Abstract
Background
Previous research demonstrated the efficacy of sustained release bupropion (bupropion SR) for smoking cessation in whites as well as moderate to heavy (≥10 cigarettes per day [CPD]) African American smokers. We evaluated whether bupropion SR was effective for smoking cessation among African American light smokers (≤10 CPD).
Methods
A randomized, double-blind placebo-controlled trial was conducted from December 27, 2007, to May 13, 2010. All participants were African American light smokers (≤10 CPD), aged 18 years or older. Participants were randomly assigned to receive 300 mg bupropion SR (150 mg once daily for 3 days and then 150 mg twice daily) (n = 270 participants) or placebo (n = 270 participants) for 7 weeks, and up to six sessions of health education counseling. Serum cotinine was measured at baseline (week 0). The primary outcome was salivary cotinine–verified 7-day point prevalence smoking abstinence at week 26; a cut point of 15 ng/mL differentiated smokers from nonsmokers. Salivary cotinine–verified smoking abstinence at end of medication treatment at week 7 was also examined. Odds ratios (OR) for smoking abstinence and 95% confidence intervals (CIs) were calculated using logistic regression models. All statistical tests were two-sided.
Results
Participants at baseline visit (week 0) smoked an average of 8.0 CPD and had a mean serum cotinine level of 275.8 ng/mL (SD = 155.8 ng/mL); most used menthol cigarettes (83.7%) and smoked within 30 minutes of waking (72.2%). After imputing those lost to follow-up as smokers, no statistically significant difference in long-term smoking abstinence rates at week 26 was observed between bupropion SR and placebo groups (13.3% vs 10.0%, OR = 1.39, 95% CI = 0.82 to 2.35, P = .23). Cotinine-verified smoking abstinence rate at end of medication week 7 was higher in the bupropion SR vs placebo group (23.7% vs 9.6%, OR = 2.92, 95% CI = 1.78 to 4.77, P < .001).
Conclusions
Bupropion SR was effective in promoting smoking cessation during the medication phase of treatment but showed no effect on long-term smoking cessation among African American light smokers. More research is needed to identify strategies for sustaining abstinence among African American light smokers.
CONTEXT AND CAVEATS
Prior knowledge
In a previously conducted randomized trial, sustained release bupropion (bupropion SR) was effective in promoting smoking abstinence in African American heavy smokers (smoked ≥10 cigarettes per day).
Study design
A randomized, double-blind placebo-controlled trial was conducted to evaluate the efficacy of bupropion SR in smoking cessation treatment of African American light smokers (smoked 1–10 cigarettes per day). Participants received 300 mg bupropion SR or placebo for 7 weeks and up to six sessions of health education counseling were provided to all. Salivary cotinine levels were measured to verify abstinence.
Contribution
The initial abstinence rate at week 7 was statistically significantly higher in the bupropion SR group compared with the placebo group (23.7% vs 9.6%), but long-term abstinence rates were not statistically significantly different in the bupropion SR and placebo groups (13.3% vs 10.0%).
Implication
During the medication phase of treatment, bupropion SR was effective in promoting smoking cessation among African American light smokers but showed no effect on long-term abstinence.
Limitation
Generalizability of the results may be limited given the inclusion of only African American light smokers.
From the Editors
Tobacco use remains the leading preventable cause of morbidity and mortality for all racial and ethnic groups in the United States (1). Indeed, smoking is responsible for greater than 30% of all cancer deaths and is a major contributor to heart, lung, and cerebrovascular disease (1). More than 50% of all African American smokers use 10 or fewer cigarettes per day (CPD) and thereby can be described as light smokers (2,3). Although African Americans smoke fewer CPD than the general population, they experience disproportionately greater tobacco-related disease burden, including the highest rates of cancer incidence and mortality (1,4). Unfortunately, African Americans are less likely to receive tobacco use treatment or to achieve abstinence when making a quit attempt, making treatment of this group a public health priority (5,6).
African Americans have been underrepresented in smoking cessation research (7–9), and only two studies have evaluated tobacco use treatment for African American light smokers (9,10). The 2008 Clinical Practice Guidelines for Treating Tobacco Use and Dependence recommend counseling and pharmacotherapy for the treatment of smokers, and call for treatment research with ethnic minority smokers and light smokers, because of limited research with these groups (11). Because most smoking cessation trials have been conducted with predominantly white (of European descent) moderate to heavy smokers, assumptions cannot be made about the benefit of pharmacotherapy for racial and ethnic minority smokers or for light smokers. In addition to smoking fewer CPD, African American smokers are more likely to smoke high-tar and mentholated cigarettes (12,13), to have slower rates of nicotine metabolism (14), and to show higher levels of cotinine (the primary metabolite of nicotine) per cigarette smoked (14–17). Such differences may influence treatment response.
This study builds upon a series of Kick It at Swope (KIS) studies focused on enhancing treatment for tobacco use in African American moderate to heavy smokers (KIS-I study) (8) and African American light smokers (KIS-II study) (9). As part of the KIS-II study, Ahluwalia et al. (9) evaluated the efficacy of nicotine gum vs placebo combined with either health education (HE) counseling or motivational interviewing. The findings demonstrated the efficacy of HE counseling in doubling smoking abstinence relative to motivational interviewing but found no measurable benefit of nicotine gum (9). The sample of light smokers demonstrated variations in daily smoking patterns and a wide range of baseline cotinine levels, suggesting that dosing using nicotine replacement therapy might be challenging. Because sustained release bupropion (bupropion SR) is a non-nicotine medication shown to be effective in producing abstinence in African American moderate to heavy smokers (≥10 CPD) (8), consideration of bupropion for light smokers was warranted. Bupropion is an effective first-line medication for tobacco use treatment, shown to approximately double the abstinence rates at 6 months compared with placebo (8,11,18). Based on previous research, bupropion treatment was expected to aid withdrawal reduction among individuals using the medication to assist quitting (19). The goal of the current Kick It at Swope III (KIS-III) study was to evaluate the efficacy of bupropion in combination with HE counseling for smoking cessation in African American light smokers.
Methods
Study Design
This KIS-III study was the third in a series of clinical trials of African American smokers conducted at an urban community-based clinic in Kansas City (MO) that serves predominantly low-income African American patients. KIS-III was a randomized, double-blind placebo-controlled study with the primary aim of evaluating the efficacy of bupropion SR in combination with HE counseling for smoking cessation among African American light smokers. A total of 540 participants, recruited from December 27, 2007, to October 27, 2009, were randomly assigned to receive a 7-week supply of active bupropion SR or placebo. All participants were scheduled to receive six sessions of HE counseling. The primary outcome was cotinine-confirmed 7-day point prevalence smoking abstinence at 6 months. A community advisory board assisted in the implementation of the study. Participants provided written informed consent, and the study procedures were approved and monitored by the University of Kansas Medical Center Human Subjects Committee. Study design, methodology, treatment intervention, and recruitment are described in further detail elsewhere (20).
Participants, Screening, and Randomization
Eligible individuals self-identified as African American, men and women aged 18 years or older, interested in quitting smoking, smoked 10 or fewer CPD for 2 or less years, smoked on 25 or more days in the past month, smoked for at least 3 years, had a home address and functioning telephone number, were willing to attend scheduled study visits, and to provide biological samples for genetic analyses related to nicotine and bupropion metabolism. Exclusion criteria included current use of bupropion; use of psychoactive medications, nicotine replacement therapy, fluoxetine, clonidine, buspirone, or doxepin in the past 30 days; history of alcohol or substance abuse within the past year; current drinking of 14 or more alcoholic drinks per week and/or drinking five or more drinks on one occasion two or more times in the past month; history of seizures or head trauma; history of bulimia or anorexia nervosa; current pregnancy (verified by over-the-counter pregnancy test kit) or contemplating pregnancy; breast feeding; myocardial infarction in the past 30 days; use of other forms of tobacco in the past 30 days; reported use of opiates, cocaine, or stimulants; or diabetes treated with oral hypoglycemics or insulin; intention to move from the Kansas City region in the next 12 months; or presence of another study participant in the household.
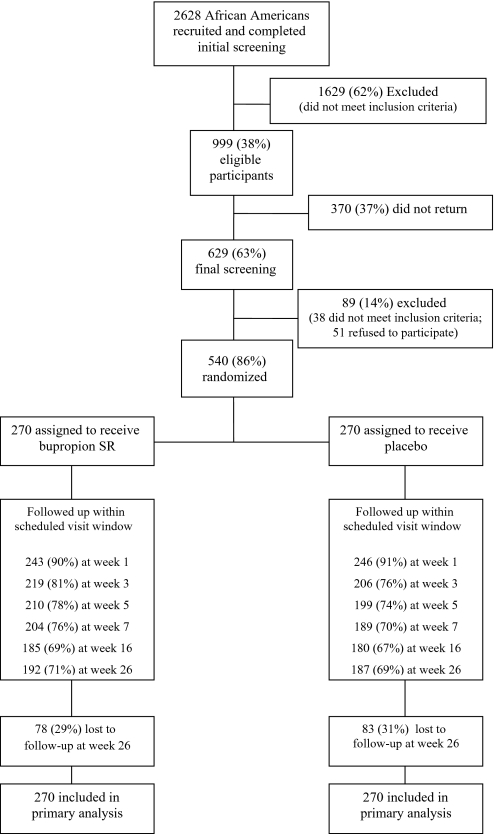
Smokers were recruited through clinic- and community-based efforts, described in detail elsewhere (20). For eligible individuals, study staff reviewed procedures and administered written informed consent at the baseline visit (week 0). A computer-generated table of random numbers was used to randomly assign 540 eligible participants into the bupropion SR (n = 270 participants) or placebo (n = 270 participants) groups between December 27, 2007, and October 27, 2009, with final 6-month follow-up completed in May 13, 2010. Study staff and participants were blinded to treatment condition. Figure 1 presents the number of individuals who completed screening, number enrolled, and participant completion of study sessions.
Figure 1.
CONSORT diagram of Kick It at Swope III (KIS-III) screening, enrollment, and retention through 6-month follow-up. Participants were enrolled between December 27, 2007, and October 27, 2009, and randomly assigned to receive a 7-week supply of active bupropion SR or placebo. Follow-up was completed on May 13, 2010.
Intervention
Participants received 7 weeks of pharmacotherapy (bupropion SR or placebo). The intervention included HE counseling through week 16 and follow-up through week 26 for all participants.
Pharmacotherapy: Bupropion SR or Placebo.
At baseline (week 0), study staff gave each participant a 7-week supply of bupropion SR (150 mg daily for 3 days and then 150 mg twice daily for the remaining 46 days) or placebo. All participants received an instruction sheet on effective use of bupropion. A quit date was scheduled to occur at week 1, after 7 days of pharmacotherapy use.
Health Education Counseling.
All participants received Kick It at Swope: Stop Smoking Guide, a 36-page culturally sensitive smoking cessation guide previously developed for African American light smokers (9). Trained counseling staff offered six sessions of HE counseling to all participants in person (weeks 0, 1, 3, 7) and via telephone (weeks 5, 16) (20,21). Counseling sessions lasted approximately 15–20 minutes. Study staff used semi-structured scripts to incorporate use of the guide within counseling sessions. Counseling information was tailored to the individual through development of personalized stop smoking plans.
Retention
Participants received telephone calls and postcard reminders before every counseling visit. For missed sessions, participants received up to six telephone calls to facilitate rescheduling. Participants were given a $20 gift card at weeks 0, 3, and 7 and $40 for follow-up at week 26 in appreciation of their time. Participants also received small tokens (eg, tote bags, t-shirts, and museum passes) for completing sessions throughout the study.
Measures
Study staff verbally administered all self-report measures. Demographic information included age, sex, marital status, income, employment status, and education. Height and weight were measured to calculate body mass index (kg/m2).
Smoking Behavior.
Baseline assessment included current CPD, type of cigarette smoked (menthol or nonmenthol), age when first smoked, age when started smoking regularly, quitting and relapse history, and home smoking restrictions. Participants rated motivation and confidence for quitting on a 10-point scale, with higher scores reflecting greater motivation or confidence. Nicotine dependence was measured using the six-item Fagerström Test of Nicotine Dependence (FTND) (22) and the 30-item Wisconsin Inventory of Smoking Dependence Motives (WISDM-30) (23,24). Nicotine withdrawal in the past 24 hours was assessed at weeks 0, 3, 7 and 26, using the Minnesota Nicotine Withdrawal Scale (MNWS): total score can range from 0 to 32, with higher scores indicating more severe withdrawal (25).
Psychosocial Measures.
The 10-item Center for Epidemiologic Studies Depression Scale (CESD-10) (26,27) assessed self-reported distress associated with depressive symptoms: total score can range from 0 to 30, with a score of 10 or greater reflecting clinically significant depression. The four-item Perceived Stress Scale (PSS-4) (28) assessed self-appraised stress experienced in the past month: total score can range from 0 to 16, with higher scores indicating greater overall stress.
Biological Measures.
Exhaled air carbon monoxide, measured in parts per million, was collected at week 0 to reflect recent tobacco smoking and smoke exposure. Blood samples were collected at week 0 for evaluation of baseline serum cotinine among all participants for the purpose of describing the level of tobacco use before intervention. Blood samples were collected at week 3 for evaluation of serum levels of total bupropion (the sum of bupropion and its three major metabolites [hydroxybupropion, threohydrobupropion, and erthrohydrobupropion]) to indicate use of study medication within the bupropion SR group. Methods of blood collection and sample analyses have been described in detail elsewhere (20). Briefly, 20 mL of blood was drawn into BD vacutainer blood collection tubes containing 100 USP units of lithium heparin (LabCorp, Kansas City, MO) mixed by inversion and centrifuged at 120 x g at room temperature for 10–15 minutes. Plasma was extracted and stored at −20°C. Following completion of all study data collection, plasma samples were thawed and assayed by solid phase extraction (29). Standard procedures were used to evaluate cotinine level in all study participants (30,31). The sum of concentrations of bupropion and its metabolites was conducted for participants in the bupropion SR group only. Salivary samples were collected at weeks 3, 7, and 26, among participants who self-reported smoking abstinence for the purpose of providing biochemical verification of smoking status. Analysis of salivary cotinine levels was conducted using a standard gas chromatography technique (32).
Treatment Measures.
At weeks 1, 3, 5, 7, and 16, adverse events (AEs) were assessed and graded using the National Cancer Institute’s Common Toxicity Criteria for Adverse Events (CTCAE), version 3.0 (grade 1 = mild AE; grade 2 = moderate AE; grade 3 = severe AE; grade 4 = life-threatening or disabling AE; grade 5 = death-related AE). Self-reported medication adherence during the medication phase (week 3) was assessed using 3-day pill recall in which participants reported the total number of study medication doses taken in the previous 3 days (out of six prescribed doses).
Smoking Abstinence.
The primary outcome was cotinine-verified 7-day point prevalence smoking abstinence defined as no cigarettes (not even a puff) in the previous 7 days at week 26, validated using salivary cotinine (33,34). Verified point prevalence abstinence was also assessed at week 3 and at end of medication treatment at week 7. The cotinine cut point of 15 ng/mL was used to differentiate smokers (>15 ng/mL) from nonsmokers (≤15 ng/mL) at all time points (17,34–36).
Statistical Analysis
Based on our previous studies (8,9), sample size was determined a priori assuming a two-sided χ2 test with a type I error rate of .05, a power of 80%, and a cotinine-verified abstinence rate of 15% in the placebo group and 25% in the bupropion SR group at week 26, with the assumption that those lost to follow-up would be imputed as smokers.
Baseline demographic, smoking-related, and psychosocial variables were summarized for both bupropion SR and placebo groups using descriptive statistics. Results were expressed as mean and standard deviation for continuous variables (age, weight, body mass index, depression, stress, serum cotinine, exhaled carbon monoxide, CPD, nicotine dependence, number of 24-hour quit attempts in the past year, age of first cigarette, age started smoking regularly, motivation, and confidence to quit) and as frequency (percentage) for categorical variables (sex [male, female], marital status [married or living with partner, other], income [<$1800 or ≥$1800 per month], education [less than high school or high school or more], time to first cigarette [≤30 minutes of waking or >30 minutes of waking], menthol use [menthol or nonmenthol cigarettes], pharmacotherapy use in most recent quit attempt [yes, no], smoke-free household [yes, no], and smoke inhalation [inhale deeply, other]). The χ2 test was used to determine whether there was a difference between treatment groups (bupropion SR vs placebo) in verified 7-day point prevalence abstinence at week 26, imputing the missing participants as smokers. Subsequently, we evaluated verified 7-day point prevalence abstinence at weeks 3 and 7, imputing missing participants as smokers. Finally, at all three time points (weeks 3, 7, and 26), we used the χ2 test to compare self-reported 7-day abstinence, imputing missing participants as smokers.
Differences in treatment engagement were assessed with a two-sample t test as measured by number of counseling sessions attended. The relationship of AEs and intervention group (bupropion SR or placebo) was examined by comparing any AEs and any grade 2 or higher AEs between bupropion SR and placebo groups using a Pearson χ2 test. Odds ratios (ORs) for smoking abstinence and corresponding 95% confidence intervals (CIs) were calculated using logistic regression modeling.
Changes in depression and withdrawal were also examined. Change was defined as the absolute difference in the score from baseline to each of the three respective follow up visits: weeks 3, 7, and 26. Depression was evaluated using total scores of the CESD-10. Withdrawal was evaluated using total scores of the MNWS. All group comparisons were conducted using a two-sample t test at each follow-up time.
All tests of statistical significance were two-sided, and all P values less than .05 were considered statistically significant. All analyses were conducted with SAS 9.2 (Cary, NC) (37).
Results
Characteristics of study participants are presented in Table 1. There were no statistically significant differences in baseline demographic, psychosocial, and smoking history characteristics between the two randomized treatment groups (bupropion SR and placebo). At the baseline visit (week 0), participants reported smoking an average of 8.0 CPD (range 1–17 CPD) in the past 7 days, used primarily menthol cigarettes (83.7%), smoked within 30 minutes of waking (72.2%), had a mean exhaled carbon monoxide of 16.4 ppm, and had a mean serum cotinine of 275.8 ng/mL (SD = 155.8 ng/mL).
Table 1.
Baseline characteristics of study participants*
| Characteristic | Bupropion SR group | Placebo group | ||
| Mean (SD) or No. (%) | No. of participants with data for each variable | Mean (SD) or No. (%) | No. of participants with data for each variable | |
| Demographic variables | ||||
| Age, mean (SD), y | 46.8 (11.1) | 270 | 46.2 (11.5) | 270 |
| Women, No. (%) | 174 (64.4) | 270 | 183 (67.8) | 270 |
| Married or living with partner, No. (%) | 91 (33.8) | 269 | 75 (27.8) | 270 |
| Monthly family income <$1800, No. (%) | 169 (63.3) | 267 | 158 (59.2) | 267 |
| Education ≥high school, No. (%) | 225 (83.6) | 269 | 229 (84.8) | 270 |
| Weight, mean (SD), lb | 196.1 (51.8) | 269 | 194.8 (54.0) | 270 |
| BMI, mean (SD), kg/m2 | 31.1 (7.6) | 269 | 31.1 (8.1) | 270 |
| Psychosocial variables | ||||
| Depression† (CESD-10) score, mean (SD) | 7.2 (4.9) | 269 | 8.2 (5.5) | 270 |
| CESD-10 ≥10, No. (%) | 76 (28.3) | 269 | 91 (33.7) | 270 |
| Stress‡ (PSS-4) score, mean (SD) | 4.9 (3.1) | 269 | 5.5 (3.3) | 270 |
| Tobacco-related variables | ||||
| Serum cotinine, mean (SD), ng/mL | 268.7 (160.2) | 269 | 283.0 (151.2) | 267 |
| Exhaled carbon monoxide§, mean (SD), ppm | 15.8 (9.4) | 202 | 17.1 (10.5) | 209 |
| Cigarettes per day, mean (SD) | 8.0 (2.6) | 270 | 7.9 (2.4) | 270 |
| FTND|| score, mean (SD) | 3.1 (1.7) | 270 | 3.3 (1.7) | 270 |
| WISDM-30¶ score, mean (SD) | 36.7 (10.9) | 270 | 37.0 (11.4) | 270 |
| Time to first cigarette, ≤30 minutes, No. (%) | 191 (70.7) | 270 | 199 (73.7) | 270 |
| Smoke menthol cigarettes, No. (%) | 224 (83.0) | 270 | 228 (84.4) | 270 |
| Number of 24-h quit attempts in the past year, mean (SD) | 3.5 (7.9) | 270 | 3.9 (7.4) | 270 |
| Pharmacotherapy use during most recent quit attempt, No. (%) | 72 (27.8) | 259 | 61 (24.2) | 252 |
| Age of first cigarette, mean (SD), y | 17.5 (5.8) | 270 | 18.1 (7.3) | 270 |
| Age started smoking regularly, mean (SD), y | 21.0 (6.7) | 269 | 21.3 (7.4) | 270 |
| Motivation to quit, mean (SD) | 9.7 (0.8) | 270 | 9.8 (0.7) | 270 |
| Confidence to quit, mean (SD) | 7.9 (2.1) | 270 | 7.8 (2.0) | 270 |
| Smoke-free household, No. (%) | 65 (24.1) | 270 | 67 (24.8) | 270 |
| Inhale deeply, No. (%) | 65 (24.1) | 270 | 61 (22.6) | 270 |
Kick It at Swope III (KIS-III) trial of 540 African American light smokers: 270 participants were randomized to receive 300 mg bupropion SR (150 mg once daily for 3 days and then 150 mg twice daily) and 270 participants were randomized to receive placebo. BMI = body mass index; CESD-10 = Center for Epidemiological Studies Short Depression Scale; FTND = Fagerström Test of Nicotine Dependence; PSS = Perceived Stress Scale; ppm = parts per million; SR = sustained release; SD = standard deviation; WISDM-30 = Wisconsin Inventory of Smoking Dependence Motives.
CESD-10 assessed distress associated with depressive symptoms: possible total score ranges from 0 to 30, with a score of 10 or greater reflecting clinically significant depression.
PSS-4 assessed self-appraised global stress: possible total score ranges from 0 to 16.
Exhaled carbon monoxide was added to baseline data collection after the study began and was collected on only 411 of 540 participants.
FTND assessed nicotine dependence: possible total score ranges from 0 to 10.
WISDM-30 assessed nicotine dependence using a multidimensional scale: possible total score ranges from 10 to 70.
Salivary cotinine–verified and self-reported smoking abstinence rates at weeks 3, 7, and 26 are presented in Table 2. At the primary endpoint of the study, which was cotinine-verified 7-day point prevalence abstinence at week 26, no statistically significant difference between the bupropion SR and placebo groups was observed. Imputing those lost to follow-up as smokers, the difference in long-term smoking abstinence rates at week 26 was not statistically significant between the bupropion SR and placebo groups (13.3% vs 10.0%, OR = 1.39, 95% CI = 0.82 to 2.35, P = .23). However, the initial smoking abstinence rate at week 3 was statistically significantly higher in the bupropion SR group compared with the placebo group (21.5% vs 9.6%, OR = 2.58, 95% CI = 1.56 to 4.22, P < .001). Similarly, at the end of the 7-week medication phase of the intervention, the smoking abstinence rate was statistically significantly higher in the bupropion SR group compared with the placebo group (23.7% vs 9.6%, OR = 2.92, 95% CI = 1.78 to 4.77, P < .001).
Table 2.
Cotinine-verified and self-reported 7-day point prevalence abstinence rates*
| Smoking abstinence | Bupropion SR, No. (%) | Placebo, No. (%) | OR (95% CI) | P† |
| Cotinine verified (<15 ng/mL)‡ | ||||
| Quit at week 3 | 58 (21.5) | 26 (9.6) | 2.58 (1.56 to 4.22) | <.001 |
| Quit at week 7 | 64 (23.7) | 26 (9.6) | 2.92 (1.78 to 4.77) | <.001 |
| Quit at week 26 | 36 (13.3) | 27 (10.0) | 1.39 (0.82 to 2.35) | .23 |
| Self-reported | ||||
| Quit at week 3 | 79 (29.3) | 39 (14.4) | 2.45 (1.60 to 3.76) | <.001 |
| Quit at week 7 | 89 (33) | 47 (17.4) | 2.33 (1.56 to 3.50) | <.001 |
| Quit at week 26 | 65 (24.1) | 45 (16.7) | 1.57 (1.04 to 2.42) | .033 |
Participants lost to follow-up were imputed as smokers. Time points reflect number of weeks following randomization (week 0): target quit date occurred at week 1. Of the 540 participants, 270 participants randomized to the bupropion SR treatment group received 300 mg bupropion per day (150 mg once daily for 3 days and then 150 mg twice daily) and 270 participants in the placebo group received matching placebo pills. Participants were instructed to begin taking study medication (bupropion SR or placebo) at randomization (week 0) for a total of 7 weeks. Week 7 reflects end of the medication phase. Week 26 signifies end of follow-up. CI = confidence interval; OR = odds ratio; SR = sustained release.
P values were calculated using a two-sided Pearson χ2 test.
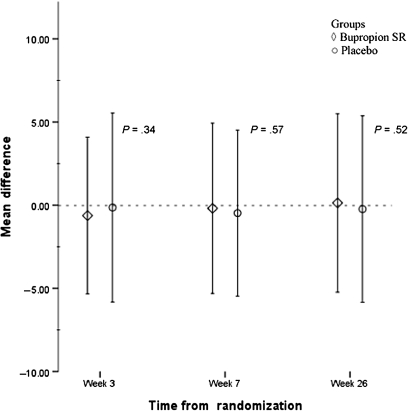
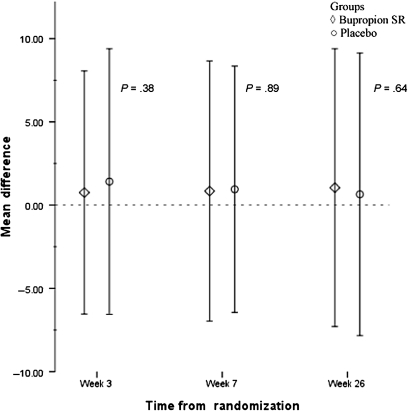
Completion of each study session did not differ between the treatment groups (retention rates shown in Figure 1). Retention of participants at week 26 was 71% (192 of 270 participants) in the bupropion SR group and 69% (187 of 270 participants) in the placebo group. No statistically significant difference in number of counseling sessions attended (range 1–6) was observed between the bupropion SR and placebo groups (mean [SD] = 4.9 [1.5] vs 4.8 [1.7], P = .24). Similarly, no difference in self-reported medication adherence at week 3 was observed based on a 3-day pill recall between bupropion SR and placebo groups (mean [SD] = 4.6 [2.1] vs 4.6 [2.3], P = .86). As shown in Table 3, the prevalence of AEs at week 3 was not different between the bupropion SR and placebo groups (P > .05 for all grades of AEs). Furthermore, an analysis of changes in symptoms of depression (Figure 2) and withdrawal (Figure 3) during the study period relative to the initial baseline self-report showed that treatment condition had no effect on changes in the level of depression or withdrawal.
Table 3.
Prevalence of adverse events at week 3 by treatment group*
| Adverse event | Bupropion SR, No. (%) | Placebo, No. (%) | P† |
| All grades (grades 1–5) | 88 (32.6) | 77 (28.5) | .30 |
| Grade ≥2 | 24 (8.9) | 28 (10.4) | .56 |
| Grades ≥3 (serious adverse events)‡ | 8 (4.4) | 13 (7.9) | .18 |
Adverse events were assessed for all 540 participants using the National Cancer Institute's Common Toxicity Criteria for Adverse Events, version 3.0 (38). SR = sustained release.
P values were calculated using a two-sided Pearson χ2 test.
No serious adverse events occurred within the first 3 weeks of bupropion SR or placebo treatment.
Figure 2.
Changes in depression scores relative to baseline at weeks 3, 7, and 26. The 10-item Center for Epidemiological Studies Depression Scale (CESD-10) assessed distress associated with depressive symptoms. CESD-10 depression scores are presented showing changes in CESD-10 score compared with baseline (horizontal dotted line). Diamonds represent the sustained release bupropion (bupropion SR) treatment group. Circles represent the placebo treatment group. Error bars represent standard deviations.
Figure 3.
Changes in withdrawal scores relative to baseline at weeks 3, 7, and 26. The eight-item Minnesota Withdrawal Scale (MNWS) assessed nicotine withdrawal. Change in withdrawal during the study period is presented showing the mean change in MNWS score compared with baseline (horizontal dotted line) for each treatment group. Diamonds represent the sustained release bupropion (bupropion SR) treatment group. Circles represent the placebo treatment group. Error bars represent standard deviations.
We examined blood levels of total bupropion at week 3 among the 270 participants within the bupropion SR treatment group, which indicated adherence to study medication during the active medication period. Within the bupropion SR treatment group, those confirmed abstinent at week 26 showed statistically significantly higher blood levels of total bupropion compared with continuing smokers (mean [SD] = 4.85 [4.06] vs 2.77 [2.34] μM, P = .013).
Discussion
The Kick It at Swope III study (KIS-III) is the first randomized, double-blind placebo-controlled clinical trial to test the efficacy of bupropion SR for smoking cessation treatment among African American light smokers. The abstinence rates at week 26 indicated no statistically significant long-term treatment effect of the standard 7-week treatment with bupropion SR compared with placebo. However, participants who received bupropion SR were more likely to achieve initial abstinence early in the treatment period and to be abstinent at the end of the medication phase of treatment compared with participants who received placebo.
To date, KIS-III is among the first studies to evaluate pharmacotherapy for light smokers (9,10,39). It is the second randomized placebo-controlled study of smoking cessation treatment for African American light smokers (9). Consistent with our previous KIS trials of African American smokers (8,9), study participants were largely low income and predominantly menthol smokers who were highly interested in stopping smoking.
In contrast to the current negative findings of long-term treatment effects, previous placebo-controlled clinical trials of bupropion have demonstrated efficacy in producing long-term abstinence in moderate to heavy smokers (≥10 CPD), with smokers using active bupropion being more than twice as likely to be abstinent at 6 months following treatment compared with those using placebo (11,18). The majority of previous bupropion trials had predominantly white participants. Like these other studies, our KIS-I placebo-controlled trial of bupropion SR for African American moderate to heavy smokers (≥10 CPD) found a statistically significant treatment effect of bupropion SR at the end of the medication phase of treatment (36.0% and 19.0% verified abstinence in bupropion SR and placebo groups, respectively) in addition to sustained abstinence at week 26 follow-up (21.0% and 13.7% in bupropion SR and placebo groups, respectively) (8). A critical question is why these long-term treatment effects seen in heavier African American smokers were not observed in this study of African American light smokers.
It is noteworthy that the current findings demonstrate initial benefit of bupropion SR for light smokers during the medication phase of the study as indicated by the higher abstinence rates in the bupropion SR group at weeks 3 and 7. African American light smokers who were given bupropion SR were almost three times as likely to quit smoking at week 7 compared with those who were given placebo (OR = 2.92, 95% CI = 1.78 to 4.77). This benefit of bupropion SR was seen early on in treatment, as evidenced by differences in abstinence rates between the study groups at week 3. The difference in abstinence rates between bupropion SR and placebo groups during the treatment phase was not related to retention or completion of counseling as no differences in these variables were seen between groups. Furthermore, although some studies have identified an impact of bupropion on reducing withdrawal and symptoms of depression (19), no such treatment effects were evident in this study. This finding is of particular interest given almost a third of our sample reported elevated symptoms of depression. While abstinence rates within the placebo group remained stable between the end of treatment at week 7 and follow-up at week 26, abstinence rates within the bupropion SR group decreased from 23.7% at week 7 to 13.3% at week 26. Notably, within the bupropion SR group, individuals who achieved long-term abstinence demonstrated higher medication adherence early in treatment, reflected in the evaluation of total bupropion levels in the blood at week 3 compared with those still smoking at week 26, suggesting a clinical benefit of bupropion use in facilitating abstinence for some light smokers. Indeed, attention to medication adherence in enhancing pharmacotherapy efficacy merits further consideration.
Limited study of smoking cessation treatment among light smokers has produced mixed findings regarding the benefit of pharmacotherapy for light smokers (9,10,39). Within the KIS-II placebo-controlled study of nicotine gum for African American light smokers (1–10 CPD), Ahluwalia et al. (9) found no statistically significant treatment effect at month 6, with cotinine-verified abstinence of 14.2% and 11.1% for nicotine and placebo groups, respectively. Lack of medication effect may have been related, in part, to challenges in underdosing or underadherence (9). In contrast, within a largely white sample of smokers who used 1–15 CPD and were randomized to placebo or nicotine lozenge, Shiffman (39) found that nicotine lozenge statistically significantly increased carbon monoxide–verified abstinence rates relative to placebo 6 weeks into treatment (45.7% vs 31.1%) and at 12-month follow-up (19.2% vs 10.0%). Gariti et al. (10) compared nicotine patch with bupropion within a racially mixed sample of light smokers (6–15 CPD; 68% African Americans) and found that within the nicotine patch group, quit rates appeared relatively stable over time, with verified abstinence rates of 26.8%, 26.0%, and 23.0% at weeks 12, 26, and 52, respectively. In contrast, bupropion produced abstinence rates of 27.1%, 18.0%, and 15.9% at weeks 12, 26, and 52, respectively, demonstrating higher early abstinence but subsequent decrease in abstinence with time (10). Across treatment groups, African Americans were less likely than whites to be abstinent at the end of the study (10). Benefit of pharmacotherapy and identification of mechanism of action for light smokers warrant additional study, with attention to enhancing initial treatment efficacy and increasing long-term abstinence for African American light smokers.
In this study, although motivation to stop smoking was high within the sample of light smokers, abstinence rates were modest. Indeed, nine of 10 light smokers who received placebo were smoking within the first 3 weeks of treatment, demonstrating that even motivated light smokers had difficulty with initial quit attempts or struggled with early relapse. These findings further support the need to identify effective treatment approaches for light smokers (11). Although this cohort of African American light smokers reported use of approximately 8.0 CPD, findings show notably high levels of cotinine, similar to those seen in our previous study of African American light smokers (9). The majority of these light smokers reported smoking soon after wakening, suggesting physical nicotine dependence (40). Given notable nicotine intake, nicotine dependence, and the early impact of bupropion SR on facilitating abstinence during the medication phase of treatment, these findings support the idea that some light smokers benefit from pharmacotherapy to aid initial cessation. Future studies with African American light smokers could examine extended use of bupropion as a means of building on the initial medication effect to support sustained abstinence over time. Although extended use of bupropion has not been established as a method of relapse prevention (41), a number of studies suggest that long-term use of bupropion (eg, up to 1 year) may promote sustained abstinence (42–44).
Successful recruitment and retention within this study support the feasibility of enrolling African American light smokers into a clinical treatment trial involving biological data collection (7,20,45). The present findings further support the importance of biochemical verification of smoking status, previously identified in KIS clinical trials (8,9), consistent with guidelines on biochemical verification (34).
This study has a few limitations. Generalizability of the findings to other smokers may be limited based on study inclusion criteria that address smoking cessation in African American light smokers motivated to stop smoking and based on study exclusion criteria that largely focus on medical eligibility to use bupropion (20). Although this study design included assessment points standard for smoking cessation treatment studies, that is, end of treatment and 6-month follow-up, the lack of assessment between these points limited the ability to characterize the process of relapse among smokers in the bupropion SR group who experienced initial abstinence. For example, it is not clear if these light smokers more commonly experienced rapid or gradual relapse following the medication phase. Interest in more comprehensive evaluation of smoking behavior change in future study will need to be weighed against considerations of participant burden and study retention.
In summary, KIS-III contributes to the limited literature on treatment of African American light smokers. Standard bupropion SR treatment did not produce long-term abstinence, despite facilitating initial abstinence during medication use. Further investigation is needed to build upon these findings to identify 1) mechanisms of action of bupropion in early use, 2) methods to enhance sustained abstinence, and 3) individuals for whom this treatment approach is most effective. The ultimate goal of this research is to advance treatment, increase long-term abstinence, and reduce tobacco-related health disparities.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (CA 091912 to L.S.C.). This work was also supported in part by the National Institute for Minority Health and Disparities (1P60MD003422 to J.S.A.). Support was also provided by the Centre for Addiction and Mental Health and by a Canada Research Chair in Pharmacogenetics (to R.F.T.).
Footnotes
The Kick It at Swope III (KIS-III) study is a federally funded registered clinical trial (ClinicalTrials.gov Identifier: NCT00666978) from the grant “Enhancing Tobacco Use Treatment for African American Light Smokers.” Dr M. S. Mayo had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We are grateful to the volunteers who participated in this research and the members of our community advisory board who supported this program. Appreciation is given to the American Jazz Museum, the Negro Baseball League Museum, and Kansas City Union Station for support of this project. The authors would like to thank the staff at Swope Health Central, Tricia Snow, Carrie Bronars, Olivia Chang, Emily Kravit, Jennifer Lipari, Ian Lynam, Heather Newhard, Cinnamon Smith, and Dr Edward Ellerbeck and Dr Gary Salzman for their efforts on this study. We also thank Ron Krebill for assistance with data analysis and Becky Clausius for assistance with article preparation.
The authors are solely responsible for the study design, data collection, analysis and interpretation of the data, writing the article, and decision to submit the article for publication.
Dr J. S. Ahluwalia serves as a consultant to Pfizer Pharmaceuticals, Inc; Dr N. L. Benowitz serves as a consultant to Pfizer Pharmaceuticals, Inc, and has been a paid expert witness in litigation against tobacco companies; Dr R. F. Tyndale holds shares in Nicogen Research, Inc, a company that is focused on novel smoking cessation treatment approaches; no Pfizer or Nicogen funds were used in this work.
References
- 1.American Cancer Society Cancer Facts and Figures 2009. Atlanta, GA: 2009. American Cancer Society; [Google Scholar]
- 2.Trinidad DR, Perez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res. 2009;11(2):203–210. doi: 10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenborn CA, Adams PF, Barnes PM, Vickerie JL, Schiller JS. Health behaviors of adults: United States, 1999-2001. Vital Health Stat 10. 2004;219:1–79. [PubMed] [Google Scholar]
- 4.CDC. Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1227–1232. [PubMed] [Google Scholar]
- 5.Fu S, Kodl M, Joseph A, et al. Racial/Ethnic disparities in the use of nicotine replacement therapy and quit ratios in lifetime smokers ages 25 to 44 years. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1640–1647. doi: 10.1158/1055-9965.EPI-07-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagan P, Moolchan ET, Lawrence D, Fernander A, Ponder PK. Identifying health disparities across the tobacco continuum. Addiction. 2007;102(suppl 2):5–29. doi: 10.1111/j.1360-0443.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 7.Cox LS, Okuyemi K, Choi WS, Ahluwalia JS. A review of tobacco use treatments in U.S. ethnic minority populations. Am J Public Health. 2011;25(Suppl 5):S11–S30. doi: 10.4278/ajhp.100610-LIT-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA. 2002;288(4):468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- 9.Ahluwalia JS, Okuyemi K, Nollen N, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 x 2 factorial design. Addiction. 2006;101(6):883–891. doi: 10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 10.Gariti P, Lynch K, Alterman A, Kampman K, Xie H, Varillo K. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. J Subst Abuse Treat. 2009;37(3):247–255. doi: 10.1016/j.jsat.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiore MC, Jaen CR, Baker TB, et al. Department of Health and Human Services. Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 update. Clinical Practice Guideline. [Google Scholar]
- 12.Allen B, Jr, Unger JB. Sociocultural correlates of menthol cigarette smoking among adult African Americans in Los Angeles. Nicotine Tob Res. 2007;9(4):447–451. doi: 10.1080/14622200701239647. [DOI] [PubMed] [Google Scholar]
- 13.Castro FG. Physiological, psychological, social, and cultural influences on the use of menthol cigarettes among Blacks and Hispanics. Nicotine Tob Res. 2004;6(suppl 1):S29–S41. doi: 10.1080/14622200310001649487. [DOI] [PubMed] [Google Scholar]
- 14.Ho MK, Mwenifumbo JC, Al Koudsi N, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85(6):635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P. Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999;291(3):1196–1203. [PubMed] [Google Scholar]
- 17.Ho MK, Faseru B, Choi WS, et al. Utility and relationships of biomarkers of smoking in African-American light smokers. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3426–3434. doi: 10.1158/1055-9965.EPI-09-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 19.West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl). 2008;197(3):371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- 20.Cox LS, Faseru B, Mayo MS, et al. Design, measures, and baseline characteristics from a randomized clinical trial of bupropion in African-American light smokers. Trials. 2011;12:22. doi: 10.1186/1745-6215-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuyemi KS, Cox LS, Nollen NL, et al. Baseline characteristics and recruitment strategies in a randomized clinical trial of African-American light smokers. Am J Health Promot. 2007;21(3):183–191. doi: 10.4278/0890-1171-21.3.183. [DOI] [PubMed] [Google Scholar]
- 22.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith SS, Piper ME, Fiore MC, Baker TB. Subscale consolidation and item reduction of the 68 item Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Proceedings of the Society for Research on Nicotine and Tobacco; 21–24 February 2007; Austin, TX. [Google Scholar]
- 24.Piper ME, Piasecki TM, Federman EB, et al. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) J Consult Clin Psychol. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- 25.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D Scale. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 27.Cole JC, Rabin AS, Smith TL, Kaufman AS. Development and validation of a Rasch-derived CES-D short form. Psychol Assess. 2004;16(4):360–372. doi: 10.1037/1040-3590.16.4.360. [DOI] [PubMed] [Google Scholar]
- 28.Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychol. 1990;9(4):466–478. doi: 10.1037//0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- 29.Stewart JJ, Berkel HJ, Parish RC, et al. Single-dose pharmacokinetics of bupropion in adolescents: effects of smoking status and gender. J Clin Pharmacol. 2001;41(7):770–778. doi: 10.1177/00912700122010564. [DOI] [PubMed] [Google Scholar]
- 30.Benowitz NL, Jacob P, III, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268(1):296–303. [PubMed] [Google Scholar]
- 31.Benowitz NL, Zevin S, Jacob P., III Sources of variability of nicotine and cotinine levels with use of nicotine nasal spray, transdermal nicotine, and cigarette smoking. Br J Clin Pharmacol. 1997;43(3):259–267. doi: 10.1111/j.1365-2125.1997.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob P, III, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222(1):61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- 33.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 34.Benowitz NL, Jacob P, Ahijevych K, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 35.Murray RP, Connett JE, Lauger GG, Voelker HT. Error in smoking measures: effects of intervention on relations of cotinine and carbon monoxide to self-reported smoking. The Lung Health Study Research Group. Am J Public Health. 1993;83(9):1251–1257. doi: 10.2105/ajph.83.9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cummings SR, Richard RJ. Optimum cutoff points for biochemical validation of smoking status. Am J Public Health. 1988;78(5):574–575. doi: 10.2105/ajph.78.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SAS/STAT User's Guide [Computer Program]. Version 8. Cary, NC: SAS Institute, Inc.; 1999. [Google Scholar]
- 38.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. 2006. http://ctep.cancer.gov/reporting/ctc.htmlAccessed February 1, 2011. [Google Scholar]
- 39.Shiffman S. Nicotine lozenge efficacy in light smokers. Drug Alcohol Depend. 2005;77(3):311–314. doi: 10.1016/j.drugalcdep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tobacco Research. 2007;9(suppl 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Croghan IT, Hurt RD, Dakhil SR, et al. Randomized comparison of nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clinic Proc. 2007;82(2):186–195. doi: 10.4065/82.2.186. [DOI] [PubMed] [Google Scholar]
- 42.Hays JT, Hurt RD, Rigotti NA, et al. Sustained release buproprion for pharmacologic relapse prevention after smoking cessation. Ann Intern Med. 2001;135:423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 43.Covey LS, Glassman AH, Jiang H, et al. A randomized trial of bupropion and/or nicotine gum as maintenance treatment for preventing smoking relapse. Addiction. 2007;102(8):1292–1302. doi: 10.1111/j.1360-0443.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 44.Killen JD, Fortmann SP, Murphy GM, et al. Extended treatment with bupropion SR for cigarette smoking cessation. J Consult Clin Psychol. 2006;74(2):286–294. doi: 10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- 45.Cox LS, Bronars CA, Thomas JL, et al. Achieving high rates of consent for genetic testing among African American smokers. Nicotine Tob Res. 2007;9(6):711–716. doi: 10.1080/14622200701365228. [DOI] [PubMed] [Google Scholar]