Abstract
We test the hypothesis that moderate calorie restriction (CR) reverses negative influences of age on molecular determinants of myocardial stress resistance. Postischemic contractile dysfunction, cellular damage, and expression of regulators of autophagy/apoptosis and of prosurvival and prodeath kinases were assessed in myocardium from young adult (YA; 2- to 4-month-old) and middle-aged (MA; 12-month-old) mice, and MA mice subjected to 14 weeks of 40% CR (MA-CR). Ventricular dysfunction after 25%±2%), as was cell death indicated by troponin I (TnI) efflux (1,701±214 ng vs. 785±102 ng in YA). MA hearts exhibited 30% and 65% reductions in postischemic Beclin1 and Parkin, respectively, yet 50% lower proapoptotic Bax and 85% higher antiapoptotic Bcl2, increasing the Bcl2/Bax ratio. Age did not influence Akt or p38-mitogen-activated protein kinase (MAPK) expression; reduced expression of increasingly phosphorylated ribosomal protein S6 kinase (p70S6K), increased expression of dephosphorylated glycogen synthase kinase 3β (GSK3β) and enhanced postischemic p38-MAPK phosphorylation. CR countered the age-related decline in ischemic tolerance, improving contractile recovery (60%±4%) and reducing cell death (123±22 ng of TnI). Protection was not associated with changes in Parkin or Bax, whereas CR partially limited the age-related decline in Beclin1 and further increased Bcl2. CR counteracted age-related changes in p70S6K, increased Akt levels, and reduced p38-MAPK (albeit increasing preischemic phosphorylation), and paradoxically reduced postischemic GSK3β phosphorylation. In summary, moderate age worsens cardiac ischemic tolerance; this is associated with reduced expression of autophagy regulators, dysregulation of p70S6K and GSK3β, and postischemic p38-MAPK activation. CR counters age effects on postischemic dysfunction/cell death; this is associated with reversal of age effects on p70S6K, augmentation of Akt and Bcl2 levels, and preischemic p38-MAPK activation. Age and CR thus impact on distinct determinants of ischemic tolerance, although p70S6K signaling presents a point of convergence.
Introduction
Age and calorie restriction (CR) exert opposing effects in the cardiovascular system, with CR effectively countering age-related cardiac and vascular changes and impacting beneficially on most major cardiovascular risk factors.1 CR is also the only stimulus that consistently increases longevity in multiple organisms, and may thus act by specifically repressing or decelerating cellular aging processes and associated molecular alterations. Indeed, there is evidence that age-dependent changes in the cardiac transcriptome are selectively reversed by CR,2 although other studies support distinct effects of age and CR on cardiac gene expression.3,4 Unraveling the basis of advanced age-related deteriorations in the heart, and of the benefit with CR in this setting, may assist the design of new therapeutic approaches in ischemic heart disease, which is prevalent in the elderly.
Experimental evidence supports a negative influence of age on ischemic tolerance,5–18 and on the efficacy of cardioprotective responses in animal models5,12,19–21 and humans.22–24 Dysfunctional postischemic remodeling may also arise with age.18,25 Detrimental effects of age on stress resistance may involve multiple mechanisms, including alterations in stress responses and signal transduction,26–28 mitochondrial control of cell death,15,26,27 autophagy,29,30 heat shock responses,31 and oxidative stress.17,32
At the level of the myocardium, CR improves resistance to injurious stressors33–37 and restores cytoprotective responses lost with age.38,39 That said, cardiac protection is not always observed after CR. For example, Ahmet et al. recently found that whereas CR inhibited cardiac and vascular changes with aging, a 3-month period of CR failed to limit infarction with permanent coronary occlusion in young rats.40 Mechanistically, CR may enhance ischemic tolerance by targeting the same processes altered with age, including mitochondrial function,34 oxidative stress,32 heat shock protein responsiveness,31 and protein kinase36 and nitric oxide (NO) signaling.37 In the present study, we tested whether CR reverses age-related changes in key molecular signaling determinants of cell survival or generates a distinct protected phenotype. Specifically, we examined the effects of moderate aging in middle-aged (MA; 12-month-old) mice and CR on myocardial outcomes from ischemia-reperfusion, on select regulators of postischemic autophagy and apoptosis, and on the expression of protein kinases implicated in control of cell survival versus death [Akt, p70S6K, p38-mitogen-activated protein kinase (MAPK), GSK3β]. Active (dephosphorylated) GSK3β promotes mitochondrial dysfunction and cell death.41 Akt and p70S6K transduce cytoprotective signals to negatively modulate GSK3β and inhibit cell death,42 whereas effects of p38-MAPK are controversial, with evidence for involvement in both cardioprotection and postischemic cell death/ remodeling.43,44
Methods
All studies were performed in accordance with the guidelines of the Animal Ethics Committee of Griffith University, which is accredited by the Queensland Government, Department of Primary Industries and Fisheries under the guidelines of The Animal Care and Protection Act 2001, Section 757.
Animals and feeding protocols
Male C57/Bl6 mice aged 2–4 months (young adult [YA]) or 12 months (MA) were housed in divided cages, enabling individual feeding while maintaining social interaction. Control mice were fed the semipure AIN-93 diet ad libitum, with food consumption measured weekly to determine normal caloric consumption. A subset of MA mice were subjected to CR (MA-CR), ingesting 40% less food (by weight) than control mice, using a version of AIN-93 specifically formulated to contain 40% more vitamins and minerals. Thus, MA-CR mice consumed 40% less calories than MA mice, yet with optimal nutrition. CR was commenced at ≈38 weeks of age and maintained for 14 weeks until experimentation (at 12 months of age).
Perfused heart preparation
Mice were anesthetized with 60 mg/kg sodium pentobarbital, and their hearts were removed and perfused as described previously.12,14,45 Briefly, the hearts were rapidly excised into ice-cold perfusion fluid, the aorta cannulated, and the coronary circulation perfused in Langendorff mode at a pressure of 80 mmHg with modified Krebs–Henseleit solution containing: 120 nM NaCl, 25 nM NaHCO3, 4.7 nM KCl, 2.5 nM CaCl2, 1.2 nM MgCl2, 1.2 nM KH2PO4, 15 nM D-glucose, and 0.5 nM EDTA. Perfusion fluid was maintained at 37°C and bubbled with a mix of 95% O2/5% CO2 at 37°C to provide a pH of 7.4 and PO2 of ≈600 mmHg at the aortic cannula over a 1- to 5-mL/min flow range. Perfusate was continuously passed through a 0.45-μm filter. The left ventricle was vented with a polyethylene apical drain, and a fluid-filled polyvinyl chloride plastic film balloon was inserted into the ventricle via the mitral valve. Balloons were connected by fluid-filled tubing to a P23 XL pressure transducer (Viggo-Spectramed, Oxnard, CA), permitting continuous assessment of contractile function. Balloon volume was increased to give an end-diastolic pressure of 5 mmHg during stabilization and not adjusted further. Coronary flow was monitored via a flow-probe in the aortic perfusion line, connected to a T206 flowmeter (Transonic Systems Inc, Ithaca, NY). Functional data were recorded at 1 KHz on an 8-channel MacLab data acquisition system (ADInstruments, Castle Hill, Australia) connected to an Apple iMac computer. Left ventricular pressure signals were digitally processed to yield diastolic, systolic, and developed pressures, +dP/dt, [rate of increase in LV systolic pressure (rate of LV contraction)], −dP/dt, and heart rate.
After preparation, hearts were immersed in perfusion fluid at 37°C in a water-jacketed organ bath. Temperatures of the perfusion fluid and organ bath were continuously monitored by two-needle thermistor probes connected to a TH-8 digital thermometer (Physitemp Instruments Inc, Clifton, NJ). Hearts were removed from study if they met one of the following exclusion criteria after stabilization: (1) Coronary flow >5 mL/min; (2) unstable (fluctuating) contractile function; (3) left ventricular systolic pressure <100 mmHg; or (4) significant cardiac arrhythmias. This amounted to <4% of all hearts perfused.
Cardiac ischemia-reperfusion
After 20 min of stabilization, hearts were switched to ventricular pacing at 420 beats/min (Grass S9 stimulator, Quincy, MA), with normalization of the rate to permit comparison of rate-dependent measures of contractile function. Baseline measurements were made after a further 10 min before subjecting hearts to 25 min of normothermic global zero-flow followed by 45 min of aerobic reperfusion. In all groups, pacing was terminated on initiation of ischemia and resumed after 1.5 min of reperfusion.14,45 To assess myocardial cell death, the total cumulative washout of cardiac troponin I (TnI) over the 45-min period of reperfusion was assayed via commercial enzyme-linked immunosorbent assay (ELISA; Life Diagnostics Inc., West Chester, PA).
Myocardial protein expression and phosphorylation
Cardiac tissue was rapidly frozen in liquid nitrogen at the end of normoxic stabilization or on completion of 45 min of reperfusion. Samples of ventricular myocardium were homogenized in lysis buffer (50 mmol/L HEPES, 150 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 1% Triton X, 10% glycerol) plus 8.6 μmol/L leupeptin, 5.8 μmol/L pepstatin A, 4 mmol/L phenylmethylsulfonyl fluoride (PMSF), 0.6 μmol/L aprotinin, 4 mmol/L sodium fluoride, and 0.8 mmol/L sodium orthovanadate, and centrifuged at 10,000×g for 15 min to remove nuclei and debris. The supernatant was centrifuged at 100,000×g to enrich for cytosolic components. Samples containing 30 μg of total protein were loaded onto precast 10% acrylamide gels and separated at 150 V for ≈1.5 hr. Proteins were subsequently transferred to polyvinylidene difluoride membranes and blocked in 5% skim milk powder in Tris-buffered saline and Tween 20 (TBST) for 60 min. Blots were incubated with primary antibody (total or phosphorylated Akt, GSK3β, p70S6K, p38-MAPK; and total Beclin1, Parkin, Bcl2, and Bax; Cell Signaling Technology Inc., Danvers, MA) overnight at 4°C. Following three washes in TBST, the blots were incubated with secondary antibody and visualized on a ChemiDoc XRS system (BioRad, Hercules, CA). For comparison purposes, data for protein expression and phospho/total protein ratios were normalized to values acquired in YA hearts.
Data analysis
A one-way analysis of variance (ANOVA) was employed to contrast data across the three groups (YA, MA, MA-CR), with a post hoc Newman–Keuls test used to identify individual effects. Evidence of statistical significance was accepted at p<0.05. All data are expressed as mean±the standard error of the mean (SEM).
Results
Effects of moderate age and CR on baseline parameters
An increase in age from 2–4 to 12 months was associated with a 50% increase in body weight (Table 1). A 14-week period of CR reduced body weight in age-matched MA animals to that for YA mice (a 35% reduction). Age was associated with a reduction in inotropic state indicated by +dP/dt (Table 1). This occurred without shifts in ventricular pressure development, −dP/dt, or coronary flow (Table 1). Induction of CR reversed the age-dependent fall in +dP/dt.
Table 1.
Baseline Function in Isolated Perfused Hearts from Young and Middle-Aged Mice±Calorie Restriction
| Young (n=11) | Middle-aged (n=10) | Middle-aged CR (n=10) | |
|---|---|---|---|
| Body weight (g) | 23.2±.4 | 35.3±1.2* | 23.3±0.7† |
| EDP (mmHg) | 4±2 | 2±2 | 3±1 |
| LVDP (mmHg) | 146±4 | 146±6 | 160±1 |
| −dP/dt (mmHg/s) | −3,441±354 | −3,690±215 | −4,022±28 |
| +dP/dt (mmHg/s) | 6,843±376 | 5,653±248* | 6,686±89† |
| Coronary flow (mL/min) | 3.2±0.3 | 3.3±0.2 | 3.3±0.1 |
Baseline functional measures were acquired immediately prior to induction of global ischemia. Data are mean±standard error of the mean (SEM).
p<0.05 versus young.
p<0.05 middle-aged versus middle-aged CR.
CR, Calorie restriction; EDP, end-diastolic pressure; LVDP, left ventricular developed pressure; −dP/dt, rate of decline in LV systolic pressure (rate of LV relaxation); +dP/dt, rate of increase in LV systolic pressure (rate of LV contraction).
Moderate age worsens whereas CR improves myocardial resistance to ischemia-reperfusion
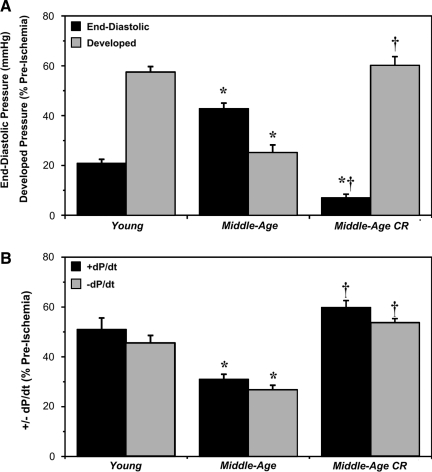
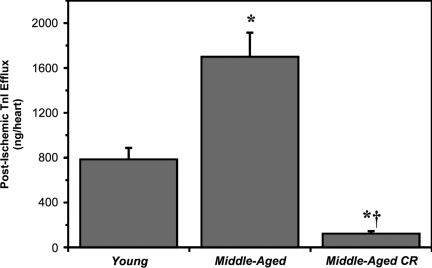
In YA hearts, a 25-min episode of ischemia followed by 45 min of reperfusion resulted in reduced pressure development (to ≈58% of preischemia), a sustained elevation in diastolic pressure (to ≈20 mmHg), and reductions in +dP/dt and −dP/dt (to 45%−50% of preischemia) (Fig. 1). MA hearts exhibited significantly greater postischemic dysfunction (Fig. 1). A period of CR improved postischemic outcomes, with final functional recoveries for MA-CR hearts equivalent or superior to recoveries in YA hearts (Fig. 1). Recovery of coronary perfusion did not differ between groups, reaching 69%±2%, 68%±4%, and 75%±3% of preischemic values in Y, MA, and MA-CR hearts, respectively. Myocardial cell damage and death, indicated by release of cardiac TnI, was significantly exacerbated in MA versus YA hearts and reduced in MA-CR hearts to levels even lower than in YA (Fig. 2).
FIG. 1.
Age reduces whereas calorie restriction (CR) enhances functional tolerance to ischemia-reperfusion. Recovery of contractile function and coronary flow was assessed at the end of 45 min reperfusion following 25 min of global ischemia in hearts from young, middle-aged, and middle-aged CR mice. Data are shown for recoveries of: (A) Left ventricular end-diastolic and developed pressures; and (B) +dP/dt and −dP/dt. Data are means±standard error of the mean (SEM). (*) p<0.05 versus young; (†) p<0.05 middle-aged versus middle-aged CR.
FIG. 2.
Age increases whereas calorie reduction (CR) reduces cardiac cell death (postischemic troponin I [TnI] efflux). Coronary washout of TnI throughout the 45-min reperfusion period following 25 min of global ischemia in hearts from young, middle-aged, and middle-aged CR mice. Data are means±standard error of the mean (SEM). (*) p<0.05 versus young; (†) p<0.05 middle-aged versus middle-aged CR.
Moderate age and CR modify regulators of postischemic autophagy and apoptosis
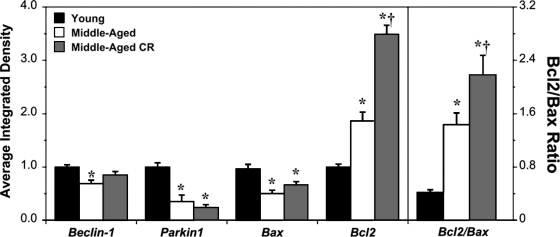
Postischemic expression of Beclin1 was significantly reduced in MA versus YA hearts (Fig. 3), a change tempered somewhat by CR (with Beclin1 in MA-CR hearts midway between, and not differing from, YA and MA values). Additionally, the key mitophagy inducer Parkin47 was repressed by 60% with age, a change insensitive to CR. Proapoptotic Bax was paradoxically reduced in MA hearts, whereas antiapoptotic Bcl2 was increased (Fig. 3). Induction of CR did not modify Bax but substantially increased Bcl2 levels (by ≈85%). As a result, the postischemic Bcl2/Bax ratio increased with moderate age and further with CR (Fig. 3).
FIG. 3.
Age and calorie restruction (CR) modify markers of autophagy and apoptosis in postischemic myocardium. Expression of Beclin1 (mammalian ortholog of yeast Atg6/Vps30, regulating autophagosome formation), Parkin (an E3 ubiquitin ligase targeting damaged mitochondria for mitophagy), and proapoptotic Bax and antiapoptotic Bcl2 was assessed in postischemic cardiac homogenates from young, middle-aged, and middle-aged CR mice (relative densities normalized to young). The ratio of Bcl2/Bax is also shown in the right panel. Left ventricular tissue was sampled after 25 min of global ischemia and 45 min of reperfusion. Data are means±standard error of the mean (SEM). (*) p<0.05 versus young; (†) p<0.05 middle-aged versus middle-aged CR.
Moderate age and CR modify protein kinase expression and phospho-regulation
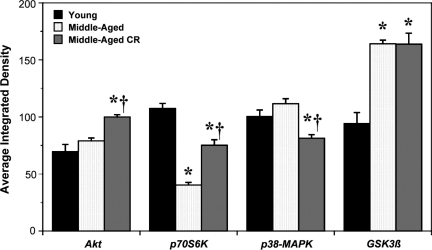
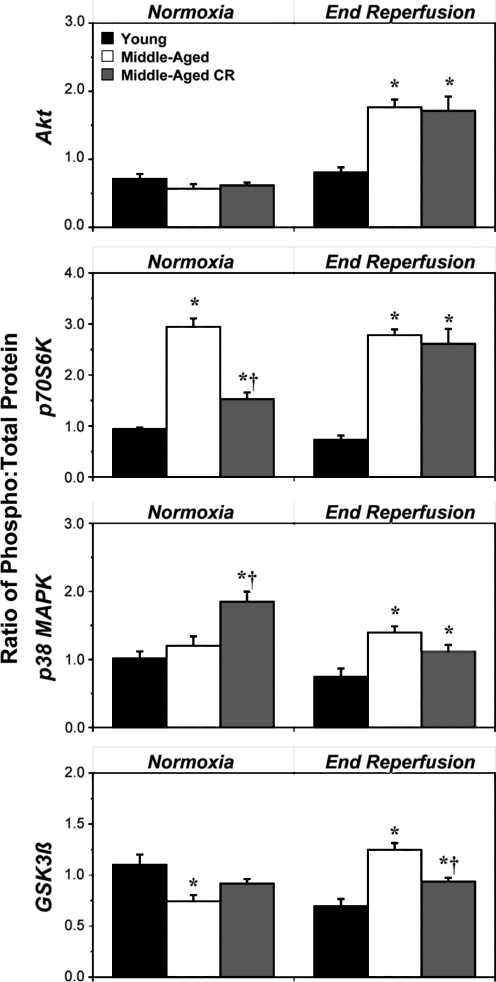
Cardiac outcomes from ischemia-reperfusion are dictated in part by expression and functionality of prosurvival versus proinjury kinases. We examined effects of age and CR on expression of key regulatory kinases—Akt, p70S6K, p38-MAPK, and GSK3β (Fig. 4). Compared with YA, MA hearts exhibited a 60% decline in p70S6K expression and 75% increase in GSK3β levels, with no changes in expression of Akt or p38-MAPK (Fig. 4). Subjecting animals to CR partially countered the effect of age on p70S6K expression, selectively increased expression of Akt by 20%, reduced expression of p38-MAPK by 25%, and was without effect on GSK3β levels (Fig. 4). Effects of age and CR on kinase expression are summarized in Table 2.
FIG. 4.
Age and calorie restriction (CR) modify cardiac expression of prosurvival and prodeath kinases. Cardiac expression of Akt, GSK3β, p70S6K, and p38-mitogen-activated protein kinase (MAPK) was assessed in left ventricular homogenates from normoxic hearts from young, middle-aged, and middle-aged CR mice (relative densities all normalized to young). Data are means±standard error of the mean (SEM). (*) p<0.05 versus young; (†) p<0.05 middle-aged versus middle-aged CR.
Table 2.
Summary of the Effects of Moderate Age and Calorie Restriction on Pre- and Postischemic Protein Kinase Signaling by Akt, GSK3β, p70S6K, and p38-MAPK
| |
Cardiac expression level |
Preischemic phosphorylation |
Postischemic phosphorylation |
|||
|---|---|---|---|---|---|---|
| Protein kinase | Age (MA vs. YA) | CR (MA-CR vs. MA) | Age (MA vs. YA) | CR (MA-CR vs. MA) | Age (MA vs. YA) | CR (MA-CR vs. MA) |
| Akt (prosurvival) | – | ↑ ↑ | – | – | ↑↑↑ | – |
| p70S6K (prosurvival) | ↓ ↓ | ↑ ↑ | ↑↑↑ | ↓ ↓ | ↑↑↑ | – |
| p38-MAPK (prosurvival/death?) | – | ↓ | – | ↑ | ↑↑↑ | – |
| GSK3β (prodeath) | ↑↑↑ | – | ↓ ↓ | – | ↑↑↑ | ↓ |
Relative changes in Akt, GSK3β, p70S6K, and p38-MAPK levels and phosphorylation in middle-aged (MA) versus young (Y) hearts, and with CR in MA hearts: (↑) increased; (↓) decreased; (−) unchanged. (↑ or ↓) 10%−40% change; (↑ ↑ or ↓ ↓) 41%−70% change; (↑↑↑ or ↓↓↓) >70+% change. Responses are derived from data in Figs. 4 and 5.
CR, Calorie reduction; YA, young adult; MAPK, mitogen-activation protein kinse.
Assessment of phosphoregulation revealed no effect of age on baseline phosphorylation of Akt or p38-MAPK, whereas p70S6K phosphorylation was enhanced and GSK3βphosphorylation reduced (Fig. 5, Table 2). Postischemic phosphorylation of all four kinases was augmented two- to three-fold in MA versus YA hearts. CR countered the effects of age on p70S6K and augmented baseline phosphorylation of p38-MAPK, whereas Akt and GSK3β phosphorylation was unaltered (Fig. 5). Postischemic kinase phosphorylation was largely unaltered by CR, although phosphorylation of GSK3β declined by ≈25% (Fig. 5, Table 2).
FIG. 5.
Age and calorie restriction (CR) modify phosphoregulation of prosurvival and prodeath kinases in normoxic and postischemic myocardium. Relative phosphorylation of Akt, p70S6K, p38-mitogen-activated protein kinase (MAPK), and GSK3β was assessed in homogenates from normoxic and postischemic hearts from young, middle-aged, and middle-aged CR mice (ratios normalized to values for young). Left ventricular tissue was sampled after normoxic perfusion or following 25 min of global ischemia and 45 min of reperfusion. Data are means±standard error of the mean (SEM). (*) p<0.05 versus young; (†) p<0.05 middle-aged versus middle-aged CR.
Discussion
The present study reveals significant impairment of ischemic tolerance in MA versus YA myocardium, and shows that CR (40% for 14 weeks) reverses this effect very effectively. The age-related decline in ischemic tolerance is associated with reduced expression of autophagy regulators (Beclin1, Parkin) and p70S6K, and enhanced expression of injurious GSK3β. Cardioprotective CR counteracts the effects of age on p70S6K (and more moderately Beclin1), selectively increases Akt expression, and further augments Bcl2 levels without modifying Parkin or Bax. Thus, opposing effects of age and CR on ischemic tolerance are associated with distinct molecular changes (age-dependent changes in GSK3β and controllers of autophagy; CR-dependent changes in Akt), together with common modulation of p70S6K signaling.
Ischemic intolerance in MA myocardium
Age is a significant risk factor for development of ischemic heart disease, and there is considerable evidence that older hearts become less resistant to ischemic insult.5–17 Age also impairs long-term outcomes and remodeling.18,25 Given the predominance of ischemic heart disease in the elderly population, it is important to delineate effects of age on the heart and its response to injurious stress. With a median life span of >800 days, 12-month C57Bl/6 mice are considered middle-aged, certainly not aged or senescent. As shown in Figs. 1 and 2, the modest increase in age from YA to MA is associated with a profound increase in postischemic contractile dysfunction and cellular damage. This augmented cell damage/death likely contributes to poor functional outcome in MA hearts, with the pattern of TnI efflux mirroring changes in contractility. However, this does not preclude a contribution from contractile dysfunction per se (stunning), with prior data demonstrating a greater degree of stunning in older hearts.14 Older myocardium, which is at greatest risk from ischemic disease, thus responds quite differently to insult and may require specific management strategies not apparent from analysis of younger hearts.
In contrast to the current data, there are reports of age-independent stress resistance in similar models.19–21,47–49 Reasons for differing observations are not readily apparent, though analysis of different end points may contribute. In these latter studies, cellular damage was assessed from infarct size determined by triphenyltetrazolium chloride (TTC) staining, whereas in our present study we assess loss of myocardial TnI (Fig. 2). Other studies confirm age-related increases in loss of intracellular proteins.5,12,14,15,50 Furthermore, McCully et al.16 and Jugdutt et al.18 also report age-related exaggeration of TTC-determined infarct size in vitro and in vivo. Overall, there seems to be a greater consensus regarding exaggerated contractile dysfunction with age, with mixed findings regarding markers of myocardial cell damage and death. Importantly, an age-related increase in either contractile dysfunction or cell death will contribute to altered remodeling and hypertrophic changes apparent in older hearts.18,25
Regulators of postischemic autophagy and apoptosis in MA myocardium
Interactions between apoptosis and autophagy may influence short- and longer-term outcomes from ischemia-reperfusion. We assessed postischemic expression of the key autophagy regulators Beclin1 and Parkin, and of pro- and antiapoptotic Bax and Bcl2. Beclin1 is essential for activation (and is a specific marker) of autophagy, whereas Parkin is key to mitophagy, translocating to depolarized or uncoupled mitochondria, ubiquitinating target proteins, and recruiting autophagosomes. A 30% fall in Beclin1 and 65% fall in Parkin implicates impaired activation of autophagy/mitophagy in postischemic tissue from older hearts (Fig. 3). This is consistent with the proposal that progressive impairment of autophagy underpins the process of cellular aging.29 Nonetheless, some investigations find no age-related changes in cardiac autophagy,51 and it is also relevant that Parkin may translocate to mitochondria after ischemic insult, reducing cytosolic expression.52
Because it is generally considered cytoprotective, a decline in autophagy may well contribute to greater postischemic dysfunction and cell death in MA hearts (Figs. 1 and 2). This is consistent with cardioprotection in response to upregulated autophagy,53 and induction of autophagy with preconditioning.54,55 However, detrimental effects of autophagy have also been reported in cardiac cells and myocardium,56–58 and repression of Beclin1-dependent autophagy may be cardioprotective.57,58 Such observations underpin the suggestion that stimulus dictates outcome: Beclin1-activated autophagy may be detrimental, whereas adenosine monophosphate-activated protein kinase (AMPK)- or Sirt1/FoxO-dependent autophagy seems protective.58 Furthermore, AMPK activation may predominate during ischemia, whereas Beclin1 activation may predominate during reperfusion. Thus, whether marked repression of postischemic Beclin1 and Parkin contributes to poor outcomes in MA hearts remains to be established. Future studies may require use of models that also permit study during late-term recovery and adaptive remodeling after ischemic injury.
Moderate aging was associated, somewhat paradoxically, with reduced expression of Bax relative to Bcl2 (Fig. 3). Bcl2 enhances cell survival, whereas Bax exerts opposing effects on signaling and apoptosis. The expression ratios of such anti- and proapoptotic molecules are important to initiation and progression of cell death with ischemia.59 As shown in Fig. 3, the Bcl2/Bax ratio increases with age, in agreement with prior evidence of age-related elevations in Bcl2/Bax or reductions in cardiac Bax.60,61 Although such changes appear inconsistent with age-related enhancement of apoptosis, the effects of age on postischemic apoptosis are not entirely clear (nor directly assessed here). For example, Liu et al.61 found that apoptosis peaks earlier in older hearts, yet is repressed later and does not differ in the long term. Some studies report associations between Bax expression and postischemic death,59,62 and others report improved postischemic outcomes in the face of increased Bax,63 or no relation between Bax expression and ischemic or hypoxic tolerance.60,61,64 This raises the question of how shifts in Bax (or Bcl2/Bax) influence postischemic death, although one might speculate that reduced postischemic Bax and increased Bcl2 may serve to dampen or counter an age-related proapoptotic phenotype.
Cardioprotective effects of CR in MA myocardium
Crandall et al.33 were among the first to assess the specific impact of CR on cardiac tolerance to injury, documenting reduced isoproterenol-induced infarction in adult rats subjected to 10 weeks of CR. A number of studies have subsequently addressed the impact of CR on myocardial stress resistance,36,65 including analysis of hearts from older animals.32,34,35,37,38,39 We show here that CR markedly enhances ischemic tolerance in MA myocardium, improving contractile recovery and largely eliminating cell death (Figs. 1 and 2). This is consistent with prior findings in young mice36 and adult to MA (6.5- to 12-month-old) rats,34,37 yet contrasts lack of effect of CR on ischemic tolerance in MA (10-month-old) and senescent (24-month-old) rats.38,39 The reasons for these differences are unclear, although the latter findings are suggestive of an age-related decline in cardiac responsiveness to CR.
Despite significant reductions in contractile dysfunction and cell death, CR failed to significantly modify postischemic expression of Bax and regulators of autophagy (Fig. 3), although the decline in Beclin1 was partly countered with CR such that Beclin1 levels no longer differed from either YA or MA. There is evidence of enhanced cardiac autophagy with starvation or CR,51,66 which may preserve ventricular function under these conditions. Our findings relate specifically to postischemic myocardium, and support an antiautophagic milieu in MA versus YA hearts, with a rather modest effect of CR on Beclin1 (but not Parkin). Interestingly, postischemic Bcl2 and the Bcl2/Bax ratio were further enhanced by CR in aged myocardium (Fig. 3). These changes support an antiapoptotic effect of CR, although there was also a rise in this ratio as a result of age alone (which is associated with worsened postischemic outcomes).
An unexpected consequence of CR was moderately improved baseline contractility (Table 1). Cardiac +dP/dt declined ≈20% with age, an effect reversed by CR. The basis of this select effect of CR on contraction velocity is not known. However, gene profiling in CR supports shifts toward reduced cardiac fibrosis and remodeling, with improved contractility and energy production,3 consistent with improved contractility observed here (Table 1). Shinmura et al.67 also recently reported that CR reverses age-related diastolic dysfunction. Thus, CR appears to restore multiple determinants of contractile function in older myocardium. The decline in +dP/dt with age is of interest, occurring in the absence of shifts in absolute force development or −dP/dt. There is good evidence for altered Ca2+ handling and impaired relaxation with age, prolonging the period of contraction.68 Lengthened contraction may well contribute to impaired +dP/dt with age, together with alterations in Ca2+ handling evidenced by impaired myocyte contraction velocity.69
Modulation of prosurvival and -injury kinases with age and CR
Age and CR are both known to modify protective kinase signaling, which may contribute to poor outcomes and refractoriness to protective stimuli in older hearts,26–28,45 and conversely to cardioprotection with CR.36,67,70 Therefore, we assessed relevant signaling components, including Akt, p70S6K, p38-MAPK, and GSK3β. The survival kinase Akt is activated by phosphoinositide 3-kinase (PI3-kinase), regulates downstream targets including eNOS, PKC, p70S6K, and GSK3β, negatively regulates autophagy, and is key to multiple cardioprotective responses.71 Age did not modify Akt expression but increased postischemic Akt phosphorylation, whereas CR specifically augmented Akt levels (Figs. 4 and 5, Table 2). These data are consistent with those of Zhu et al.,49 who reported no effect of age on myocardial Akt phosphorylation, and Sung et al.,70 who reported that protective CR increased activation of Akt in young hearts. Thus, although age-related ischemic intolerance does not involve repressed Akt signaling, cardioprotection via CR may involve enhanced Akt signaling.
Downstream p70S6K is activated by Akt/mammalian target of rapamycin (mTOR) paths and impacts on cell cycle regulation, ribosomal peptide translation, autophagy, and prosurvival signaling (the latter via phosphorylation and sequestration of Bad).72 Although the importance of p70S6K in ischemic tolerance remains to be firmly established, there is support for involvement in protection in response to preconditioning,73 postconditioning,74,75 and opioid and adenosine receptor agonism.76,77 Our data support age-related repression of p70S6K to less than 50% of levels in YA hearts, a change that may well influence ischemic tolerance (Fig. 4). Whereas relative phosphorylation was increased (Fig. 5), partly compensating for the fall in p70S6K expression, these changes will collectively restrict capacity to activate p70S6K signaling (for example via Akt or mTOR), because there is considerably less total and nonphosphorylated p70S6K substrate available for activation in older hearts. Subjecting animals to CR countered the negative influence of age on p70S6K signaling, the only clear point of convergence between age and CR (Figs. 4 and 5). The positive effects of a 14-week period of CR on p70S6K signaling in older hearts contrasts the study of Shinmura et al.,67 who reported that a much longer period of CR can represses baseline mTOR activation and thus downstream p70S6K phosphorylation.
The role of p38-MAPK in cardioprotection is also controversial, with evidence for both beneficial and detrimental functions.44,71 These differences may stem from time- and isoform-dependent actions of p38-MAPK. Although age did not modify p38-MAPK expression, postischemic phosphorylation (activation) was increased (Fig. 3, Table 2). Given evidence that p38-MAPK induces cardiac fibrosis and dysfunction78–80 and mediates detrimental remodeling postischemia,81,82 enhanced postischemic activation may contribute to age-dependent changes in postischemic remodeling. Clinical and experimental data support adverse remodeling in aged myocardium from humans and animal models.18,25 Interestingly, induction of CR modified pre- but not postischemic p38-MAPK activation, and preischemic activation has been shown to limit cell death during subsequent ischemia-reperfusion.83 Therefore, it is possible that pre- versus postischemic p38-MAPK activation contributes to the positive effects of CR versus the negative effects of age on ischemic tolerance.
Modulation of GSK3β, constitutively active within the cytosol and inactivated by phosphorylation, is a critical component of many protective responses.41 While underlying mechanisms remain to be elucidated, active GSK3β promotes mitochondrial permeability transition pore activity, and may modulate functionality of proapoptotic Bcl-2 proteins such as Bax.41,49 Moderate age was associated with a 60%−70% increase in GSK3β expression and a 25% fall in baseline phosphorylation. Thus, MA hearts exhibit much higher active GSK3β, consistent with enhanced cell death and dysfunction. Because p70S6K inactivates GSK3β,42 impaired expression of p70S6K may further promote GSK3β activation in older hearts. On the other hand, postischemic phosphorylation of GSK3β was enhanced in older hearts, supporting later repression of GSK3β activity. Increased postischemic phosphorylation agrees with the findings of Zhu et al.,49 although they also observed increased baseline phosphorylation (without reporting total expression values). Subjecting MA mice to protective CR did not modify GSK3β expression, but reduced postischemic phosphorylation (Fig. 3). These effects are inconsistent with mechanistic involvement in CR-associated cardioprotection. In contrast, Sung et al.70 found that CR increased baseline phosphorylation of GSK3β in YA hearts, supporting a role for phospho-dependent regulation of GSK3β in younger tissue. Thus, the effects of CR may be mediated via distinct mechanisms in older versus young tissue. Age-dependent shifts in mechanisms of injury or protection have been reported,48 and age significantly modifies regulatory cell signaling as shown here (Figs. 3–5) and in prior studies.26–28,45 In this respect, Giani et al.84 showed that a prolonged 12-month period of CR increased Akt phosphorylation in older hearts, contrasting effects of CR in the young,70 and differing from our findings regarding more abbreviated CR in MA hearts (increased Akt expression but not phosphorylation). Thus, both duration of CR and tissue age may be important determinants of molecular adaptations to CR in the heart.
Limitations
There are a number of limitations to the current study worth mentioning. First, although we were interested in potential roles of Beclin1 and Parkin, which are less well studied in myocardium, we did not directly assess autophagy or measure conventional markers such as LC3 or Sqstm1/p62. Changes in these regulatory proteins are consistent with the proposed role of autophagy in aging; for example, further work is warranted to specifically address the impact of age, CR, and ischemia on activity of cardiac autophagy. Furthermore, we here assess changes in autophagy, apoptosis, and signaling proteins at a single time point of reperfusion (45 min), when substantial cellular injury/death is apparent and processes of tissue injury and repair will be relevant to outcome. Nonetheless, temporal changes in cellular injury and repair processes are likely to be important to outcome from ischemic insult, and future work may address patterns of change in regulatory proteins over time.
Finally, heart weights were not recorded owing to the need to freeze tissue rapidly for analysis of protein expression and phosphorylation. Cardiac mass does appear to fall with long-term CR, dependent upon duration and severity of CR. As indicated by the work of Faulks et al., cardiac mass normalized to body weight is relatively invariant with 3-month periods of moderate-to-profound CR.84 Other studies report relatively fixed heart/body weight ratios with CR.35,85 On this basis, we predict as much as a 30%−35% decline in cardiac mass with the current CR regime (based on the fall in body mass). How a fall in cardiac mass impacts on ischemic tolerance is not clear, although a rise in mass with hypertrophy can be detrimental.
Conclusions
In summary, the present data support emergence of cardiac ischemic intolerance by middle age in male C57/Bl6 mice, and show that moderate CR (40% for 14 weeks) counters this effect, restoring ischemic tolerance to levels observed in young hearts. Age-related postischemic dysfunction and cell damage are associated with repressed expression of autophagy regulators (Beclin1, Parkin) and p70S6K, increased expression of active GSK3β, and increased postischemic activation of p38-MAPK. In contrast, cardioprotection with CR is associated with a distinct phenotype, involving reversal of age-related changes in p70S6K, augmented Akt expression, preischemic activation of p38-MAPK, partial inhibition of changes in Beclin1, and exaggeration of Bcl2 levels. Thus, whereas different molecular adaptations may contribute to differential effects of age and CR on ischemic tolerance, transduction of Akt/mTOR signaling via p70S6K may represent a key point of regulatory convergence.
Acknowledgments
The authors gratefully acknowledge grant support from the National Heart Foundation of Australia (G 08B 3971; G 05B 2029) and National Health and Medical Research Council of Australia (NHMRC). J.N.P. was the recipient of CDA fellowship support from the NHMRC, and an ARC Future Fellowship.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Marzetti E. Wohlgemuth SE. Anton SD. Bernabei R. Carter CS. Leeuwenburgh C. Cellular mechanisms of cardioprotection by calorie restriction: State of the science and future perspectives. Clin Geriatr Med. 2009;25:715–732. doi: 10.1016/j.cger.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CK. Allison DB. Brand J. Weindruch R. Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci USA. 2002;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhahbi JM. Tsuchiya T. Kim HJ. Mote PL. Spindler SR. Gene expression and physiologic responses of the heart to the initiation and withdrawal of caloric restriction. J Gerontol A Biol Sci Med Sci. 2006;61:218–231. doi: 10.1093/gerona/61.3.218. [DOI] [PubMed] [Google Scholar]
- 4.Linford NJ. Beyer RP. Gollahon K. Krajcik RA. Malloy VL. Demas V. Burmer GC. Rabinovitch PS. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell. 2007;6:673–688. doi: 10.1111/j.1474-9726.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 5.Tani M. Suganuma Y. Hasegawa H. Shinmura K. Hayashi Y. Guo X. Nakamura Y. Changes in ischemic tolerance and effects of ischemic preconditioning in middle-aged rat hearts. Circulation. 1997;95:2559–2566. doi: 10.1161/01.cir.95.11.2559. [DOI] [PubMed] [Google Scholar]
- 6.Assayag P. CHarlemagne D. Marty I. de Leiris J. Lompré AM. Boucher F. Valére PE. Lortet S. Swynghedauw B. Besse S. Effects of sustained low-flow ischemia on myocardial function and calcium-regulating proteins in adult and senescent rat hearts. Cardiovasc Res. 1998;38:169–180. doi: 10.1016/s0008-6363(97)00283-6. [DOI] [PubMed] [Google Scholar]
- 7.Boucher F. Tanguy S. Besse S. Tresallet N. Favier A. de Leiris J. Age-dependent changes in myocardial susceptibility to zero flow ischemia and reperfusion in isolated perfused rat hearts: Relation to antioxidant status. Mech Ageing Dev. 1998;103:301–316. doi: 10.1016/s0047-6374(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 8.Pepe S. Tsuchiya N. Lakatta EG. Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol Heart Circ Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 9.Frolkis VV. Frolkis RA. Mkhitarian LS. Fraifeld VE. Age-dependent effects of ischemia and reperfusion on cardiac function and Ca2+ transport in myocardium. Gerontology. 1991;37:233–239. doi: 10.1159/000213266. [DOI] [PubMed] [Google Scholar]
- 10.Headrick JP. Aging impairs functional, metabolic and ionic recovery from ischemia-reperfusion and hypoxia-reoxygenation. J Mol Cell Cardiol. 1998;30:1415–1430. doi: 10.1006/jmcc.1998.0710. [DOI] [PubMed] [Google Scholar]
- 11.Mariani J. Ou R. Bailey M. Rowland M. Nagley P. Rosenfeldt F. Pepe S. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg. 2000;120:660–667. doi: 10.1067/mtc.2000.106528. [DOI] [PubMed] [Google Scholar]
- 12.Headrick JP. Willems L. Ashton KJ. Holmgren K. Peart J. Matherne GP. Ischaemic tolerance in aged mouse myocardium: The role of adenosine and effects of A1 adenosine receptor overexpression. J Physiol. 2003;549:823–833. doi: 10.1113/jphysiol.2003.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu P. Xu B. Cavalieri TA. Hock CE. Attenuation of antioxidative capacity enhances reperfusion injury in aged rat myocardium after MI/R. Am J Physiol Heart Circ Physiol. 2004;287:H2719–H2727. doi: 10.1152/ajpheart.00317.2004. [DOI] [PubMed] [Google Scholar]
- 14.Willems L. Zatta A. Holmgren K. Ashton KJ. Headrick JP. Age-related changes in ischemic tolerance in male and female mouse hearts. J Mol Cell Cardiol. 2005;38:245–256. doi: 10.1016/j.yjmcc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Lesnefsky EJ. He D. Moghaddas S. Hoppel CL. Reversal of mitochondrial defects before ischemia protects the aged heart. FASEB J. 2006;20:1543–1545. doi: 10.1096/fj.05-4535fje. [DOI] [PubMed] [Google Scholar]
- 16.McCully JD. Toyoda Y. Wakiyama H. Rousou AJ. Parker RA. Levitsky S. Age- and gender-related differences in ischemia/reperfusion injury and cardioprotection: Effects of diazoxide. Ann Thorac Surg. 2006;82:117–123. doi: 10.1016/j.athoracsur.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oudot A. Martin C. Busseuil D. Vergely C. Demaison L. Rochette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med. 2006;40:2214–2222. doi: 10.1016/j.freeradbiomed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Jugdutt BI. Jelani A. Palaniyappan A. Idikio H. Uweira RE. Menon V. Jugdutt CE. Aging-related early changes in markers of ventricular and matrix remodeling after reperfused ST-segment elevation myocardial infarction in the canine model: Effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation. 2010;122:341–351. doi: 10.1161/CIRCULATIONAHA.110.948190. [DOI] [PubMed] [Google Scholar]
- 19.Fenton RA. Dickson EW. Meyer TE. Dobson JG., Jr Aging reduces the cardioprotective effect of ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 2000;32:1371–1375. doi: 10.1006/jmcc.2000.1189. [DOI] [PubMed] [Google Scholar]
- 20.Boengler K. Konietzka I. Buechert A. Heinen Y. Garcia-Dorado D. Heusch G. Schulz R. Loss of ischemic preconditioning's cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292:H1764–H1769. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- 21.Przyklenk K. Maynard M. Darling CE. Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1393–1398. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 22.Mariani J. Ou R. Bailey M. Rowland M. Nagley P. Rosenfeldt F. Pepe S. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg. 2000;120:660–667. doi: 10.1067/mtc.2000.106528. [DOI] [PubMed] [Google Scholar]
- 23.Lee TM. Su SF. Chou TF. Lee YT. Tsai CH. Loss of preconditioning by attenuated activation of myocardial ATP-sensitive potassium channels in elderly patients undergoing coronary angioplasty. Circulation. 2002;105:334–340. doi: 10.1161/hc0302.102572. [DOI] [PubMed] [Google Scholar]
- 24.Bartling B. Friedrich I. Silber RE. Simm A. Ischemic preconditioning is not cardioprotective in senescent human myocardium. Ann Thorac Surg. 2003;76:105–111. doi: 10.1016/s0003-4975(03)00186-3. [DOI] [PubMed] [Google Scholar]
- 25.Bujak M. Kweon HJ. Chatila K. Li N. Taffet G. Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juhaszova M. Rabuel C. Zorov DB. Lakatta EG. Sollott SJ. Protection in the aged heart: Preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Boengler K. Schulz R. Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 28.Peart JN. Headrick JP. Clinical cardioprotection and the value of conditioning responses. Am J Physiol Heart Circ Physiol. 2009;296:H1705–H1720. doi: 10.1152/ajpheart.00162.2009. [DOI] [PubMed] [Google Scholar]
- 29.Terman A. Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res. 2005;68:355–365. doi: 10.1016/j.cardiores.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb RA. Gustafsson AB. Mitochondrial turnover in the heart. Biochim Biophys Acta. 2011;1813:1295–1301. doi: 10.1016/j.bbamcr.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frier B. Locke M. Preservation of heat stress induced myocardial hsp 72 in aged animals following caloric restriction. Exp Gerontol. 2005;40:615–617. doi: 10.1016/j.exger.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Gredilla R. Sanz A. Lopez-Torres M. Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- 33.Crandall DL. Feirer RP. Griffith DR. Beitz DC. Relative role of caloric restriction and exercise training upon susceptibility to isoproterenol-induced myocardial infarction in male rats. Am J Clin Nutr. 1981;34:841–847. doi: 10.1093/ajcn/34.5.841. [DOI] [PubMed] [Google Scholar]
- 34.Broderick TL. Belke T. Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem. 2002;233:119–125. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- 35.Shinmura K. Tamaki K. Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Shinmura K. Tamaki K. Saito K. Nakano Y. Tobe T. Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 37.Shinmura K. Tamaki K. Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: Possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abete P. Testa G. Galizia G. Mazzella F. Della Morte D. de Santis D. Calabrese C. Cacciatore F. Gargiulo G. Ferrara N. Rengo G. Sica V. Napoli C. Rengo F. Tandem action of exercise training and food restriction completely preserves ischemic preconditioning in the aging heart. Exp Gerontol. 2005;40:43–50. doi: 10.1016/j.exger.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Long P. Nguyen Q. Thurow C. Broderick TL. Caloric restriction restores the cardioprotective effect of preconditioning in the rat heart. Mech Ageing Dev. 2002;123:1411–1413. doi: 10.1016/s0047-6374(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 40.Ahmet I. Tae HJ. de Cabo R. Lakatta EG. Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol. 2011;51:263–271. doi: 10.1016/j.yjmcc.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juhaszova M. Zorov DB. Yaniv Y. Nuss HB. Wang S. Sollott SJ. Role of glycogen synthase kinase-3β in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutherland C. Cohen P. The {alpha}-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70s6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994;338:37–42. doi: 10.1016/0014-5793(94)80112-6. [DOI] [PubMed] [Google Scholar]
- 43.Lochner A. Marais E. Genade S. Huisamen B. du Toit EF. Moolman JA. Protection of the ischaemic heart: Investigations into the phenomenon of ischaemic preconditioning. Cardiovasc J Afr. 2009;20:43–51. [PMC free article] [PubMed] [Google Scholar]
- 44.Rose BA. Force T. Wang Y. Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peart JN. Gross ER. Headrick JP. Gross GJ. Impaired p38-MAPK/HSP27 signaling underlies aging-related failure in opioid-mediated cardioprotection. J Mol Cell Cardiol. 2007;42:972–980. doi: 10.1016/j.yjmcc.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka A. Parkin-mediated selective mitochondrial autophagy, mitophagy: Parkin purges damaged organelles from the vital mitochondrial network. FEBS Lett. 2010;584:1386–1392. doi: 10.1016/j.febslet.2010.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulman D. Latchman DS. Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281:H1630–H1636. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- 48.Przyklenk K. Li G. Simkhovich BZ. Kloner RA. Mechanisms of myocardial ischemic preconditioning are age related: PKC-epsilon does not play a requisite role in old rabbits. J Appl Physiol. 2003;95:2563–2569. doi: 10.1152/japplphysiol.00404.2003. [DOI] [PubMed] [Google Scholar]
- 49.Zhu J. Rebecchi MJ. Tan M. Glass PS. Brink PR. Liu L. Age-associated differences in activation of Akt/GSK-3beta signaling pathways and inhibition of mitochondrial permeability transition pore opening in the rat heart. J Gerontol A Biol Sci Med Sci. 2010;65:611–619. doi: 10.1093/gerona/glq035. [DOI] [PubMed] [Google Scholar]
- 50.Lesnefsky EJ. Gallo DS. Ye J. Whittingham TS. Lust WD. Aging increases ischemia-reperfusion injury in the isolated, buffer-perfused heart. J Lab Clin Med. 1994;124:843–851. [PubMed] [Google Scholar]
- 51.Wohlgemuth SE. Julian D. Akin DE. Fried J. Toscano K. Leeuwenburgh C. Dunn WA., Jr Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 52.Huang C. Andres AM. Ratliff EP. Hernandez G. Lee P. Gottlieb RA. Preconditioning Involves Selective Mitophagy Mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamacher-Brady A. Brady NR. Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 54.Gurusamy N. Lekli I. Gorbunov NV. Gherghiceanu M. Popescu LM. Das DK. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373–387. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang C. Yitzhaki S. Perry CN. Liu W. Giricz Z. Mentzer RM., Jr Gottlieb RA. Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Transl Res. 2010;3:365–373. doi: 10.1007/s12265-010-9189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aki T. Yamaguchi K. Fujimiya T. Mizukami Y. Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line H9c2. Oncogene. 2003;22:8529–8535. doi: 10.1038/sj.onc.1207197. [DOI] [PubMed] [Google Scholar]
- 57.Valentim L. Laurence KM. Townsend PA. Carroll CJ. Soond S. Scarabelli TM. Knight RA. Latchman DS. Stephanou A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 58.Matsui Y. Takagi H. Qu X. Abdellatif M. Sakoda H. Asano T. Levine B. Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 59.Misao J. Hayakawa Y. Ohno M. Kato S. Fujiwara T. Fujiwara H. Expression of bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996;94:1506–1512. doi: 10.1161/01.cir.94.7.1506. [DOI] [PubMed] [Google Scholar]
- 60.Azhar G. Liu L. Zhang X. Wei JY. Influence of age on hypoxia/reoxygenation-induced DNA fragmentation and bcl-2, bcl-xl, bax and fas in the rat heart and brain. Mech Ageing Dev. 1999;112:5–25. doi: 10.1016/s0047-6374(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 61.Liu L. Azhar G. Gao W. Zhang X. Wei JY. Bcl-2 and Bax expression in adult rat hearts after coronary occlusion: age-associated differences. Am J Physiol. 1998;275:R315–R322. doi: 10.1152/ajpregu.1998.275.1.R315. [DOI] [PubMed] [Google Scholar]
- 62.Centurione L. Antonucci A. Miscia S. Grilli A. Rapino M. Grifone G. Di Giacomo V. Di Giulio C. Falconi M. Cataldi A. Age-related death-survival balance in myocardium: An immunohistochemical and biochemical study. Mech Ageing Dev. 2002;123:341–350. doi: 10.1016/s0047-6374(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 63.Huang C. Gu H. Zhang W. Herrmann JL. Wang M. Testosterone-down-regulated Akt pathway during cardiac ischemia/reperfusion: A mechanism involving BAD, Bcl-2 and FOXO3a. J Surg Res. 2010;164:e1–e11. doi: 10.1016/j.jss.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazou A. Iliodromitis EK. Cieslak D. Voskarides K. Mousikos S. Bofilis E. Kremastinos DT. Ischemic but not mechanical preconditioning attenuates ischemia/ reperfusion induced myocardial apoptosis in anaesthetized rabbits: The role of Bcl-2 family proteins and ERK1/2. Apoptosis. 2006;11:2195–2204. doi: 10.1007/s10495-006-0292-5. [DOI] [PubMed] [Google Scholar]
- 65.Katare RG. Kakinuma Y. Arikawa M. Yamasaki F. Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–412. doi: 10.1016/j.yjmcc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 66.Hariharan N. Maejima Y. Nakae J. Paik J. Depinho RA. Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shinmura K. Tamaki K. Sano M. Murata M. Yamakawa H. Ishida H. Fukuda K. Impact of long-term caloric restriction on cardiac senescence: Caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50:117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 68.Janczewski AM. Lakatta EG. Modulation of sarcoplasmic reticulum Ca2+ cycling in systolic and diastolic heart failure associated with aging. Heart Fail Rev. 2010;15:431–445. doi: 10.1007/s10741-010-9167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nair RR. Nair P. Age-dependent variation in contractility of adult cardiac myocytes. Int J Biochem Cell Biol. 2001;33:119–125. doi: 10.1016/s1357-2725(00)00077-7. [DOI] [PubMed] [Google Scholar]
- 70.Sung MM. Soltys CL. Masson G. Boisvenue JJ. Dyck JR. Improved cardiac metabolism and activation of the RISK pathway contributes to improved post-ischemic recovery in calorie restricted mice. J Mol Med. 2011;89:291–302. doi: 10.1007/s00109-010-0703-5. [DOI] [PubMed] [Google Scholar]
- 71.Hausenloy DJ. Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 72.Harada H. Andersen JS. Mann M. Terada N. Korsmeyer p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci USA. 2001;98:9666–9670. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hausenloy DJ. Mocanu MM. Yellon DM. Cross-talk between the survival kinases during early reperfusion: Its contribution to ischemic preconditioning. Cardiovasc Res. 2004;63:305–312. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 74.Tsang A. Hausenloy DJ. Mocanu MM. Yellon DM. Postconditioning: A form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 75.Zhu M. Feng J. Lucchinetti E. Fischer G. Xu L. Pedrazzini T. Schaub MC. Zaugg M. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc Res. 2006;72:152–162. doi: 10.1016/j.cardiores.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 76.Gross ER. Hsu AK. Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 77.Förster K. Paul I. Solenkova N. Staudt A. Cohen MV. Downey JM. Felix SB. Krieg T. NECA at reperfusion limits infarction and inhibits formation of the mitochondrial permeability transition pore by activating p70S6 kinase. Basic Res Cardiol. 2006;101:319–326. doi: 10.1007/s00395-006-0593-4. [DOI] [PubMed] [Google Scholar]
- 78.Liao P. Georgakopoulos D. Kovacs A. Zheng M. Lerner D. Pu H. Saffitz J. Chien KR. Xiao R. Kass D. Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein G. Schaefer A. Hilfiker-Kleiner D. Oppermann D. Shukla P. Quint A. Podewski E. Hilfiker A. Schrode F. Leitges M. Drexler H. Increased collagen deposition and diastolic dysfunction but preserved myocardial hypertrophy after pressure overload in mice lacking PKCɛ. Circ Res. 2005;96:748–755. doi: 10.1161/01.RES.0000161999.86198.1e. [DOI] [PubMed] [Google Scholar]
- 80.Kompa AR. See F. Lewis DA. Adrahtas A. Cantwell DM. Wang BH. Krum H. Long-term but not short-term p38 mitogen-activated protein kinase inhibition improves cardiac function and reduces cardiac remodeling post-myocardial infarction. J Pharmacol Exp Ther. 2008;325:741–750. doi: 10.1124/jpet.107.133546. [DOI] [PubMed] [Google Scholar]
- 81.See F. Thomas W. Way K. Tzanidis A. Kompa A. Lewis D. Itescu S. Krum H. p38 Mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 82.Liu YH. Wang D. Rhaleb NE. Yang XP. Xu J. Sankey SS. Rudolph AE. Carretero OA. Inhibition of p38 mitogen-activated protein kinase protects the heart against cardiac remodeling in mice with heart failure resulting from myocardial infarction. J Cardiac Failure. 2005;11:74–81. doi: 10.1016/j.cardfail.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 83.Moolman JA. Hartley S. Van Wyk J. Marais E. Lochner A. Inhibition of myocardial apoptosis by ischaemic and beta-adrenergic preconditioning is dependent on p38 MAPK. Cardiovasc Drugs Ther. 2006;20:13–25. doi: 10.1007/s10557-006-6257-7. [DOI] [PubMed] [Google Scholar]
- 84.Giani JF. Bonkowski MS. Muñoz MC. Masternak MM. Turyn D. Bartke A. Dominici FP. Insulin signaling cascade in the hearts of long-lived growth hormone receptor knockout mice: Effects of calorie restriction. J Gerontol A Biol Sci Med Sci. 2008;63:788–797. doi: 10.1093/gerona/63.8.788. [DOI] [PubMed] [Google Scholar]
- 85.Faulks SC. Turner N. Else PL. Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. J Gerontol A Biol Sci Med Sci. 2006;61:781–794. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]