Abstract
This study examines the effects of combination therapy of collagen scaffolds and human marrow stromal cells (hMSCs) on the expression of tissue plasminogen activator (tPA) after traumatic brain injury (TBI) in rats. Adult male Wistar rats (n=48) were injured with controlled cortical impact and treated either with scaffolds suffused with hMSCs (3×106) or hMSCs (3×106) alone transplanted into the lesion cavity 1 week after TBI. A control group was treated with saline. Neurological function was assessed using the Morris Water Maze test (MWM) and modified Neurological Severity Scores (mNSS). The rats were sacrificed 14 days after TBI and brain samples were processed for immunohistochemical analysis and quantitative Western blot and quantitative real-time polymerase chain reaction (qRT-PCR) studies. Enhanced functional improvement was observed on both the mNSS and MWM tests in the scaffold+hMSC-treated group compared to the other two groups. Immunostaining with anti-human mitochondrial antibody (E5204) showed more hMSCs in the injury zone of the scaffold+hMSC group compared to the hMSC-alone group. Triple staining showed that more neurons were tPA-positive in the scaffold+hMSC group compared to the other two groups (p<0.05). Western blot analysis and qRT-PCR showed that scaffold+hMSC and hMSC-alone treatment enhanced the expression of tPA compared to controls (p<0.05), but tPA expression was significantly greater in the scaffold+hMSC group. The induction of tPA by hMSCs after TBI may be one of the mechanisms involved in promoting functional improvement after TBI.
Key words: collagen scaffolds, marrow stromal cells, tissue plasminogen activator, traumatic brain injury
Introduction
Human marrow stromal cells (hMSCs) have shown efficacy in improving functional outcome after traumatic brain injury (TBI; Lu et al., 2001; Mahmood et al., 2001a, 2001b, 2002, 2003, 2005, 2006). The beneficial effects of hMSCs have been demonstrated after direct intracerebral administration, as well as by systemic administration (Lu et al., 2001; Mahmood et al., 2001b, 2002, 2003, 2005, 2006). To enhance the functional efficacy of hMSCs, we suffused collagen scaffolds with hMSCs and transplanted them into the lesion core (Lu et al., 2007; Qu et al., 2009; Xiong et al., 2009). Our results have shown that this mode of hMSC delivery significantly enhances the beneficial effects of hMSC therapy (Lu et al., 2007; Qu et al., 2009; Xiong et al., 2009). Our present report is part of mechanistic studies that are being conducted to elucidate the molecular basis of functional benefits observed with hMSC treatment. We are studying the effects of hMSC therapy on the activity of the plasminogen activator system in the central nervous system (CNS) and its relationship to functional improvement after TBI. These effects are observed after hMSC delivery alone or in combination with collagen scaffolds.
There are two molecularly distinct plasminogen activators: tissue plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA; Ellis, 2003). In the CNS, tPA is the major plasminogen activator (Sappino et al., 1993). In addition to being a plasminogen activator, tPA performs many other neurorestorative functions in the CNS (Barnes and Thomas, 2008; Bruno and Cuello, 2006; Nagai and Ureno, 2008; Park et al., 2008). Stein and associates (2009) administered tPA bound to erythrocytes after lateral fluid percussion injury in rats and found reductions in cortical injury and hippocampal loss, indicating that tPA is neuroprotective after TBI. However, the role of endogenous tPA in neural restoration after TBI and its modification by cell therapy has not been investigated. We have demonstrated that hMSC therapy increases tPA activity after stroke, and thereby promotes neurite outgrowth and functional improvement (Xin et al., 2010). In vivo administration of hMSCs to mice after stroke significantly increases activation of tPA in the ischemic boundary zone compared with control mice, and thereby increases myelinated axons and synaptic protein expression. In vitro studies also support the hMSC activation of tPA as contributing to neurite outgrowth. These data prompted us to investigate the effects of hMSCs on tPA under the condition of TBI.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Animal model
A controlled cortical impact model in rats was used in the present study (Dixon et al., 1991; Mahmood et al., 2001a, 2001b). Forty-eight male Wistar rats were anesthetized with an IP injection of chloral hydrate (300–350 mg/kg body weight). Rectal temperature was maintained at 37°C by using a feedback-regulated water-heating pad. A controlled cortical impact device was used to induce injury. The rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies, one in each hemisphere, were performed adjacent to the central suture, midway between the lambda and the bregma. The second craniotomy allowed for lateral movement of cortical tissue. The dura mater was kept intact over the cortex. Injury was induced by impacting the left (ipsilateral) cortex with a pneumatic piston containing a 6-mm-diameter flat tip at a rate of 4 m/sec and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer. Brain injury in this model is characterized by cystic cavity formation in the cortex, which causes asymmetric neurological deficits (Lu et al., 2003a), and selective cell damage in the hippocampal formation, causing spatial memory dysfunction (Lu et al., 2004). Therefore, sensorimotor and spatial memory tests were used to evaluate the functional response to injury and treatment after TBI.
Experimental groups
Adult male Wistar rats (n=48) were injured with controlled cortical impact and divided into three equal groups. The first group (n=16) was treated with scaffolds suffused with hMSCs (3×106) transplanted into the lesion cavity 1 week after TBI. The second group (n=16) was transplanted with hMSCs alone, and a third group (control, n=16) was injected with saline into the lesion cavity. The assignment to each group was done in a random fashion. A modified Morris Water Maze test (MWM) and modified Neurological Severity Scores (mNSS) were measured to evaluate spatial learning and motor-sensory functions. The rats were sacrificed 14 days after TBI. The brain samples from half the animals in each group were processed for immunohistochemical studies. The expression of tPA was studied in the lesion boundary zone with immunohistochemistry. The brain samples from the remaining half of each group were frozen and used for quantitative Western blot and quantitative real-time polymerase chain reaction (qRT-PCR) studies. We used Western blotting and qRT-PCR to evaluate changes in tPA, plasminogen activator inhibitor-1 (PAI-1), and uPA in boundary zone tissue from the three experimental groups at 14 days after TBI. The assignment of animals for immunohistochemical analysis or for Western blot and qRT-PCR studies was done in a random fashion, and the person performing these measurements was blinded as to which group the animals belonged. Six animals, one in saline, two in the scaffold+hMSC group, and three in the hMSC-alone group died early in the experiment. They were excluded from the trial since their deaths occurred early in the study before any functional, histological, or biochemical data could be collected.
Scaffold preparation and cell seeding
Ultrafoam scaffolds, collagen type I, were obtained from commercial sources (Davol, Warwick, RI). Scaffolds were pre-wetted overnight at 4°C (approximately 12 h) in culture medium consisting of DMEM supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, 0.1 mM non-essential amino acids, and 1 ng/mL of basic fibroblast growth factor (bFGF; Life Technologies, Rockville, MD). The scaffolds were then aseptically transferred using tweezers (1 scaffold per tube) to 50-mL sterile centrifuge tubes, allowing the scaffolds to sit at the bottom of the tubes. Following trypsinization of hMSCs from ex vivo expansion conditions, hMSCs were resuspended thoroughly and transferred gently (3×106 hMSCs per scaffold) into 200 μL of culture medium. Culture medium (100 μL) was then applied two times successively to opposite sides of the body of the cylindrical scaffold. The scaffold and cell solution were incubated for 30 min in a humidified incubator to facilitate primary cell seeding. The scaffolds were agitated gently within the solution manually twice every 15 min during this time. Following primary seeding, the centrifuge tubes were filled with an additional 3 mL of culture medium and placed in a humidified incubator overnight until scaffold implantation (Xiong et al., 2009).
Transplantation
The scaffolds were seeded with 3×106 hMSCs and transplanted into the lesion cavity of rats 7 days after TBI. Under aseptic conditions and general anesthesia with chloral hydrate injected IP (350 mg/kg body weight), a 1-cm incision was made along the mid-line of the scalp. The lesion cavity induced by TBI was exposed in the left hemisphere. A scaffold seeded with hMSCs was placed directly into the cavity without removal of additional brain tissue, and subsequently covered by surgical foam (polyurethane foam), and the incision was closed with 4-0 absorbable gut surgical suture.
The group of animals treated with hMSCs alone was injected with hMSCs in solution into the lesion cavity under the same conditions (Xiong et al., 2009). The control group animals were treated with saline. The animals were sacrificed 14 days after TBI.
Sensorimotor functional test
The measurement of sensorimotor function was performed using mNSS (Chen et al., 2001; Lu et al., 2002, 2003b; Sinz et al., 1999). This measure was conducted in all rats before injury and on days 1, 5, 8, and 14 after TBI. The mNSS is a composite of motor (i.e., muscle status and abnormal movement), sensory (i.e., visual, tactile, and proprioceptive), beam balance, and reflex tests.
Spatial learning memory test
Our spatial memory testing procedure was a modification of the MWM, as described previously (Day and Schallert, 1996; Day et al., 1999; Lu et al., 2004; Yamada et al., 1999). Data collection was automated using the HVS Image 2020 Plus Tracking System (US HVS Image, San Diego, CA). The rats were tested on days 10, 11, 12, 13, and 14 after TBI. At the start of a trial, the rat was randomly placed at one of four fixed starting points, randomly facing toward the wall (designated north, south, east, and west), and was allowed to swim for 90 sec or until it found the platform. The platform was located in a randomly-changing position within the northeast quadrant throughout the test period (i.e., sometimes equidistant from the center and the edge of the pool, against the wall, near the center of the pool, or at the edge of the northeast quadrant). If the animal was unable to find the platform within 90 sec, the experiment was terminated and a maximal score of 90 sec was assigned. The percentage of time traveled within the northeast (correct) quadrant was calculated relative to the total amount of time spent swimming before reaching the platform.
Tissue preparation
Fifteen days after TBI, the 24 rats were anesthetized with ketamine and xylazine administered IP and perfused transcardially with saline solution containing heparin. After perfusion with saline, the tissue from boundary zone of these rats was stored in the freezer at −80°C for Western blotting and qRT-PCR. For histological studies the animals (n=24) were perfused with 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Their brains were removed, post-fixed in 10% formalin for 1–2 days at room temperature, and then processed for paraffin sectioning. A series of 6-mm-thick tissue sections were cut using a microtome through each of seven standard blocks. A section from every block was stained with hematoxylin and eosin (H&E) for the calculation of lesion volume.
Immunofluorescence staining
The expression of microtubule-associated protein-2 (MAP-2) and tPA was employed to detect the expression of tPA in neurons after the different treatments. After dehydration, tissue sections were boiled in 1% citric acid buffer (pH 6) in a microwave oven for 10 min, cooled to room temperature, and incubated in 1% saponin for 3 h. Subsequently, the sections were incubated in 1% bovine serum albumin to block the nonspecific signals. Using the same buffer solution, the sections were incubated overnight at 4°C in primary antibody (monoclonal mouse anti-MAP2, dilution 1:400; Chemicon, Temecula, CA), followed by 2 h at room temperature in corresponding fluorochrome-conjugated secondary antibody (anti-mouse fluorescein isothiocyanate; Jackson ImmunoResearch, West Grove, PA). Another primary antibody (rabbit anti-tPA, dilution 1:50, SC-15346; Santa Cruz Biotechnology, Santa Cruz, CA) was used to incubate these slides overnight at 4°C, followed by a secondary antibody (anti-rabbit Cy3; Jackson ImmunoResearch). Each of the aforementioned steps was followed by four 5-min rinses in PBS. The sections were counterstained with 4′,6-diamidino-2-phenyl-indole dihydrochloride for the identification of nuclei. Tissue sections were mounted on slides with ProLong antifade medium (Molecular Probes, Eugene, OR). Sections were observed with the aid of a fluorescence microscope.
Immunoperoxidase staining
To identify the transplanted hMSCs, brain tissue sections, after being deparaffinized and boiled in 1% citric acid buffer (pH 6), were incubated in 1% bovine serum albumin (BSA)/PBS at room temperature for 30 min and subsequently treated with mouse anti-human mitochondrial antibody (Dako Cytomation, Carpinteria, CA) diluted to 1:200 in PBS at 4°C overnight. The primary antibody (rabbit anti-tPA, dilution 1:50 in 1% BSA in PBS, SC-15346; Santa Cruz Biotechnology) was incubated 1 h at room temperature to detect the changes in tPA in the boundary zone of injured animal brain. Following sequential incubation with biotin-conjugated anti-mouse immunoglobulin G (dilution 1:100; Dakopatts, Carpinteria, CA), the sections were treated with an avidin-biotin-peroxidase system (ABC kit; Vector Laboratories, Inc., Burlingame, CA). Diaminobenzidine was then used as a sensitive chromogen for light microscopy. The percentage of positive area for tPA in the boundary zone of injured brain in rats was measured with the help of MetaMorph (Molecular Devices, Downington, PA).
Western blot analysis
Brain tissues from the lesion boundary zone were washed once in 1×PBS and suspended in lysis buffer (20 mM Tris [pH 7.6], 100 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 1% deoxycholic acid, 10% glycerol, 1 mM EDTA, 1 mM NaVO3, 50 mM NaF, and cocktail I of protease inhibitors from Calbiochem, San Diego, CA). After sonication, soluble protein was obtained by centrifugation at 13,000g for 15 min at 4°C. The protein concentration of each sample was determined by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). For immunoblotting, equal amounts of cell lysate were subjected to SDS-polyacrylamide electrophoresis on Novex Tris-Glycine pre-cast gels (Invitrogen, Carlsbad, CA), and separated proteins were then electrotransferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 2% I-Block (Applied Biosystems, Foster City, CA) in PBS plus 0.1% Tween 20 for 1 h at room temperature, and then incubated with different primary antibodies overnight at 4°C. The following antibodies were used: anti-tPA (H-90: sc-15364), anti-uPA (H-140: sc-14019), anti-PAI-1(H-135: sc-8979, 1:1000 dilution), and anti-actin (I-19) (1:2000; Santa Cruz Biotechnology). After washing, the membranes were incubated with HRP-conjugated secondary antibodies (1:2500; Jackson ImmunoResearch Laboratories) in blocking buffer for 2 h at room temperature. Specific proteins were visualized using the SuperSignal West Pico chemiluminescence substrate system (Pierce). The intensity of the bands was measured using Scion image analysis software (Scion Cooperation, Frederick, MD).
Quantitative real-time polymerase chain reaction
Total RNAs were extracted and DNase digested using the RNeasy mini kit (Qiagen, Santa Clarita, CA). Two-step quantitative real-time PCR was performed in this study. The reverse transcription (RT) reaction was carried out using 2 μg of total RNA following the protocol of the TaqMan Reverse Transcription Master Mix kit (Applied Biosystems). A primer optimization step was tested for each set of primers to determine the optimal primer concentrations. Once the optimal primer concentrations were determined, primers, 12.5 μL 2×SYBR Green Master Mix (Applied Biosystems), and 20–100 ng of cDNA sample were applied to a total volume of 25 μL PCR amplification. Reactions were run on an ABI Prism 7000 Sequence Detection System (Applied Biosystems). The cycling conditions comprised 10-min polymerase activation at 95°C, 40 cycles at 95°C for 15 sec, and 60°C for 60 sec. Cycle threshold (Ct) values were obtained from the ABI 7000 software. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was also determined for each RNA sample as control. The fold change of relative mRNA expression was determined using the 2−ΔΔCt method (Livak and Schmittgen, 2001). Total RNA isolated from the tissues of differently treated groups (i.e., hMSCs+scaffold, hMSCs alone, and saline) were used for real-time PCR. The following primers for real-time PCR were designed using primer design software (Invitrogen): GAPDH, sense 5′-GGCATTGCTCTCAATGACAA-3′, antisense 5′-TGTGAGGGAGATGCTCAGTG-3′; tPA, sense 5′-GCAAAATGAAGGGAGAGCTG-3′, antisense 5′-GGGACGTAGCCATGACTGAT-3’; u-PA sense 5′-CACTCTGAAGGTGGCAGTG, antisense 5′-CGGCCTTTGGTGTCAGTATT; and PAI-1 sense 5′-AGGGGCAGCAGATAGACAGA-3′, antisense 5′ CACAGGGAGACCCAGGATAA-3′.
Statistical analysis
All data are presented as the means±standard deviation. Data were analyzed using an analysis of variance (ANOVA) for repeated measures of functional test data (Lu et al., 2003b, 2003c). A paired t-test was used to consider the difference in cell counts, as well as in the ipsilateral hemisphere between the hMSC-scaffold, hMSC alone, and saline groups. All measures were analyzed by observers blinded to the individual treatments.
Results
Neurological and sensorimotor functional responses
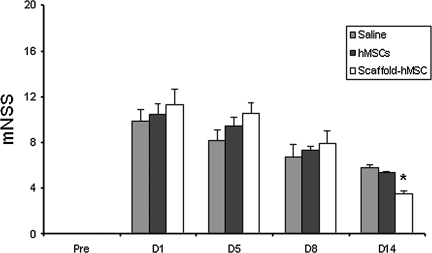
Injury in the left hemispheric cortex of rats caused neurological functional deficits as measured by mNSS. The higher the mNSS score, the worse the sensorimotor function. Figure 1 shows the changes of sensorimotor function in injured rats after the different treatments. Compared to the hMSC-alone and saline groups, the group treated with scaffold-populated hMSCs demonstrated significantly improved scores on day 14 (7 days after transplantation; p<0.0001). There was no significant difference in mNSS scores among the scaffold+hMSC, hMSC alone, and saline groups on days 1, 5, and 8 after TBI.
FIG. 1.
The bar graph shows the sensorimotor function measured with the modified Neurological Severity Score (mNSS) after the different treatments. Treatment was performed on day 7 after traumatic brain injury (TBI), and the scaffold+hMSC group showed functional improvement on day 14 compared with the saline and hMSC-alone groups (*p<0.0001 versus the hMSC-alone and saline-treated groups; hMSC, human marrow stromal cell).
Scaffold+hMSC treatment improves spatial learning
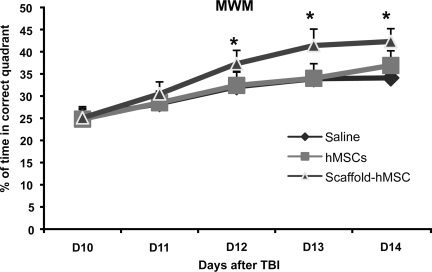
To test whether scaffold+hMSC treatment promotes spatial learning function, the rats were tested on the MWM during the last 5 days (from days 10–12 after TBI) before sacrifice. The mean percentages of time spent in the correct quadrant were significantly higher in the scaffold+hMSC-treated group than in the saline-treated and hMSC-alone groups at days 12 (p=0.016 versus saline, p=0.02 versus hMSCs alone), 13 (p=0.04 versus saline, p=0.004 versus hMSCs alone), and 14 (p=0.036 versus saline, p=0.018 versus hMSC alone) after TBI (Fig. 2). These data demonstrate that scaffold+hMSC treatment promotes spatial learning after TBI.
FIG. 2.
The plot shows the spatial learning function after the different treatments. The scaffold+hMSC-treated group had functional improvement from days 12–14 after traumatic brain injury (TBI), compared to the saline- and hMSC-alone-treated groups (day 12 *p=0.016 versus saline, *p=0.02 versus hMSCs alone; day 13 *p=0.04 versus saline, *p=0.004 versus hMSCs alone; day 14 *p=0.036 versus saline, *p=0.018 versus hMSCs alone; *p=versus hMSCs-alone and saline-treated groups; hMSC, human marrow stromal cell).
Scaffold+hMSC treatment significantly increases tPA expression after TBI in rats
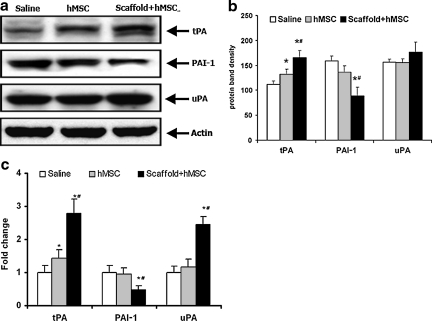
The expression of the plasminogen activators tPA and uPA, as well as PAI-1, were measured in frozen specimens with qRT-PCR and Western blot analysis (Fig. 3). Western blot analysis showed that scaffold+hMSCs and hMSC-alone treatment enhanced the expression of tPA, but expression was significantly greater in the scaffold+hMSC group compared to the hMSC-alone group (Fig. 3B: tPA, p=0.004 for hMSCs versus saline, p=0.0075 for scaffold+hMSC versus hMSCs, p<0.0001 for scaffold+hMSCs versus saline). qRT-PCR showed similar results (Fig. 3C: tPA, p=0.0011 for hMSCs versus saline, p<0.0001 for scaffold+hMSCs versus saline, p<0.0001 for scaffold+hMSCs versus hMSCs alone). The scaffold+hMSC group suppressed the expression of PAI-1, whereas the hMSC-alone group had no significant effect on PAI-1 expression as shown by Western blot analysis (Fig. 3B: PAI-1, p=0.0003 for scaffold+hMSCs versus saline, p<0.0001 for scaffold+hMSCs versus hMSCs), as well as qRT-PCR studies (Fig. 3C: PAI-1, p=0.003 for scaffold+hMSCs versus saline, p<0.0001 for scaffold+hMSCs vs hMSCs). There was no significant difference in the expression of uPA in all three groups with Western blot analysis, whereas qRT-PCR studies showed a significant increase in the expression of uPA in the scaffold+hMSC group (Fig. 3C: uPA, p<0.0001 for scaffold+hMSCs versus saline, p<0.0001 for scaffold+hMSCs versus hMSCs alone).
FIG. 3.
Western blot analysis (a and b) and quantitative real-time polymerase chain reaction (qRT-PCR) studies (c) show the expression of tPA, PAI-1, and uPA in the scaffold+hMSCs, hMSCs-alone, and saline-treated groups. Western blot analysis showed that scaffold+hMSC and hMSC-alone treatment significantly enhanced the expression of tPA, though the scaffold+hMSC group showed greater enhancement than the hMSC-alone group. There was no difference in the expression of uPA. The expression of PAI-1 was also significantly decreased by scaffold+hMSC treatment, but not by hMSC-alone treatment (b: tPA *p=0.004 for hMSCs alone versus saline, #p=0.0075 for scaffold+hMSCs versus hMSCs alone, *p<0.0001 for scaffold+hMSCs versus saline; PAI-1 *p=0.0003 for scaffold+hMSCs versus saline, #p<0.0001 for scaffold+hMSCs versus hMSCs alone). (c) qRT-PCR studies also showed that scaffold+hMSC and hMSC-alone treatment increased the expression of tPA, with the scaffold+hMSC group showing significantly stronger expression than the hMSC-alone group. The expression of uPA and PAI-1 was significantly affected only by the scaffold+hMSC group (c: uPA *p<0.0001 for scaffold+hMSCs versus saline, #p<0.0001 for scaffold+hMSCs versus hMSCs alone; tPA *p=0.0011 for hMSCs alone versus saline, *p<0.0001 for scaffold+hMSCs versus saline, #p<0.0001 for scaffold+hMSCs versus hMSCs alone; PAI-1 *p=0.0003 for scaffold+hMSCs versus saline, #p<0.0001 for scaffold+hMSCs versus hMSCs alone, (*p= versus saline and #p= versus hMSCs alone; hMSC, human marrow stromal cell; tPA, tissue plasminogen activator; uPA, urokinase-type plasminogen activator; PAI-1, plasminogen activator inhibitor-1).
Scaffold+hMSC treatment promotes the presence of hMSCs in the injured brain
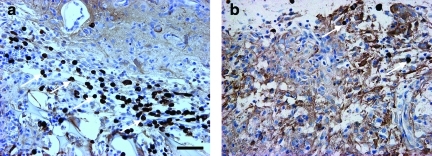
Immunostaining with anti-human mitochondrial antibody (E5204) showed many hMSCs in the boundary zone of the scaffold+hMSCs group. However, very few hMSCs were visible in the hMSC-alone group (Fig. 4).
FIG. 4.
Immunostaining shows human marrow stromal cells (hMSCs, brown) in the boundary zone. (a) The image shows many hMSCs in the scaffold+hMSC treatment group (arrows). (b) There are very few positive hMSCs visible in the hMSC-alone treatment group (scale bar in a=50 μm; hMSC, human marrow stromal cell). Color image is available online at www.liebertpub.com/neu
Scaffold+hMSC treatment increases tPA expression in the injured brain
The percentage of area with positive tPA staining, including positive cells and intercellular spaces, was measured with immunostaining. The area of positive tPA was significantly larger in the scaffold+hMSC group than in the hMSC-alone and saline-treated groups, though the hMSC-alone treated group had a larger area of tPA than that of the saline-treated group (Fig. 5). The expression of tPA in neurons was detected with triple staining, including MAP-2 and 4′,6-diamidino-2-phenyl-indole dihydrochloride. Triple staining showed that more neurons were tPA-positive in the scaffold+hMSC-treated group compared to the other two groups, and the hMSC-alone treated group had more tPA-positive neurons than the saline-treated group (Fig. 6).
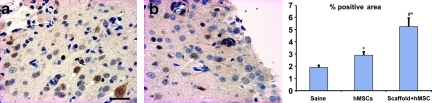
FIG. 5.
Micrograph shows the expression of tissue plasminogen activator (tPA) in the scaffold+hMSCs (a) and hMSC-alone (b) groups in the boundary zone. Bar graph shows that the percentage of tPA-positive area is significantly increased in the scaffold+hMSC group, compared to the saline (*p<0.0001) and hMSC-alone (#p=0.0016) groups. The hMSC-alone group has a larger positive area than the saline (*p=0.0015) group (*p=versus saline, #p= versus hMSCs alone; n=8; scale bar in a=25 μm; hMSC, human marrow stromal cell). Color image is available online at www.liebertpub.com/neu
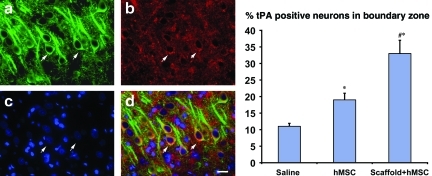
FIG. 6.
Triple immunostaining shows the neurons in the injured area. (a) MAP-2 (green). (b) tPA (red). (c) Nuclei (blue). (d) Merged image shows the tPA-positive neurons in yellow (scale bar in d=25 μm). The bar graph shows that scaffold+hMSC treatment increases the number of tPA-positive neurons compared to the hMSC-alone (#p=0.002) and saline groups (*p<0.001), whereas the positive neurons in the hMSC-alone group were also significantly more than in the saline group (*p=0.00015; *p=versus saline; #p=versus hMSCs alone; n=8; hMSC, human marrow stromal cell; MAP-2, microtubule-associated protein-2; tPA, tissue plasminogen activator). Color image is available online at www.liebertpub.com/neu
Discussion
Our data show that (1) scaffold+hMSC and hMSC-alone treatment enhanced the expression of tPA, but tPA activity in the scaffold+hMSCs group was significantly greater compared to the hMSC-alone group; (2) the scaffold+hMSCs group also suppressed the expression of PAI-1, whereas the hMSC-alone group had no significant effect on PAI-1 expression; and (3) significant functional improvement was seen with scaffold+hMSCs treatment of TBI.
This study shows that implanting hMSCs with scaffolds is more effective in inducing tPA activity than implanting hMSCs alone. Whether this is secondary to scaffolds increasing the survival and concentration of donor hMSCs at the injury site, or if scaffolds also modify the genetic structure of hMSCs is unknown. In our previous study, hMSC-alone treatment of TBI induced significant functional improvement (Lu et al., 2007), although these functional benefits were inferior to scaffold+hMSC treatment. However, in that study, which was of 35 days duration, the functional benefits of hMSC-alone treatment were not visible until 10 days after treatment, whereas in the present study it only lasted until 7 days after treatment, and the lack of efficacy of hMSC-alone treatment may be because of the short duration of the present study. The present study was designed as a short-term trial, since its primary focus was to elucidate the effects of hMSC treatment on tPA expression, and cerebral ischemia studies have shown that changes in tPA after neural injury occur acutely or subacutely (Xin et al., 2010). We realize that extended trials to evaluate long-term changes in tPA after TBI are still necessary, and they will be conducted in the near future.
Endogenous tPA is synthesized and released in the CNS by neurons and endothelial and glial cells (Nagai and Ureno, 2008; Park et al., 2008). The expression of the tPA gene is enhanced by several physiological and pathological stimuli, such as neuronal depolarization (Sappino et al., 1993), and brain or peripheral nerve injury (Rogove and Tsirka, 1998; Siconolfi and Seeds, 2001). Inhibition of tPA activity occurs through PAIs and neuroserpin, of which the most important in the CNS is PAI-1, produced primarily by reactive astrocytes (Li et al., 2008; Teesalu et al., 2003). Interaction between tPA and its inhibitors regulate the final production of plasmin (Li et al., 2008; Teesalu et al., 2003). Plasmin induces proteolysis of proteins such as laminin and neural cell adhesion molecules (Nagai and Ureno, 2008), but most importantly, it plays an important role in the activation of neurotrophic factors such as BDNF (Barnes and Thomas, 2008) and NGF (Bruno and Cuello, 2006). Plasmin cleaves pro-BDNF and pro-NGF into active forms (Barnes and Thomas, 2008; Bruno and Cuello, 2006). The physiological relevance of this catalytic reaction is demonstrated by the fact that effective cleavage and maturation of pro-BDNF was observed in hippocampal lysates of wild-type mice, but not in those from tPA−/− or Plg−/− mice (Pang et al., 2004). BDNF and NGF growth factors have been shown to be induced by MSCs (Lu et al., 2003a, 2003b; Mahmood et al., 2004, 2005), and they play an important role in neuroprotection and neurorestoration after neural injury. It seems probable that MSC induction of BDNF and NGF is to a large extent mediated through the tPA pathway. In a closely related study we have shown that scaffold+hMSC treatment increases the expression of vascular endothelial growth factor (VEGF; Qu et al, 2011). However, further studies are necessary to clearly define the role of tPA in the induction of neurotrophic factors.
tPA also possesses many other neuroprotective functions that are independent of plasminogen activation. It plays a key role in synaptic plasticity. The tPA gene is induced early after neuronal stimulation associated with seizure, kindling, and long-term potentiation (LTP) (Qian et al, 1993). LTP is closely associated with memory, and an increase in tPA gene expression in neurons is directly related to the acceleration of LTP and synaptic growth (Madani et al., 1999). tPA also plays a role in synaptic remodeling, which includes clearance of established synapses, leading to new synapse generation (Mataga et al., 2002, 2004). This synaptic remodeling is an important part of synaptic plasticity. Part of the effects of tPA on synaptic plasticity are mediated by modification of the N-methyl-d-aspartate (NMDA) receptor (Nagai and Ureno, 2008). NMDA is a calcium channel-coupled glutamate receptor that is involved in synaptic plasticity, learning, and excitotoxicity (Nagai and Ureno, 2008), and tPA has been shown to increase NMDA receptor gene expression (Pawlak et al., 2005). tPA is also involved in motor learning in the cerebellum (Seeds et al., 1995). Seeds and associates (1995) showed that tPA mRNA and protein were induced in granular/Purkinje neurons in the cerebellum during the learning of complex motor skills. In addition to a neuroprotective role in the CNS, tPA has demonstrated similar neuroprotective properties in the peripheral nervous system. tPA is upregulated in dorsal root ganglion after sciatic nerve crush, and exogenously-administered tPA enhances nerve regeneration and functional recovery after sciatic nerve crush (Siconolfi and Seeds, 2001; Zou et al., 2006). tPA plays an important role in neuronal migration and neurite outgrowth (Moonen et al., 1995). In the developing cerebellum tPA is strongly expressed in granule neurons during migration (Ware et al., 1995), and their neuronal migration is decreased in tPA−/− mice (Seeds et al., 1999). Also, hippocampal neurite outgrowth associated with seizure formation is suppressed in tPA−/− mice (Wu et al., 2000).
In summary, treatment with scaffold+hMSCs is superior to treatment with hMSCs alone in inducing expression of tPA in the injured brain after TBI, and this may be partially responsible for the enhanced functional recovery seen with scaffold+hMSC treatment.
Author Disclosure Statement
No competing financial interests exist.
References
- Barnes P. Thomas K. Proteolysis of pro BDNF is a key regulator in the formation of memory. PLOS. 2008;3:1–10. doi: 10.1371/journal.pone.0003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M.A. Cuello C. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor and its degradation by a protease cascade. PNAS. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Li Y. Wang L. Zhang Z. Lu D. Lu M. Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Day L.B. Schallert T. Anticholinergic effects on acquisition of place learning in the Morris water task: spatial mapping deficit or inability to inhibit nonplace strategies? Behav. Neurosci. 1996;110:998–1005. doi: 10.1037//0735-7044.110.5.998. [DOI] [PubMed] [Google Scholar]
- Day L.B. Weisand M. Sutherland R.J. Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behav. Neurosci. 1999;113:914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Ellis V. Plasminogen activator: structure and function. In: Waisman D.M., editor. Plasminogen: Structure, Activation and Regulation. Kluwer Academic/Plenum Publishers; New York: 2003. pp. 19–45. [Google Scholar]
- Li L. Lundkvist A. Andersson D. Whilhelmsson U. Nagai N. Pardo A.C. Nodin C. Stahlberg A. Aprico K. Larsson K. Yabe T. Moons L. Fotheringham A. Davies I. Carmeliet P. Schwartz J.P. Pekna M. Kubista M. Blamstrand F. Maragakis N. Nilsson M. Pekny J. Protective role of reactive astrocytes in brain ischemia. J. Cereb. Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu D. Goussev A. Chen J. Pannu P. Li Y. Mahmood A. Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J. Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- Lu D. Li Y. Mahmood A. Wang L. Rafiq T. Chopp M. Neural and marrow-derived stromal cell sphere transplantation in a rat model of traumatic brain injury. J. Neurosurg. 2002;97:935–940. doi: 10.3171/jns.2002.97.4.0935. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Chopp M. Biological transplantation and neurotrophic induced neuroplasticity after traumatic brain injury. J. Head Trauma Rehabil. 2003a;18:357–376. doi: 10.1097/00001199-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Li Y. Chopp M. Adult bone marrow stromal cell administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Qu C. Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61:596–602. doi: 10.1227/01.NEU.0000290908.38438.B2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Zhang R. Chopp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J. Neurosurg. 2003b;99:351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- Lu M. Chen J. Lu D. Yi L. Mahmood A. Chopp M. Global test statistics for treatment effect of stroke and traumatic brain injury in rats with administration of bone marrow stromal cells. J. Neurosci. Methods. 2003c;128:183–190. doi: 10.1016/s0165-0270(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Madani R. Hulo S. Toni N. Madani H. Steimer T. Muller D. Vassalli J.D. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma. 2004;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Chopp M. Treatment of traumatic brain injury in adult rats with human bone marrow stromal cells. Neurosurgery. 2003;53:697–700. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Li Y. Chopp M. Intracerebral transplantation of marrow stromal cells (MSCs) improves outcome after traumatic brain injury. J. Neurotrauma. 2002;19:1609–1617. doi: 10.1089/089771502762300265. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Qu C. Goussev A. Chopp M. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57:1026–1031. doi: 10.1227/01.neu.0000181369.76323.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Qu C. Goussev A. Chopp M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J. Neurosurg. 2006;104:272–277. doi: 10.3171/jns.2006.104.2.272. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Wang L. Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001a;49:1196–1204. [PubMed] [Google Scholar]
- Mahmood A. Lu D. Yi L. Chen J.L. Chopp M. Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. J. Neurosurg. 2001b;94:589–595. doi: 10.3171/jns.2001.94.4.0589. [DOI] [PubMed] [Google Scholar]
- Mataga N. Mizuguchi Y. Hensch T.K. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Mataga N. Nagai N. Hensch T.K. Permissive proteolytic activity for visual cortical plasticity. Proc. Natl. Acad. Sci. USA. 2002;99:7717–7721. doi: 10.1073/pnas.102088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen G. Grau-Wagemans M.P. Selak I. Plasminogen activator-plasmin system and neuronal migration. Nature. 1995;298:753–755. doi: 10.1038/298753a0. [DOI] [PubMed] [Google Scholar]
- Nagai N. Ureno T. Role of tPA in the neural system. In: Tanaka K., editor. Recent Advances in Thrombosis and Hemostasis. Springer; Japan: 2008. pp. 314–326. [Google Scholar]
- Pang P.T. Teng H.K. Zaitsev E. Woo N.T. Sakata K. Zhen S. Teng K.K. Yung W.H. Hempstead B.L. Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Park L. Gallo E. Anrather J. Wang G. Norris E. Paul J. Strickland S. Iadecola C. Key role of tissue plasminogen activator in neurovascular coupling. PNAS. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak R. Melchor J.P. Matys T. Skrzypiec A.E. Strickland S. Ethanol-withdrawal seizures are controlled by tissue plasminogen activator via modulation of NR2B-containing NMDA receptors. Proc. Natl. Acad. Sci. USA. 2005;102:443–448. doi: 10.1073/pnas.0406454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z. Gilbert M.E. Colicos M.A. Kandel E.R. Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Qu C. Mahmood A. Kaplan D. Goussev A. Xiong Y. Ning R. Chopp M. Treatment of traumatic brain injury in mice with bone marrow stromal cell-impregnated collagen scaffolds. J. Neurosurg. 2009;111:658–665. doi: 10.3171/2009.4.JNS081681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C. Mahmood A. Liu X.S. Xiong Y. Wang L. Wu H. Li B. Zhang Z.G. Kaplan D.L. Chopp M. The treatment of TBI with human marrow stromal cells impregnated into collagen scaffold. Functional outcome and gene expression profile. Brain Res. 2011;1371:129–139. doi: 10.1016/j.brainres.2010.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogove A.D. Tsirka S.E. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Curr. Biol. 1998;8:19–25. doi: 10.1016/s0960-9822(98)70016-8. [DOI] [PubMed] [Google Scholar]
- Sappino A.P. Madani R. Huarte J. Belin D. Kiss J.Z. Wohlwend A. Vassalli J.D. Extracellular proteolysis in the adult murine brain. J. Clin. Invest. 1993;92:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N.W. Basham M.E. Haffke S.P. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc. Natl. Acad. Sci. USA. 1999;96:14118–14123. doi: 10.1073/pnas.96.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N.W. Williams B.L. Bickford P.C. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- Siconolfi L.B. Seeds N.W. Induction of the plasminogen activator system accompanies peripheral nerve regeneration after sciatic nerve crush. J. Neurosci. 2001;21:4336–4347. doi: 10.1523/JNEUROSCI.21-12-04336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinz E.H. Kochanek P.M. Dixon C.E. Clark R.S. Carcillo J.A. Schiding J.K. Chen M. Wisniewski S.R. Carlos T.M. Williams D. Dekosky S.T. Watkins S.C. Marion D.W. Billiar T.R. Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J. Clin. Invest. 1999;104:647–656. doi: 10.1172/JCI6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S.C. Ganguly K. Belfield C.M. Xu X. Swanson E.W. Chen X.H. Browne K.D. Johnson V.E. Smith D.H. LeBold D.G. Cines D.B. Muzykantov V.R. Erythrocyte bound tissue plasminogen activator (tPA) is neuroprotective in experimental traumatic brain injury. J. Neurotrauma. 2009;26:1585–1592. doi: 10.1089/neu.2008.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teesalu T. Kulla A. Asser T. Simisker A. Vaheri A. Plasminogen activators in CNS physiology and disease. In: Waisman D.M., editor. Plasminogen: Structure, Activation and Regulation. Kluwer Academic/Plenum Publishers; New York: 2003. pp. 251–267. [Google Scholar]
- Ware J.H. DiBenedetto A.J. Pittman R.N. Localization of tissue plasminogen activator mRNA in the developing rat cerebellum and effects of inhibiting tissue plasminogen activator on granule cell migration. J. Neurobiol. 1995;28:9–22. doi: 10.1002/neu.480280103. [DOI] [PubMed] [Google Scholar]
- Wu Y.P. Siao C.J. Lu W. Sung T.C. Frohman M.A. Milev P. Bugge T.H. Degen J.L. Levine J.M. Margolis R.U. The tissue plasminogen activator (tPA)/plasmin extracellular proteolytic system regulates seizure induced hippocampal mossy fiber outgrowth through a proteoglycan substrate. J. Cell. Biol. 2000;148:1295–1304. doi: 10.1083/jcb.148.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H. Li Y. Shen H. Wang X. Liu X. Zhang J. Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in mouse. PLoS One. 2010;3:e9027. doi: 10.1371/journal.pone.0009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y. Qu C. Mahmood A. Liu Z. Ning R. Li Y. Kaplan D.L. Schallert T. Chopp M. Delayed transplantation of human marrow stromal cell-seeded scaffolds increases transcallosal neural fiber length, angiogenesis, and hippocampal neuronal survival and improves functional outcome after traumatic brain injury in rats. Brain Res. 2009;1263:183–191. doi: 10.1016/j.brainres.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. Tanaka T. Mamiya T. Shiotani T. Kameyama T. Nabeshima T. Improvement by nefiracetam of beta-amyloid-(1-42)-induced learning and memory impairments in rats. Br. J. Pharmacol. 1999;126:235–244. doi: 10.1038/sj.bjp.0702309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou T. Ling C. Xiao Y. Tao X. Ma D. Chen Z.L. Strickland S. Song H. Exogenous tissue plasminogen activator enhances peripheral nerve regeneration and functional recovery after injury in mice. J. Neuropathol. Exp. Neurol. 2006;65:78–86. doi: 10.1097/01.jnen.0000195942.25163.f5. [DOI] [PubMed] [Google Scholar]