Abstract
Significance Protein misfolding within the endoplasmic reticulum (ER) is managed by an ER quality control system that retro-translocates aberrant proteins into the cytosol for proteasomal destruction. This process, known as ER-associated degradation, utilizes the action of ER redox enzymes to accommodate the disulfide-bonded nature of misfolded proteins. Strikingly, various pathogenic viruses and toxins co-opt these redox components to reach the cytosol during entry. These redox factors thus regulate critical cellular homeostasis and host–pathogen interactions. Recent Advances: Recent studies identify specific members of the protein disulfide isomerase (PDI) family, which use their chaperone and catalytic activities, in engaging both misfolded ER proteins and pathogens. Critical Issues: The precise molecular mechanism by which a dedicated PDI family member disrupts the disulfide bonds in the misfolded ER proteins and pathogens, as well as how they act to unfold these substrates to promote their ER-to-cytosol membrane transport, remain poorly characterized. Future Directions: How PDI family members distinguish folded versus misfolded ER substrates remains enigmatic. What physical characteristics surrounding a substrate's disulfide bond instruct PDI that it is mispaired or native? For the pathogens, as their disulfide bonds normally serve a critical role in providing physical support, what conformational changes experienced in the host enable their disulfide bonds to be disrupted? A combination of more rigorous biochemical and high-resolution structural studies should begin to address these questions. Antioxid. Redox Signal. 16, 809–818.
Introduction
The endoplasmic reticulum (ER) is the cellular organelle serving as the starting point for the anterograde secretory pathway responsible for sorting and transporting proteins. Folding and maturation of these proteins in the ER accounts for ∼30% of all cellular proteins, yet the ER comprises only 10% of the total cellular volume. This heavy protein folding burden requires a complex quality control system to ensure that misfolded proteins are retained in the ER until properly folded, or in the case of terminally misfolded/damaged proteins, efficiently degraded. The latter scenario utilizes a process known as ER-associated degradation (ERAD) (56).
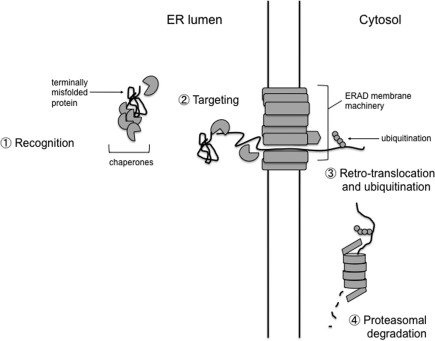
ERAD is an organized process by which defective ER proteins are recognized, targeted to ER membrane machinery, retro-translocated to the cytosol, ubiquitinated, and degraded by the proteasome (Fig. 1). Each step is coupled by specific protein–protein interactions. The recognition and accommodation of a diverse population of potential substrates necessitate a large number of ERAD components. In addition to whether a substrate is membrane integrated or soluble, specific ER post-translational modifications, including glycosylation and disulfide bond formation, contribute to this diversity. While glycosylation normally mediates proper protein folding, the specific glycosylation state of a misfolded substrate could be recognized by ERAD components as a degradation signal, promoting destruction of the substrate (56). As not all substrates are glycosylated, there must be additional mechanisms to distinguish aberrant proteins from ones on a correct folding path.
FIG. 1.
General steps involved in ERAD pathways. (1) Recognition. If a nascent protein cannot adopt its proper conformation and becomes terminally misfolded, many endoplasmic reticulum (ER)-resident chaperones recognize the misfolded protein as an ER-associated degradation (ERAD) substrate. (2) Targeting. The misfolded substrate is targeted to membrane localized ERAD machinery by virtue of the ability of the chaperones to bind substrates while also interacting with membrane components involved in retro-translocation. (3) Retro-translocation and ubiquitination. Substrates targeted to the membrane machinery are unfolded and retro-translocated through a protein channel, although in some cases substrates may remain folded and intact. Once exposed to the cytosol, E3 ubiquitin ligases attach ubiquitin molecules to the substrates, allowing the substrate to be recognized and degraded by the proteasome. (4) Proteasomal degradation. An unfolded substrate is extracted into the cytosol and maintained in a soluble state by cytosolic chaperones, before de-ubiquitination and proteasomal degradation.
Another critical feature of misfolded ER substrates is the nature of their disulfide bonds. Disulfide bonds are covalent linkages between two cysteines (Cys) that provide proteins with the proper conformation and stability required for their secretion or transport to various cellular destinations. In contrast to the reducing environment of the cytosol, the ER maintains an oxidizing environment, allowing for disulfide bond formation. Protein disulfide isomerase (PDI) and other PDI family members are ER-resident enzymes that catalyze disulfide bond formation. In some instances, these enzymes reduce and isomerize these bonds. In addition to this catalytic function, PDI proteins also possess chaperone activity, aiding in protein folding and unfolding reactions. Both PDI's catalytic and chaperone activities have been implicated in ERAD of misfolded proteins (14, 17, 25, 32, 55, 57, 59). PDI's physical proximity to newly synthesized substrates entering the ER and its interaction with ERAD machinery allow it to function in this capacity.
This review highlights the mechanism by which ER redox factors regulate ERAD. We will also discuss how pathogens co-opt redox factors in the ER to gain entry into the host cytosol during infection. The utilization of PDI proteins' catalytic and chaperone functions during ERAD and pathogen entry underscores the importance of ER redox reactions in maintaining normal cellular homeostasis and in facilitating host–pathogen interactions.
Catalytic Function of PDI Family Proteins During ERAD
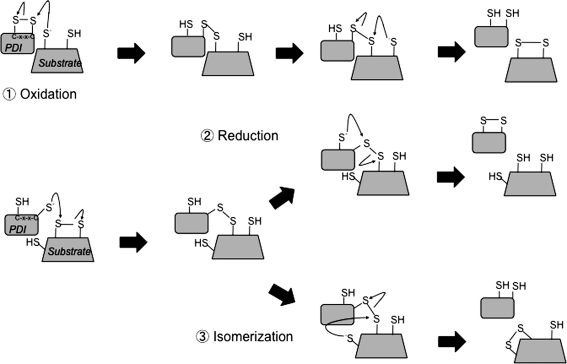
During oxidative folding in the ER, newly synthesized proteins engage PDI family members. This family of at least 20 proteins shares in common ER localization and the presence of thioredoxin-like domains. Thioredoxin domains often contain a catalytic Cys-x-x-Cys motif responsible for transfer of electrons with other Cys during oxidation, reduction, or isomerization reactions (Fig. 2). For instance, canonical PDI contains two thioredoxin domains with this catalytic motif.
FIG. 2.
Thiol–disulfide exchange reactions between protein disulfide isomerase (PDI) proteins and substrates. (1) Oxidation. Oxidized PDI engages reduced substrate and allows for a mixed disulfide to form between PDI and the substrate. This mixed disulfide is resolved by a nucleophilic attack of a cysteine residue's thiolate anion on the substrate. This reaction results in oxidized substrate and reduced PDI. (2) Reduction. Reduced PDI forms a mixed disulfide bond with an oxidized substrate, which is resolved by a cysteine residue on PDI and results in oxidized PDI and reduced substrate. (3) Isomerization. Reduced PDI again forms a mixed disulfide with a substrate but instead is resolved by a cysteine residue on the substrate forming a different disulfide bond. This reaction results in reduced PDI and isomerized substrate.
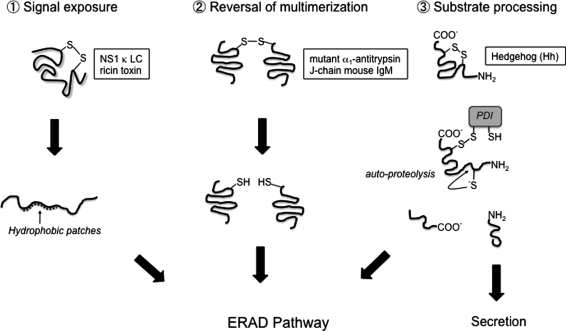
Early data implicated ER protein degradation as a redox-dependent process (50, 62), suggesting that a misfolded substrate's redox state plays a crucial role during ERAD. How might a misfolded protein's redox property affect ERAD? In this context, disulfide bond disruption serves at least three potential roles (Fig. 3). First, reduction of a disulfide bond may expose a previously obscured signal that indicates the protein is terminally misfolded, such as an amount of hydrophobic residues that reaches the threshold required for binding to the ERAD machinery. For example, the Ig light chain mutant, NS1 κ LC, is an ERAD substrate that exists in the fully or partially oxidized form. While the oxidoreductase controlling NS1 κ LC's redox state is unidentified, the ER-resident Hsp70 ATPase (BiP) is known to preferentially engage partially but not fully oxidized NS1 κ LC (24). One explanation for this specific interaction is that partially oxidized NS1 κ LC exposes more hydrophobic surfaces allowing BiP to bind. Upon binding, BiP recruits the substrate to ER membrane ERAD components, including Derlin-1, the E3 ubiquitin ligase Hrd1, and HERP (36).
FIG. 3.
Disulfide disruption as a prerequisite for ERAD. (1) Disruption of disulfide bonds within an ER protein leads to more complete unfolding and exposure of hydrophobic patches. This increased hydrophobicity allows for the recruitment of factors involved in selecting substrates for ERAD. (2) Removal of disulfide bonds can increase the efficiency of ERAD. Substrates that are erroneously multimerized by disulfide bonds are disrupted to allow more complete unfolding and processing by ERAD membrane machinery including the retro-translocation channel. (3) Disulfide bond disruption can allow auto-catalytic processing of Hedgehog (Hh). This processing produces an unused C-terminal fragment, which is constitutively degraded by an ERAD pathway.
Disulfide bond disruption during ERAD may also allow for efficient retro-translocation. Improper disulfide bonds can lead to unwanted substrate oligomerization/aggregation caused by aberrant hydrophobic interactions. ER factors that normally handle misfolded proteins in preparation for retro-translocation may not properly engage highly oligomerized substrates. Resolution of these erroneous disulfide bonds could reverse substrate multimerization, allowing proper unfolding before retro-translocation.
An excellent example of a reductase activity serving in this capacity has been observed in studies with ERdj5. ERdj5 was originally discovered as a stress-induced PDI family member containing thioredoxin domains with catalytic Cys-x-x-Cys motifs (9). The presence of a J domain, which stimulates BiP's ATPase activity to enable substrate binding, makes ERdj5 unique among the PDI family. Importantly, ERdj5 is the most reductive of PDI proteins studied thus far, allowing it to play a more dominant role in disulfide bond disruption than in formation. ERdj5 acts on the ERAD substrates, mutant α1-antitrypsin, and the J chain of mouse immunoglobulin M (55). These two substrates contain aberrant disulfide bonds, producing dimeric and oligomeric species, respectively (55). When ERdj5 was overexpressed in cells, both substrates' degradation rates accelerated due to increased disruption of the aberrant disulfide bonds (55). Conversely, ERdj5 knockdown blocked substrate degradation, leading to disulfide-linked dimer and oligomer accumulation (55). These findings demonstrate that ERdj5 plays a critical role in ERAD by reversing substrate oligomerization via disruption of incorrect disulfide bonds. A recent determination of ERdj5's crystal structure suggests that after reduction, the substrate is transferred to BiP via ERdj5's J domain (19). BiP holds the substrate in a soluble state or imposes additional unfolding, eventually presenting the substrate to membrane retro-translocation components such as SEL1 for subsequent transport to the cytosol (19).
A third purpose of disulfide bond disruption enables substrate auto-processing required for efficient ERAD. A salient example is observed in PDI-mediated auto-processing of the Hedgehog (Hh) signaling molecule (6). PDI disrupts a disulfide bond in the Hh precursor, freeing a catalytic Cys on the substrate. This Cys is critical during an auto-proteolytic reaction that produces Hh N- and C-terminal fragments. In contrast to the signaling competent N-terminal fragment, the C-terminal fragment is not secreted but rather is degraded in a constitutive manner via ERAD.
The ability of a PDI family member to reduce disulfides is dependent on the overall redox environment of the ER. Recent work has begun to elucidate how small molecules such as glutathione function with several enzymes dedicated to regulating the redox status of PDI proteins (5). In the context of ERAD, little is known about how reductive pathways are supported and controlled. However, at least one enzyme, an ER flavoprotein termed ERFAD has been implicated in ERAD and interacts with ERdj5 (43). ERFAD contains a motif for binding the reductive molecule NADPH and may use this cofactor to directly reduce the active sites of ERdj5, allowing ultimately for reduction of ERAD substrates. It remains to be answered whether additional electron-donating enzymes paired with PDI proteins function specifically during ERAD.
Chaperone Function of PDI Family Proteins During ERAD
In addition to catalyzing thiol–disulfide exchange reactions, many PDI family members are bona fide chaperones that bind proteins irrespective of their thiol content. Working coordinately with other ER chaperones, PDI family members are critical to productive folding. Not surprisingly, the central role of PDI proteins in protein maturation enables them to be the first to recognize terminally misfolded substrates.
PDI's chaperone activity during ERAD was first implicated in retro-translocation of a misfolded protein lacking Cys in yeast (17). That retro-translocation of a Cys-less substrate requires PDI demonstrates PDI's noncatalytic activity is crucial during ERAD. A more recent finding strengthens this idea, demonstrating PDI's chaperone, not redox activity, is required for US2-dependent MHC class I degradation (25). US2 is a viral protein encoded by human cytomegalovirus that facilitates retro-translocation of host cell MHC class I heavy chains as part of an immune evasion strategy (61).
PDI also operates as a chaperone during ERAD of glyco-proteins. In yeast, PDI forms a complex with the mannosidase Htm1. This interaction is functionally important because PDI stochastically chaperones misfolded glyco-proteins to Htm1, which subsequently modifies the substrate's glycosylation status (16). This glycan modification produces a signal recognizable to ERAD components that facilitates retro-translocation and degradation of the substrate.
In addition to soluble PDI proteins, the membrane-integrated PDI family member Eps1 was identified in yeast as an ERAD component that facilitates degradation of the misfolded membrane substrate Pma1-D478N (59). Eps1 binds to Pma1-D478N in a manner dependent on the catalytic Cys-x-x-Cys motifs and likely recruits this substrate to an E3 ubiquitin ligase. To date, a mammalian Eps1 homolog involved in ERAD has yet to be identified. Nonetheless, the above examples clearly implicate PDI's nonenzymatic chaperone activity in elimination of a diverse range of misfolded proteins from the ER. As described in the following sections, PDI's function is also hijacked by pathogenic toxins and viruses during host entry.
Toxins Co-Opting ERAD Pathways Require Disulfide Bond Disruption for Membrane Translocation
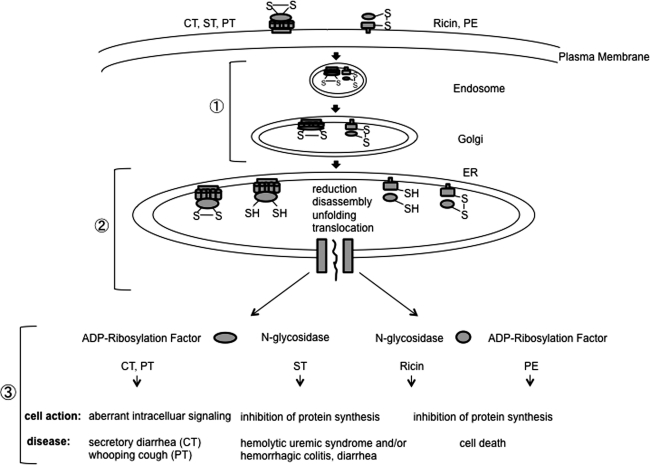
In addition to cellular misfolded substrates, members of the AB5 family of toxins also rely on disruption of their disulfide bonds for proper translocation across the ER membrane during intoxication. The members of this toxin family consists of five receptor-binding B subunits (B5) and a single catalytic A component (2, 28). To cause infection, the toxins enter the target cell and arrive in the ER via retrograde transport from the cell surface (Fig. 4, step 1). In the ER, the toxins are thought to disguise themselves as misfolded proteins, co-opting ERAD machineries to access the cytosol. A key toxin disulfide bond is disrupted during ER-to-cytosol transport (Fig. 4, step 2). By inhibiting protein synthesis or modulating essential signaling cascades in the host cytosol, these toxins exert catastrophic effects on their human hosts (Fig. 4, step 3).
FIG. 4.
General toxin intoxication pathway. Step 1. AB5 (cholera toxin [CT], shiga toxin, and Pertussis toxin [PT]) and AB toxins (ricin and Pseudomonas exotoxin A [PE]) attach to receptors on the surface of the host cell and are transported to the ER via retrograde transport. Step 2. A key disulfide bond in each toxin is reduced to aid in the disassembly, unfolding and translocation of each toxin's catalytic subunit into the cytosol. Step 3. The catalytic subunit of each toxin in the cytosol elicits its toxic effects.
Structurally, AB5 toxins have a disulfide bond within their A chain that is necessary for the toxin's assembly in its native organism and its stability as it traffics to the ER in the host cell. After proteolytic cleavage at the host cell surface or in endosomes, disulfide bond reduction in the ER is required for complete disassembly of the toxin and stimulation of its catalytic activities (31, 34, 38). Studies focused on elucidating toxin reduction have implicated PDI proteins as factors responsible for reducing the disulfide bond for a subset of AB5 toxins. Although only those toxins that translocate across the ER membrane will be discussed here, disulfide bond disruption in toxins that translocate across endosomal membranes, such as diphtheria toxin (8), is also important for their catalytic activity.
Cholera toxin (CT) is a prototype AB5 toxin containing a catalytic A subunit (CTA) and five receptor-binding B subunits (CTB5). A fragment of CTA called CTA1, generated when CTA's disulfide bond is reduced, undergoes ER-to-cytosol transport to induce cytotoxicity. Recent findings have revealed the mechanisms utilized by CT in the ER to prepare CTA1 for retro-translocation. Initial in vitro evidence suggested that PDI reduced CTA to generate CTA1 (40). However, when PDI was down-regulated in cells by siRNA, a concomitant decrease in CTA1 formation was not seen (14), suggesting that PDI does not reduce CTA in cells, or other factors compensate for this reductive event in PDI's absence.
Importantly, instead of functioning as a reductase, PDI was found to act as a redox-driven unfoldase as it engages CTA1 (14, 53, 54). In its reduced state, PDI exhibits a conformation that allows for tight binding to and unfolding of CTA1 via its bb′a′ domains (13, 54). Upon oxidation of PDI's C-terminal disulfide bond by the PDI oxidase Ero1α, PDI undergoes a conformational change that releases unfolded CTA1 for retro-translocation (33, 53).
PDI's redox-regulated chaperone activity is strictly controlled by its molar ratio with Ero1α. Changing this ratio blocks CTA1 retro-translocation (33). Specifically, PDI's binding to CTA1 is inhibited in cells overexpressing Ero1α, as PDI is held preferentially in an oxidized state. Conversely, cells lacking Ero1α due to siRNA knockdown also exhibit decreased CTA1 retro-translocation. Under these conditions, the substrate is locked onto reduced PDI and is unable to be released (33). As a recent finding demonstrates that PDI controls regulatory disulfides on Ero1α (1), changing the Ero1α level could potentially affect PDI's ability to properly control this feedback mechanism.
Reduced PDI also interacts preferentially with Derlin-1, a membrane ERAD component that facilitates CTA1 retro-translocation (3, 11). This finding suggests that PDI's localization within the ER is also redox-dependent (33). By placing reduced PDI next to the retro-translocation machinery on the ER membrane, PDI could efficiently reduce disulfide bonds present in ERAD substrates and facilitates their retro-translocation.
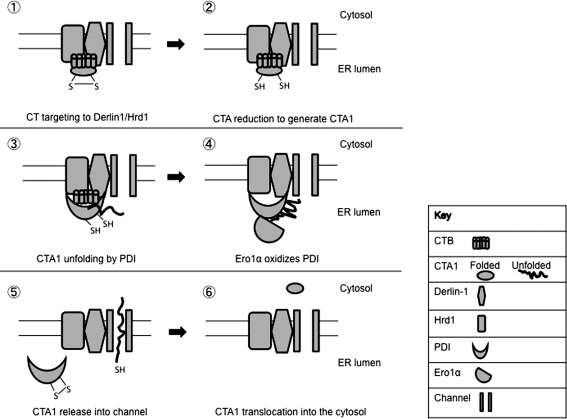
PDI's redox-dependent unfolding activity on CTA1 and binding to Derlin-1 afford a clearer picture of the CTA1 retro-translocation mechanism, as depicted in Figure 5. After ER arrival, CTB targets the holotoxin to the Derlin-1/Hrd1 complex (Fig. 5, step 1) (4). CTA is then reduced by an unidentified reductase (Fig. 5, step 2). The bb′a′ domains of reduced PDI (bound to the Derlin-1/Hrd1 complex) unfold CTA1 (Fig. 5, step 3), holding the toxin in a retro-translocation-competent unfolded state. Upon oxidation of its a′ domain by Ero1α (Fig. 5, step 4), PDI releases CTA1 (Fig. 5, step 5). As CTA1 can refold spontaneously in vitro (44), CTA1 is likely released directly into the retro-translocation channel to prevent refolding. How the toxin is extracted into the cytosol and evades proteasomal degradation remains unclear (Fig. 5, step 6).
FIG. 5.
ER events promoting cholera toxin subunit A 1 (CTA1) retro-translocation. Step 1. CT is targeted to the Derlin-1/Hrd1 complex via cholera toxin subunit B (CTB). Step 2.The CTA disulfide bond is reduced by an unidentified reductase to generate CTA1. Step 3. CTA1 is then unfolded by the bb′a′ domains of reduced protein disulfide isomerase (PDI) attached to the Derlin-1/Hrd1 complex. Step 4. Ero1α oxidizes PDI's a′ domain. Step 5. Oxidized PDI releases CTA1 into the retro-translocation channel and detaches from the Derlin-1/Hrd1 complex. Step 6. CTA1 avoids proteasomal degradation and refolds spontaneously in the cytosol to elicit its toxic effects.
PDI's redox-driven chaperone activity appears to be required for several other ERAD substrates. For instance, the ERAD substrates BACE457 and the non-glycosylated variant of pro-alpha-factor bind tightly to reduced PDI during ERAD and require a change in PDI's redox state to be retro-translocated (32, 57). Furthermore, PDI's ability to act as a chaperone in the ER when transferring peptides from transporter associated with antigen processing to MHC class I is also redox-driven (7). As some substrates do not bind to PDI in a redox-dependent manner (34), PDI's ability to act as a redox-triggered chaperone may be substrate dependent.
Similar to CT, pertussis toxin (PT) and shiga toxin (ST) are members of the AB5 family with catalytic subunits S1 and STA1, respectively (2). While ATP may disassemble PT initially in the ER (35), the precise reductase responsible for reducing its disulfide bond, as well as the sequence of events coupling disassembly, reduction, and unfolding, are not defined. S1's disulfide bond is highly resistant to reduction in ATP's absence, suggesting that the disassembly and conformational change induced by ATP binding may expose the disulfide bond for reduction (34). PT's crystal structure suggests that reduction exposes a segment on S1 that inserts into the membrane (49), allowing S1 retro-translocation into the cytosol. In support of this idea, reducing PT's disulfide bond increased its interaction with model membranes (20). Exposure of a hydrophobic domain in STA1 after reduction may also facilitate ST retro-translocation. In this case, reduction of STA1's disulfide bond by an unidentified reductase would presumably aid STA1 disassembly, unfolding, and retro-translocation regulated by the ERAD factors HEDj/ERdj3, BiP, and Sec61 (15, 63)
In addition to AB5 toxins, AB toxins in which a catalytic A chain is linked via a disulfide bond to the receptor-binding B chain, such as the plant toxin ricin and bacterial toxin Pseudomonas exotoxin A (PE), also require reduction of a key disulfide bond for complete disassembly and stimulation of their catalytic activity (26, 28, 39). For both ricin and PE, PDI accomplishes this reduction (30, 47). Ricin reduction likely occurs before initial dissociation of the toxin as PDI was found to act on the holotoxin (47). Reduction and unfolding of ricin's catalytic A chain (RTA) may expose a critical hydrophobic domain important for its interaction with the ERAD component EDEM1, which facilitates RTA retro-translocation (28, 46). This final event involving hydrophobic exposure before retro-translocation is conceptually similar to how endogenous substrates may be handled during ERAD (Fig. 3).
Unlike ricin, PDI appears to reduce PE's disulfide bond after toxin unfolding has initiated (30). The toxin's crystal structure indicates a hidden disulfide bond (60); therefore, some unfolding must presumably occur to expose the linkage. Indeed, when the toxin is exposed to moderate heat in vitro, structural unfolding exposes the disulfide bond, allowing access by PDI for reduction (30). Despite these insights, the cellular factors and mechanism responsible for unfolding PE to expose the disulfide bond are unknown.
A central theme thus emerges in these toxin studies demonstrating the importance of disulfide bond disruption in promoting ER membrane translocation and toxin activity. Additional studies are essential to identify unknown reductases and to clarify the sequence of events coupling reduction, disassembly, and unfolding before retro-translocation.
Polyomavirus Family Members Use PDI Family Members and Other ERAD Components for Entry
Similar to toxins, members of the polyomavirus family co-opt ER oxidoreductases, chaperones, and other ERAD machinery to enter host cells and cause infection (52). This nonenveloped virus family includes simian virus 40 (SV40) and murine polyomavirus (Py), as well as the human polyomaviruses BK, JC, WU, KI, and Merkel cell. SV40 and Py are model viruses for studying cell entry of this virus family.
An early observation revealed that SV40, upon entry, traffics from the cell surface to the ER (23). To reach the ER, polyomaviruses first bind to glycolipid ganglioside receptors on the plasma membrane and are endocytosed (51). Vesicular transport through the endolysosomes brings the viral particles to the ER (12, 41). The viruses then cross the ER membrane to reach the cytosol and ultimately deliver their viral DNA into the nucleus where transcription and replication of the viral genome initiate.
Structurally, polyomaviruses are stabilized by several forces that allow them to withstand the harsh extracellular environment. However, these forces must be disassembled systematically during cell entry. Lacking a lipid bilayer on their surface, polyomaviruses are unable to enter the host cell via fusion. Instead, conformational changes to the viral capsid induced by cellular factors in the ER allow for passage through the limiting ER membrane. The capsid's outer surface is comprised of 72 pentamers of the VP1 protein arranged in an icosahedral geometry (27, 48). Each pentamer interacts with neighboring pentamers by virtue of VP1's C-terminus, which interlocks with an adjacent VP1 C-terminus. This interaction is further stabilized by calcium ion-binding (48). Additionally, the capsid is supported by a network of inter- and intra-pentameric disulfide bonds. Beneath each VP1 pentamer resides one copy of either the internal proteins VP2 or VP3, the exposure of which is hypothesized to be critical for penetration of the ER membrane (10, 42).
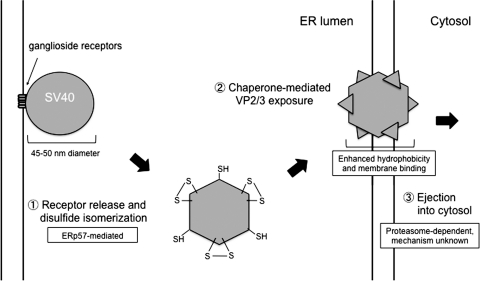
The mechanism controlling polyomavirus ER-to-cytosol membrane penetration is slowly becoming clear through accumulating data. Upon reaching the ER, SV40 detaches from the ganglioside receptor and is released into the ER lumen (Fig. 6, step 1) (21). Once in the ER lumen, redox reactions partially disassemble the viral particle (Fig. 6, step 1) (21, 45). Critical for this event is ERp57, a PDI family member that is normally involved in assisting the folding and maturation of nascent glyco-proteins (37). Using an unpaired VP1 Cys, ERp57 acts as an isomerase to disrupt specific inter-pentameric disulfide bonds (45). This reaction effectively releases a subset of VP1 from the viral particle. Interestingly, PDI acts as a chaperone but not as a reductase/isomerase to facilitate entry at this stage (45). Subsequent to the actions of ERp57 and PDI, SV40 engages BiP in a reaction controlled by the ER-resident J protein ERdj3 (18). The culmination of these reactions and possibly other unknown ER events lead to the exposure of VP2 and VP3 (Fig. 6, step 2) (17). Because these viral proteins contain hydrophobic moieties and can integrate into the ER membrane (10), their exposure enables viral binding to the ER membrane. The process of membrane integration initiates nonenveloped virus membrane penetration. Remarkably, SV40 reaches the cytosol as a large particle (21), suggesting that the virus either ruptures a portion of the ER membrane or travels through a large protein-conducting channel. More studies are necessary to understand mechanistically how a large viral particle is ejected across the ER membrane into the cytosol (Fig. 6, step 3).
FIG. 6.
ER events facilitating ER-to-cytosol transport of polyomaviruses. Polyomaviruses traffic from the cell surface to the ER attached to the ganglioside receptor. Step 1. Viral particles are released from the receptor into the ER lumen. The PDI family member ERp57 isomerizes viral disulfide bonds, releasing a subset of the major capsid VP1. Step 2. Additional conformational changes are conferred to the viral particle by other ER chaperones including ERp29, PDI, BiP, and ERdj3. These reactions expose the minor capsid proteins VP2 and VP3, increasing the viral hydrophobicity. Step 3. The viral particle binds to the ER membrane and is ejected into the cytosol as an intact particle by unknown mechanisms.
Although Py has a slightly different disulfide bond arrangement than SV40, Py uses a similar sequence of events to penetrate the ER membrane: thiol–disulfide exchange followed by chaperone-induced conformational changes prime the virus for membrane penetration to the cytosol. However, in contrast to SV40, an additional PDI family member known as ERp29 is co-opted by Py. ERp29 is a redox-inactive PDI protein that extrudes the interlocking VP1 C-termini (29). Disruption of the viral disulfide bonds by PDI and ERp57 (58) is a prerequisite for the ERp29 chaperone activity, likely due to presence of an intra-pentameric disulfide bond that clamps down the interlocking VP1 C-termini. The ERp29-induced Py conformational change exposes VP2, allowing the virus to bind and perforate the ER membrane (42). Finally, drawing another parallel to cellular ERAD substrates, arrival of polyomaviruses to the cytosol requires functional Derlin-1 members and proteasome activity (21, 22, 45). Whether additional cytosolic components aid in this final translocation step or stimulate complete disassembly of the capsid in the cytosol before nuclear entry remains to be determined.
Conclusion and Future Directions
Classically, disulfide bond formation has been viewed as an integral part of the protein folding process as substrates translocate from the cytosol into the ER. Not surprisingly, studies in the last decade have demonstrated that disulfide bond disruption, the opposite of disulfide bond formation, is linked functionally to the reverse translocation event, in which misfolded substrates are targeted from the ER to the cytosol for proteasomal degradation in a process called ERAD. ER-resident PDI family members are largely responsible for disrupting the disulfide bonds in the aberrant substrate. In addition to acting as enzymes, these PDI proteins also employ their chaperone function during ERAD. Because there are more than 20 PDI family members, it remains to be established whether all or only a subset of them are dedicated to ERAD. Moreover, are there additional PDI proteins that will be uncovered that control ERAD? Finally, a major question remains: as PDI proteins promote disulfide bond formation and substrate folding, how do they know when to catalyze disulfide bond reduction and substrate unfolding? What precise structural feature surrounding a disulfide bond informs PDI that it is native or mispaired? Clearly, high-resolution structures of a substrate containing either a native or mispaired disulfide bond will begin to address this question.
What has been striking over the past decade is the observation that pathogenic viruses and toxins hijack ER redox factors to gain entry into the cytosol to cause infection. During assembly, disulfide bond formation in these toxic agents provides vital structural support. Yet during host entry, these same bonds are broken to allow disassembly, enabling the toxic agents to cross a membrane barrier and induce cytotoxicity. What is so vastly different in the host cell that allows these disulfide bonds to be broken, which normally provide important physical support, is unknown. A systematic approach to probe both the environment in which a toxic agent is assembled or disassembled, and the conformation of the toxic agents in the context of these environments, should shed light on this conundrum.
Abbreviations Used
- CT
cholera toxin
- CTA
cholera toxin subunit A
- CTA1
cholera toxin subunit A1
- CTB
cholera toxin subunit B
- Cys
cysteine
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- Hh
Hedgehog
- NADPH
nicotinamide adenine dinucleotide phosphate
- PDI
protein disulfide isomerase
- PE
Pseudomonas exotoxin A
- PT
pertussis toxin
- Py
murine polyomavirus
- RTA
ricin toxin subunit A
- ST
shiga toxin
- STA1
shiga toxin subunit A1
- SV40
simian virus 40
Acknowledgments
We thank Dr. Emily Rainey-Barger (University of Michigan) for critical review of this article. The authors apologize for not citing the work of our colleagues due to space constraints. C.P.W. is funded partially by the Cellular and Molecular Biology program (NIH T32-GM007315) and the Rackham Graduate School at the University of Michigan. B.T. is a Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease and is supported by the NIH.
References
- 1.Appenzeller-Herzog C. Riemer J. Christensen B. Sorensen ES. Ellgaard L. A novel disulphide switch mechanism in Ero1alpha balances ER oxidation in human cells. EMBO J. 2008;27:2977–2987. doi: 10.1038/emboj.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beddoe T. Paton AW. Le Nours J. Rossjohn J. Paton JC. Structure, biological functions and applications of the AB5 toxins. Trends Biochem Sci. 2010;35:411–418. doi: 10.1016/j.tibs.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardi KM. Forster ML. Lencer WI. Tsai B. Derlin-1 facilitates the retro-translocation of cholera toxin. Mol Biol Cell. 2008;19:877–884. doi: 10.1091/mbc.E07-08-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardi KM. Williams JM. Kikkert M. van Voorden S. Wiertz EJ. Ye Y. Tsai B. The E3 ubiquitin ligases Hrd1 and gp78 bind to and promote cholera toxin retro-translocation. Mol Biol Cell. 2010;21:140–151. doi: 10.1091/mbc.E09-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulleid NJ. Ellgaard L. Multiple ways to make disulfides. Trends Biochem Sci. 2011;36:485–492. doi: 10.1016/j.tibs.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Chen X. Tukachinsky H. Huang CH. Jao C. Chu YR. Tang HY. Mueller B. Schulman S. Rapoport TA. Salic A. Processing and turnover of the Hedgehog protein in the endoplasmic reticulum. J Cell Biol. 2011;192:825–838. doi: 10.1083/jcb.201008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho K. Cho S. Lee SO. Oh C. Kang K. Ryoo J. Lee S. Kang S. Ahn K. Redox-regulated Peptide transfer from the transporter associated with antigen processing to major histocompatibility complex class I molecules by protein disulfide isomerase. Antioxid Redox Signal. 2011;15:621–633. doi: 10.1089/ars.2010.3756. [DOI] [PubMed] [Google Scholar]
- 8.Collier RJ. Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971;246:1496–1503. [PubMed] [Google Scholar]
- 9.Cunnea PM. Miranda-Vizuete A. Bertoli G. Simmen T. Damdimopoulos AE. Hermann S. Leinonen S. Huikko MP. Gustafsson JA. Sitia R. Spyrou G. ERdj5, an endoplasmic reticulum (ER)-resident protein containing DnaJ and thioredoxin domains, is expressed in secretory cells or following ER stress. J Biol Chem. 2003;278:1059–1066. doi: 10.1074/jbc.M206995200. [DOI] [PubMed] [Google Scholar]
- 10.Daniels R. Rusan NM. Wadsworth P. Hebert DN. SV40 VP2 and VP3 insertion into ER membranes is controlled by the capsid protein VP1: implications for DNA translocation out of the ER. Mol Cell. 2006;24:955–966. doi: 10.1016/j.molcel.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Dixit G. Mikoryak C. Hayslett T. Bhat A. Draper RK. Cholera toxin up-regulates endoplasmic reticulum proteins that correlate with sensitivity to the toxin. Exp Biol Med (Maywood) 2008;233:163–175. doi: 10.3181/0705-RM-132. [DOI] [PubMed] [Google Scholar]
- 12.Engel S. Heger T. Mancini R. Herzog F. Kartenbeck J. Hayer A. Helenius A. Role of endosomes in simian virus 40 entry and infection. J Virol. 2011;85:4198–4211. doi: 10.1128/JVI.02179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster ML. Mahn JJ. Tsai B. Generating an unfoldase from thioredoxin-like domains. J Biol Chem. 2009;284:13045–13056. doi: 10.1074/jbc.M808352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster ML. Sivick K. Park YN. Arvan P. Lencer WI. Tsai B. Protein disulfide isomerase-like proteins play opposing roles during retrotranslocation. J Cell Biol. 2006;173:853–859. doi: 10.1083/jcb.200602046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garred O. Dubinina E. Polesskaya A. Olsnes S. Kozlov J. Sandvig K. Role of the disulfide bond in Shiga toxin A-chain for toxin entry into cells. J Biol Chem. 1997;272:11414–11419. doi: 10.1074/jbc.272.17.11414. [DOI] [PubMed] [Google Scholar]
- 16.Gauss R. Kanehara K. Carvalho P. Ng DT. Aebi M. A complex of pdi1p and the mannosidase htm1p initiates clearance of unfolded glycoproteins from the endoplasmic reticulum. Mol Cell. 2011;42:782–793. doi: 10.1016/j.molcel.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Gillece P. Luz JM. Lennarz WJ. de La Cruz FJ. Romisch K. Export of a cysteine-free misfolded secretory protein from the endoplasmic reticulum for degradation requires interaction with protein disulfide isomerase. J Cell Biol. 1999;147:1443–1456. doi: 10.1083/jcb.147.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin EC. Lipovsky A. Inoue T. Magaldi TG. Edwards AP. Van Goor KE. Paton AW. Paton JC. Atwood WJ. Tsai B. Dimaio D. BiP and multiple DNAJ molecular chaperones in the endoplasmic reticulum are required for efficient simian virus 40 infection. MBio. 2011;2:e00101–e00111. doi: 10.1128/mBio.00101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagiwara M. Maegawa K. Suzuki M. Ushioda R. Araki K. Matsumoto Y. Hoseki J. Nagata K. Inaba K. Structural basis of an ERAD pathway mediated by the ER-resident protein disulfide reductase ERdj5. Mol Cell. 2011;41:432–444. doi: 10.1016/j.molcel.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Hausman SZ. Burns DL. Interaction of pertussis toxin with cells and model membranes. J Biol Chem. 1992;267:13735–13739. [PubMed] [Google Scholar]
- 21.Inoue T. Tsai B. A large and intact viral particle penetrates the endoplasmic reticulum membrane to reach the cytosol. PLoS Pathog. 2011;7:e1002037. doi: 10.1371/journal.ppat.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang M. Abend JR. Tsai B. Imperiale MJ. Early events during BK virus entry and disassembly. J Virol. 2009;83:1350–1358. doi: 10.1128/JVI.02169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kartenbeck J. Stukenbrok H. Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989;109:2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knittler MR. Dirks S. Haas IG. Molecular chaperones involved in protein degradation in the endoplasmic reticulum: quantitative interaction of the heat shock cognate protein BiP with partially folded immunoglobulin light chains that are degraded in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1995;92:1764–1768. doi: 10.1073/pnas.92.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SO. Cho K. Cho S. Kim I. Oh C. Ahn K. Protein disulphide isomerase is required for signal peptide peptidase-mediated protein degradation. EMBO J. 2010;29:363–375. doi: 10.1038/emboj.2009.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppla SH. Martin OC. Muehl LA. The exotoxin P. aeruginosa: a proenzyme having an unusual mode of activation. Biochem Biophys Res Commun. 1978;81:532–538. doi: 10.1016/0006-291x(78)91567-x. [DOI] [PubMed] [Google Scholar]
- 27.Liddington RC. Yan Y. Moulai J. Sahli R. Benjamin TL. Harrison SC. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991;354:278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 28.Lord JM. Roberts LM. Lencer WI. Entry of protein toxins into mammalian cells by crossing the endoplasmic reticulum membrane: co-opting basic mechanisms of endoplasmic reticulum-associated degradation. Curr Top Microbiol Immunol. 2005;300:149–168. doi: 10.1007/3-540-28007-3_7. [DOI] [PubMed] [Google Scholar]
- 29.Magnuson B. Rainey EK. Benjamin T. Baryshev M. Mkrtchian S. Tsai B. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol Cell. 2005;20:289–300. doi: 10.1016/j.molcel.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 30.McKee ML. FitzGerald DJ. Reduction of furin-nicked Pseudomonas exotoxin A: an unfolding story. Biochemistry. 1999;38:16507–16513. doi: 10.1021/bi991308+. [DOI] [PubMed] [Google Scholar]
- 31.Mekalanos JJ. Collier RJ. Romig WR. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979;254:5855–5861. [PubMed] [Google Scholar]
- 32.Molinari M. Galli C. Piccaluga V. Pieren M. Paganetti P. Sequential assistance of molecular chaperones and transient formation of covalent complexes during protein degradation from the ER. J Cell Biol. 2002;158:247–257. doi: 10.1083/jcb.200204122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore P. Bernardi KM. Tsai B. The Ero1alpha-PDI redox cycle regulates retro-translocation of cholera toxin. Mol Biol Cell. 2010;21:1305–1313. doi: 10.1091/mbc.E09-09-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss J. Stanley SJ. Burns DL. Hsia JA. Yost DA. Myers GA. Hewlett EL. Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet-activating protein) J Biol Chem. 1983;258:11879–11882. [PubMed] [Google Scholar]
- 35.Moss J. Stanley SJ. Watkins PA. Burns DL. Manclark CR. Kaslow HR. Hewlett EL. Stimulation of the thiol-dependent ADP-ribosyltransferase and NAD glycohydrolase activities of Bordetella pertussis toxin by adenine nucleotides, phospholipids, and detergents. Biochemistry. 1986;25:2720–2725. doi: 10.1021/bi00357a066. [DOI] [PubMed] [Google Scholar]
- 36.Okuda-Shimizu Y. Hendershot LM. Characterization of an ERAD pathway for nonglycosylated BiP substrates, which require Herp. Mol Cell. 2007;28:544–554. doi: 10.1016/j.molcel.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliver JD. van der Wal FJ. Bulleid NJ. High S. Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science. 1997;275:86–88. doi: 10.1126/science.275.5296.86. [DOI] [PubMed] [Google Scholar]
- 38.Olsnes S. Reisbig R. Eiklid K. Subunit structure of Shigella cytotoxin. J Biol Chem. 1981;256:8732–8738. [PubMed] [Google Scholar]
- 39.Olsnes S. Sandvig K. Refsnes K. Pihl A. Rates of different steps involved in the inhibition of protein synthesis by the toxic lectins abrin and ricin. J Biol Chem. 1976;251:3985–3992. [PubMed] [Google Scholar]
- 40.Orlandi PA. Protein-disulfide isomerase-mediated reduction of the A subunit of cholera toxin in a human intestinal cell line. J Biol Chem. 1997;272:4591–4599. [PubMed] [Google Scholar]
- 41.Qian M. Cai D. Verhey KJ. Tsai B. A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection. PLoS Pathog. 2009;5:e1000465. doi: 10.1371/journal.ppat.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rainey-Barger EK. Magnuson B. Tsai B. A chaperone-activated nonenveloped virus perforates the physiologically relevant endoplasmic reticulum membrane. J Virol. 2007;81:12996–13004. doi: 10.1128/JVI.01037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riemer J. Appenzeller-Herzog C. Johansson L. Bodenmiller B. Hartmann-Petersen R. Ellgaard L. A luminal flavoprotein in endoplasmic reticulum-associated degradation. Proc Natl Acad Sci U S A. 2009;106:14831–14836. doi: 10.1073/pnas.0900742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodighiero C. Tsai B. Rapoport TA. Lencer WI. Role of ubiquitination in retro-translocation of cholera toxin and escape of cytosolic degradation. EMBO Rep. 2002;3:1222–1227. doi: 10.1093/embo-reports/kvf239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schelhaas M. Malmstrom J. Pelkmans L. Haugstetter J. Ellgaard L. Grunewald K. Helenius A. Simian virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell. 2007;131:516–529. doi: 10.1016/j.cell.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 46.Slominska-Wojewodzka M. Gregers TF. Walchli S. Sandvig K. EDEM is involved in retrotranslocation of ricin from the endoplasmic reticulum to the cytosol. Mol Biol Cell. 2006;17:1664–1675. doi: 10.1091/mbc.E05-10-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spooner RA. Watson PD. Marsden CJ. Smith DC. Moore KA. Cook JP. Lord JM. Roberts LM. Protein disulphide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum. Biochem J. 2004;383:285–293. doi: 10.1042/BJ20040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stehle T. Gamblin SJ. Yan Y. Harrison SC. The structure of simian virus 40 refined at 3.1 A resolution. Structure. 1996;4:165–182. doi: 10.1016/s0969-2126(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 49.Stein PE. Boodhoo A. Armstrong GD. Cockle SA. Klein MH. Read RJ. The crystal structure of pertussis toxin. Structure. 1994;2:45–57. doi: 10.1016/s0969-2126(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 50.Tortorella D. Story CM. Huppa JB. Wiertz EJ. Jones TR. Bacik I. Bennink JR. Yewdell JW. Ploegh HL. Dislocation of type I membrane proteins from the ER to the cytosol is sensitive to changes in redox potential. J Cell Biol. 1998;142:365–376. doi: 10.1083/jcb.142.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai B. Gilbert JM. Stehle T. Lencer W. Benjamin TL. Rapoport TA. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003;22:4346–4355. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai B. Qian M. Cellular entry of polyomaviruses. Curr Top Microbiol Immunol. 2010;343:177–194. doi: 10.1007/82_2010_38. [DOI] [PubMed] [Google Scholar]
- 53.Tsai B. Rapoport TA. Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J Cell Biol. 2002;159:207–216. doi: 10.1083/jcb.200207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai B. Rodighiero C. Lencer WI. Rapoport TA. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell. 2001;104:937–948. doi: 10.1016/s0092-8674(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 55.Ushioda R. Hoseki J. Araki K. Jansen G. Thomas DY. Nagata K. ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science. 2008;321:569–572. doi: 10.1126/science.1159293. [DOI] [PubMed] [Google Scholar]
- 56.Vembar SS. Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wahlman J. DeMartino GN. Skach WR. Bulleid NJ. Brodsky JL. Johnson AE. Real-time fluorescence detection of ERAD substrate retrotranslocation in a mammalian in vitro system. Cell. 2007;129:943–955. doi: 10.1016/j.cell.2007.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walczak CP. Tsai B. A PDI family network acts distinctly and coordinately with ERp29 to facilitate polyomavirus infection. J Virol. 2011;85:2386–2396. doi: 10.1128/JVI.01855-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q. Chang A. Substrate recognition in ER-associated degradation mediated by Eps1, a member of the protein disulfide isomerase family. EMBO J. 2003;22:3792–3802. doi: 10.1093/emboj/cdg378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wedekind JE. Trame CB. Dorywalska M. Koehl P. Raschke TM. McKee M. FitzGerald D. Collier RJ. McKay DB. Refined crystallographic structure of Pseudomonas aeruginosa exotoxin A and its implications for the molecular mechanism of toxicity. J Mol Biol. 2001;314:823–837. doi: 10.1006/jmbi.2001.5195. [DOI] [PubMed] [Google Scholar]
- 61.Wiertz EJ. Tortorella D. Bogyo M. Yu J. Mothes W. Jones TR. Rapoport TA. Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 62.Young J. Kane LP. Exley M. Wileman T. Regulation of selective protein degradation in the endoplasmic reticulum by redox potential. J Biol Chem. 1993;268:19810–19818. [PubMed] [Google Scholar]
- 63.Yu M. Haslam DB. Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone HEDJ/ERdj3. Infect Immun. 2005;73:2524–2532. doi: 10.1128/IAI.73.4.2524-2532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]