagu M., Inzé D. Characterization of highly repetitive sequences of Arabidopsis thaliana. Nucleic Acids Res. 1988 Jul 25;16(14B):6753–6766. doi: 10.1093/nar/16.14.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Base sequence and evolution of guinea-pig alpha-satellite DNA. Nature. 1970 Aug 22;227(5260):794–798. doi: 10.1038/227794a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
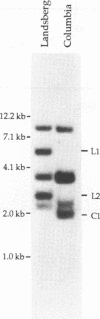
- Walmsley R. W., Chan C. S., Tye B. K., Petes T. D. Unusual DNA sequences associated with the ends of yeast chromosomes. Nature. 1984 Jul 12;310(5973):157–160. doi: 10.1038/310157a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]