Abstract
There is growing evidence that among the different conduct disorder (CD) behaviors, physical aggression, but not theft, links to low neurocognitive abilities. Specifically, physical aggression has consistently been found to be negatively related to neurocognitive abilities, whereas theft has been shown to be either positively or not related to neurocognition. The specificity of these links needs further examination because attention deficit hyperactivity disorder (ADHD) links to both physical aggression and neurocognitive variation. The development of self-reported physical aggression and theft, from age 11 to 17 years, was studied in a prospective at-risk male cohort via a dual process latent growth curve model. Seven neurocognitive tests at age 20 were regressed on the growth parameters of physical aggression and theft. The links between neurocognition and the growth parameters of physical aggression and theft were adjusted for ADHD symptoms at ages 11 and 15 (parent, child and teacher reports). Results indicated that verbal abilities were negatively related to physical aggression while they were positively associated with theft. However, inductive reasoning was negatively associated with increases in theft across adolescence. Symptoms of ADHD accounted for part of the neurocognitive test links with physical aggression but did not account for the associations with theft. These differences emphasize the importance of examining specific CD behaviors to better understand their neurodevelopmental mechanisms. They also suggest that youth who engage in different levels of physical aggression or theft behaviors may require different preventive and corrective interventions.
Keywords: conduct disorder, physical aggression, theft, ADHD, neurocognition, latent growth curves
INTRODUCTION
Adolescent antisocial behavior (ASB) affects society in a wide and penetrating manner; the victimization and distress to individuals, impairment of life opportunities and cost burden to society is staggering [Loeber and Hay, 1997; Tremblay, 2010]. One approach to understanding causes and correlates of ASB has involved the assessment of neurocognition [Moffitt, 1993; Séguin et al., 2007a,b]. Indeed, the idea that there may be variations in the links between neurocognitive functioning and various forms of ASB had been raised well over 60 years ago, when it was proposed IQ levels may influence the type of delinquent behaviors youths primarily engage in [Burt, 1944; McCord et al., 1959]. In a nutshell, lower IQ delinquents were thought to engage in more aggressive and violent behaviors, in contrast to the “high IQ” delinquents, described as engaging in more covert, monetary directed antisocial behaviors [Gath and Tennent, 1972; Merrill, 1947; Tennent and Gath, 1975].
More recently, in an attempt to help clarify the link between aggression and neurocognitive impairment, this focus has gradually shifted to consider attention deficit hyperactivity disorder (ADHD) symptoms [Pennington and Ozonoff, 1996]. ADHD symptoms include excessive activity and impulsive behaviors as well as difficulties in sustaining attention, distractibility and cognitive disorganization [American Psychiatric Association, 2000]. Two frequently cited developmental child psychiatry models suggest that the combination of neurocognitive impairment and ADHD increase the risk for chronic engagement in serious conduct disorder (CD) behaviors such as physical aggression. The first model states that a childhood onset of CD behaviors is characterized by aggressive and ADHD behaviors as well as by mild neurocognitive impairments [Moffitt, 1993; Moffitt et al., 2002, 2008]. In contrast, nonaggressive forms of CD behaviors, such as theft, are believed to do be relatively independent of neurocognitive deficits or ADHD. A second model suggests that neurocognitive deficits and ADHD should associate to more serious physical aggression behaviors, and that deceptive behaviors, such as theft without the presence of physical aggression, may relate to relatively higher levels of neurocognition but lower levels of ADHD [Loeber et al., 1993; Loeber and Stouthamer-Loeber, 1998].
Indeed, reviews of empirical studies independently conclude that neurocognitive functions, including impairments in working memory—such as planning (the capacity to organize the steps that need to be executed in order to attain a goal) and interference control (the capacity to follow a plan despite distractions)—are characteristic of both CD [Morgan and Lilienfeld, 2000] and ADHD [Willcutt et al., 2005]. However, not all CD studies report these impairments [Fairchild et al., 2009; Pennington and Ozonoff, 1996]. The variation in results may be due to the fact that CD is often comorbid with ADHD, yet studies seldom examined their comorbidity [Déry et al., 1999; Diamantopoulou et al., 2007; Séguin et al., 2004, 2009; Waschbusch, 2002].
Another reason for the inconsistent findings is that—as stated—CD behaviors are heterogeneous [Loeber et al., 2000] and include aggressive and nonaggressive behaviors, such as destruction of property, deceitfulness or theft, and serious rule violations, as defined by the American Psychiatric Association [2000], which may relate differently to neurocognitive function. Of note, although the majority of studies collapse all CD behaviors together [Tremblay, 2010], factor analytic [Tackett et al., 2005], genetically informed [Barker et al., 2009; Burt, 2009a,b; Monuteaux et al., 2009], and longitudinal phenotype [Bongers et al., 2004; Craig et al., 2002; Lacourse et al., 2002; Loeber et al., 2000; van Lier et al., 2009] studies support a distinction between aggressive and non aggressive CD behaviors.
The evidence for a neurocognitive distinction between aggressive and nonaggressive CD behaviors, however, is not as robust. For example, two studies found specific relations of neurocognitive function with aggressive but not with nonaggressive conduct problems [Giancola et al., 1998; Hancock et al., 2010], whereas two other studies failed to find such a distinction [Baker and Ireland, 2007; Hoaken et al., 2007]. One potential explanation for this discrepancy is the aggregation of different types of nonaggressive conduct problems, which assumes that each type of nonaggressive misbehavior is equally linked to neurocognitive function. Further unpacking nonaggressive conduct problems with a focus on theft may be a useful line of inquiry. For instance, theft in particular, may require a degree of working memory (i.e., surveying the environment while holding a plan in mind and adjusting to unexpected events requiring strategy change) and inhibition (i.e., waiting for the optimal moment to steal an object) not necessary for the use of physical aggression.
Two studies indeed suggest that theft and physical aggression may relate differently to neurocognitive function. In a clinical sample of adjudicated adolescent males, one study has reported that IQ was negatively linked violent crimes but positively linked with property crimes [Walsh, 1987]. Property crimes—as measured in the Walsh study—included mainly theft behaviors; however, the main neurocognitive measure was global IQ score, which may not adequately capture the neurocognition of theft. In an attempt to further clarify the neurocognitive correlates of theft and physical aggression, another study examined a community sample of males followed from early adolescence into adulthood. Verbal IQ and executive function problems were found to be negatively related to physical aggression, while the relation of these two tests to theft, although positive, only approached significance [Barker et al., 2007]. Thus the negative relation to physical aggression noted by Walsh [1987] was replicated by Barker et al. [2007], but the observed positive relation between neurocognition and theft did not fully reach significance. There are two potential reasons for the trend in the association between verbal IQ, executive function and theft: (1) the measures of neurocognitive function may have been less sensitive to working memory, which may be an especially important function that is sensitive to theft and (2) the study was an accelerated cohort sequential design consisting of three cohorts spanning adolescence. Hence, Barker et al. [2007] inferred developmental patterns, but were limited in the assessment of the association between neurocognition and developmental growth of adolescent aggression and theft. In summary, converging evidence suggests a clear neurocognitive differentiation between CD behaviors: poor neurocognitive function is consistently related to physical aggression. The neurocognition underlying theft specifically remains inconsistent, though the link has never been reported as negative (i.e., higher theft, lower test scores).
To further elucidate the potential differences in the associations between neurocognition and theft and physical aggression, it is first important to broaden the assessment of neurocognitive function. Reliance on global IQ scores [Walsh, 1987] and tests of verbal ability and interference control [Barker et al., 2007] may not sufficiently tap specific neurocognitive profiles that will differentiate physical aggression from theft. We consider here a broad set of neurocognitive domains that tap short-term memory and working memory, including associative learning and IQ estimates. These tests may better link to the impulsive use of physical aggression and to the more instrumental and calculated aspects of theft. In addition, our understanding of the specificity of neurocognitive function to physical aggression and theft may be enhanced by controlling for ADHD— that is, to assess the extent to which neurocognitive variation may be shared by aggression and ADHD but not with theft. Finally, some of the inconsistencies in research may have been due to lack of consideration of the development of physical aggression and theft. We will therefore examine the association between change in these behaviors during adolescence (11–17) and a neurcocognitive test battery at age 20. Following theoretical models and the previous research, we expected poor neurocognitive performance to be associated with high levels of physical aggression, even after controlling for its likely relation with ADHD, whereas working memory is expected to positively relate to theft.
METHOD
Participants
Participants were part of a French-speaking community longitudinal study of males (N=1,037) from disadvantaged areas of Montréal, Canada. At age 20 years (Range: 18.47–22.02), a sample of 494 young men were selected from the larger study on the basis of teacher-reported histories of physical aggression and hyperactivity since kindergarten (age 6 years) with the aim of recruiting 300 based on an a priori power analysis (303 accepted) to come into a laboratory for neurocognitive testing [Séguin et al., 2004]. The association between physical aggression and hyperactivity in the laboratory sample represented well the same association across the larger sample (ordinal by ordinal Spearman r=.64, P<.0001) Informed consent was obtained from all participants. These young men had already reported on their physically aggressive behavior and theft annually from ages 11 to 17 years.
Measures
Self-reports of physical aggression and theft were assessed with the Self-Report Delinquency scale [LeBlanc and Fréchette, 1989], between ages 11 and 17 years. At each assessment, the participants reported whether they had been involved over the last 12 months in acts of physical violence (e.g., Threatening to attack someone, Fist fighting; mean Cronbach α from age 11 to 17=.74), and theft (e.g., Stealing from a store, Breaking and entering; mean α=.82). Items were scored on a 4-point Likert scale (0=never, 1=rarely, 2=sometimes, or 3=often).
Symptoms of ADHD were assessed with a French version of the Diagnostic Interview Schedule for Children (DISC) a structured psychiatric interview designed to assess DSM-III-R childhood psychiatric disorders [Breton et al., 1998]. Reliability and validity information, within this sample, are reported elsewhere [Romano et al., 2004]. The DISC was administered to the youth, their mothers and teachers at age 11 by trained interviewers, and to the youth and their mothers between the ages of 14 and 16. Fourteen ADHD symptoms were assessed including symptoms across the domains of hyperactivity– impulsivity (e.g., fidgets, restless) and inattention (e.g., difficulty sustaining attention, easily distracted), and were summed to create a global ADHD symptom score. An algorithm combining informants and using a sum of symptoms across the three dimensions of ADHD has been found to be sensitive to both physical aggression and neurocognitive function [Romano et al., 2004].
Neurocognitive function was measured with seven tests. High scores represented success for all tests. Methodological details are available elsewhere [Séguin et al., 2004] and are summarized here. Verbal and performance IQ estimates were obtained with the Vocabulary test (VIQ) and Block Design test (PIQ) from French Canadian equivalents [Chevrier, 1989] of the Vocabulary (VT) and Block Design (BD) tests of the Wechsler Adult Intelligence Scale -III [Wechsler, 1997]. The VT requires respondents to define words of gradually increasing difficulty. The BD subtest requires the reproduction of two-dimensional patterns of red and white blocks. BD ranks highest on the g factor, it measures visuospatial organization and planning and is related to brain circuitry involving frontal, parietal and occipital grey matter [Colom et al., 2006], and not uniquely based in the frontal lobes [Duncan et al., 2000]. Short-term auditory memory functions associated with the hippocampus and medial temporal lobe [Lezak et al., 2004], were assessed with subtests of the Wechsler Memory Scales—Revised [Wechsler, 1987]: Paired Associate Learning (PAL) [Ojemann et al., 2010] and Digit Span (DS) [Alpherts et al., 2008; Moore and Baker, 1996]. Such tests are recommended neurocognitive controls because they minimize demands on strategic retrieval in comparison to more demanding working memory tests [Petrides et al., 1995]. The PAL requires listening to easy or difficult word pairs. The first word of the pair is then provided to cue recall for the second word. The DS task requires repeating digits in increasing spans (forward and backward orders). Working memory was measured with two validated mid-dorsolateral frontal lobe tasks and two validated posterior dorsolateral task. They were respectively: Number Randomization (NR), Self-Ordered Pointing (SOP) [Petrides et al., 1993a,b], and Spatial and nonspatial Conditional Association tasks (CAS) [Petrides, 1985; Petrides et al., 1993a,b]. In the NR task, a range of numbers is provided, and all numbers must then be selected without using repetition, pattern, or more than two consecutive numbers. The SOP consists of 12 arrays of the same 12 stimuli where one must select a new stimulus in each array. Repetitions are counted as errors. The CAS tasks require inductive reasoning to uncover predetermined patterns of association between a button and a light, or a color and an abstract symbol. Increases in all neurocognitive scores represent greater ability. Functionally, the NR and SOP require deductive reasoning–following explicit rules. And the CAS tasks require inductive reasoning—figuring out the implicit rules and applying them). The neurocognitive tests were significantly associated (mean r=.39, range=.32–.48).
Attrition and Missing Data
Covariance coverage for the repeated measures of aggression, theft, ADHD and the neurocognitive tests ranged from 89 to 100%, which indicated that participant retention was quite good. Data missingness was considered missing at random. Missing data were handled through Full Information Maximum Likelihood.
Data Analysis
The analysis proceeded in three steps. In the first step, we examined the relationships between physical aggression, theft and neurocognition, respectively. The growth of physical aggression and theft were modeled using latent growth parameters. In this study, the intercept represents the initial level of problems at age 11 years, whereas the slope represents the linear change (i.e., increasing, decreasing) in physical aggression and theft with age (i.e., age 12–17). The growth parameters of physical aggression and theft were correlated, thus adjusting all parameter estimates for the co-occurrence between them.
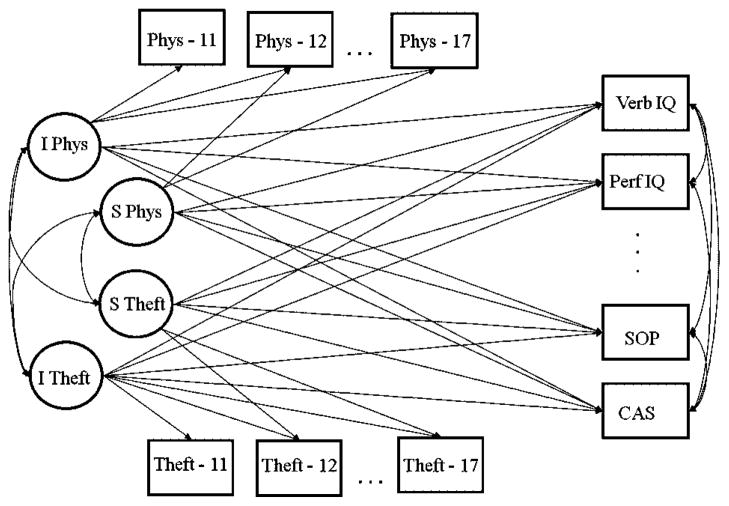
In step 2, the seven neurocognitive tests (age 20) were regressed on the growth parameters of physical aggression, and theft (aged 11–17). The dual process model is presented in Figure 1. To examine the most parsimonious model, we conducted nested model comparisons. That is, if certain neurocognitive tests did not significantly associate to the intercept of slope of physical aggression and/or theft, we examined if restricting these parameters to zero significantly reduced model fit. Nested models were tested with the Satorra–Bentler Scaled Chi-square [Satorra, 2000] because, a Robust Maximum Likelihood estimator was used to estimate the models.
Fig. 1.
The dual process growth curve model and the neurocognitive tests.
In the Step 3, we examined whether ADHD symptom scores accounted for the relationships between physical aggression and neurocognition, but not the relationships between theft and neurocognition. In this step, we (a) used the identified and parsimonious model from the Step 2, and (b) controlled for ADHD by regressing the symptom scores on the growth parameters of physical aggression and theft, as well as on the seven neurocognitive tests.
The model chi-square, the comparative fit index (CFI, critical value≥.90) [Bentler and Bonett, 1980], and the root mean squared estimate of approximation (RMSEA, critical value≤.08) [Browne and Cudeck, 1993] were used to determine model fit. To account for the nonnormal distributions of the physical aggression, theft and ADHD (age 15) scores, we used a Robust Maximum Likelihood estimator (i.e., the Huber–White variance adjustment) [Muthén and Muthén, 2000].
RESULTS
Descriptive Statistics
The raw means and standard deviations of physical violence, and theft at ages 11–17 years are in Table I. The physical aggression and theft scores, by year, were correlated at a moderate effect size (mean r=.57; range: .49–.63). ADHD symptoms at age 11 were significantly related with theft (mean r=.14; range: .06–.22), physical aggression (mean r=.21; range: .12–.26) and the neurocognitive battery (mean r=−.27; range: −.16 to .39). ADHD symptoms at age 15 were also related with theft (mean r=.28; range: .20–.35), physical aggression (mean r=.29; range: .24–.25), and the neurocognitive battery (mean r=−.18; range: −.11 to .28).
TABLE I.
Summary Statistics of ADHD, Physical Aggression, Theft and the Aggregate Score by Age
| Age (years) | ADHD
|
Physical aggression
|
Theft
|
|||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| 11 | 4.34 | 2.50 | 1.94 | 2.33 | 3.26 | 4.35 |
| 12 | – | – | 1.80 | 2.44 | 3.09 | 4.78 |
| 13 | – | – | 1.67 | 2.43 | 3.26 | 4.71 |
| 14 | – | – | 2.04 | 2.58 | 4.25 | 5.55 |
| 15 | 2.15 | 2.53 | 1.94 | 2.39 | 4.40 | 5.17 |
| 16 | – | – | 1.64 | 2.46 | 4.00 | 5.74 |
| 17 | – | – | 1.33 | 2.13 | 3.60 | 5.45 |
Step 1: Developmental Associations Between Physical Aggression and Theft
The development of physical aggression and theft were simultaneously estimated, allowing for correlations between the growth parameters. This model had adequate fit to the data (χ2(df=99, N=299)= 1,458.71, P<.01, CFI=.96, RMSEA=.05).1 The slope for physical aggression was negative, indicating a decrease from age 11 to 17 (Bslope=−.06, SE=.03, t=−2.40). The slope for theft was positive, indicating an increase from age 11 to 17 (Bslope=.21, SE=.04, t=5.03). Significant associations between the intercepts (r=.86, P<.001) and slopes (r=.75, P<.001) were identified, suggesting baseline levels and growth rates in one type of antisocial behavior were associated to baseline levels and growth rates in the other type of behavior. Higher levels of physical aggression at age 11 (intercept) was not significantly related to growth (slope) in theft (r=−.10, P>.05). However, higher levels of theft at age 11 (intercept) was related to a significant linear decrease (slope) in physical aggression (r=−.64, P<.001).
Step 2: Links Between the Seven Neurocognitive Tests, and Physical Aggression and Theft
In the next step we regressed the seven tests (age 20) on the slopes and intercepts of physical aggression and theft (aged 11–17). To allow for the most parsimonious model, the nonsignificant regressions between the neurocognitive tests and the growth parameters were deleted from the model. The two models, with and without nonsignificant paths, did not significantly differ (Satorra-Bentler Scaled χ2(df=12, N=303)=12.52, P=.41), thereby suggesting that eliminating the nonsignificant parameters (i.e., the regressions involving the slopes of physical aggression and theft) did not significantly reduce model fit (χ2(df=161, N=299)=282.65, P<.0001, CFI=.95, RMSEA=.05).
Examination of the regressions (see Table II, top) suggests that, in general, associations with the neurocognitive tests were found only for the intercepts, but not the slopes, of physical aggression and theft (an exception for a slope effect of theft is noted below). For physical aggression, the relations between baseline levels (intercept) and the tests were negative, indicating lower success on four of seven tests: the VIQ, PIQ, PAL and the DS. For theft, the sign of the relationships between baseline levels (intercept) and the neurocognitive tests were positive for two of the seven tests (VIQ and PAL). Notice, however, in Table II (top), for the rate of increase in theft (slope) and the CAS, there was a significant negative relationship. Hence, increasing levels of theft related to lower test scores on the CAS.
TABLE II.
Latent Growth Parameters Regressed on the Neurocognitive Test Battery
| Physical aggression
|
Theft
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept
|
Slope
|
Intercept
|
Slope
|
|||||||||
| B | SE B | B | B | SE B | β | B | SE B | β | B | SE B | β | |
| Step 1: Dual process | – | |||||||||||
| Verbal IQ (VIQ) | −.56* | .17 | −.84 | – | – | – | .33* | .14 | .61 | −.122 | .14 | −.06 |
| Performance IQ (PIQ) | −.36* | .17 | −.55 | – | – | – | .22 | .13 | .41 | – | – | – |
| Paired Ass. Learning (PAL) | −.44* | .16 | −.61 | – | – | – | .30* | .12 | .50 | – | – | – |
| Digit Span (DS) | −.26* | .13 | −.40 | – | – | – | .18 | .11 | .33 | – | – | – |
| Number Randomization (NR) | −.23 | .15 | −.35 | – | – | – | .21 | .12 | .39 | – | – | – |
| Self-Ordered Pointing (SOP) | −.28 | .15 | −.40 | – | – | – | .15 | .13 | .28 | – | – | – |
| Cond. Association Task (CAS) | −.15 | .14 | −.23 | – | – | – | .02 | .14 | .04 | −.34* | .14 | −.17 |
| Step 2: Adding ADHD | – | |||||||||||
| Verbal IQ (VIQ) | −.46* | .16 | −.70 | – | – | – | .34* | .15 | .58 | −.17 | .19 | −.06 |
| Performance IQ (PIQ) | −.25 | .15 | −.39 | – | – | – | .20 | .12 | .35 | – | – | – |
| Paired Ass. Learning (PAL) | −.33* | .15 | −.50 | – | – | – | .25* | .13 | .43 | – | – | – |
| Digit Span (DS) | −.22 | .13 | −.34 | – | – | – | .20 | .11 | .33 | – | – | – |
| Number Randomization (NR) | −.16 | .15 | −.25 | – | – | – | .20 | .13 | .34 | – | – | – |
| Self-Ordered Pointing (SOP) | −.17 | .15 | −.26 | – | – | – | .12 | .13 | .20 | – | – | – |
| Cond. Association Task (CAS) | −.12 | .15 | −.18 | – | – | – | .03 | .13 | .05 | −.52 * | .22 | −.19 |
Note: –, excluded from the model.
P<.05.
Step 3: Adding ADHD Symptoms
In Step 3 we controlled for ADHD symptoms at ages 11 and 15; we regress the ADHD symptoms scores on the growth parameters of physical aggression and theft, and the neurocognitive test scores. This model fits the data adequately (χ2(df=194, N=299)=365.66, P<.0001, CFI=.93, RMSEA=.05). The relation of the parameter estimates of the neurocognitive tests with the growth parameters of physical aggression and theft, while controlling for ADHD are in Table II (bottom). For physical aggression, two of the previously four significant associations with neurocognitive tests were no longer significant (i.e., PIQ and DS), whereas the two significant effects identified for theft all remained significant. In summary, ADHD symptoms at age 11 years accounted for the relationship of two significant associations between physical aggression and the neurocognitive test scores (i.e., the PIQ and DS, but not VIQ and PAL), but not for theft.
With regard to associations of the ADHD scores, ADHD symptom scores at ages 11 and 15 did not significantly relate to the slope of physical aggression or to the intercept or slope of theft. ADHD symptoms at age 11 significantly associated to four of the seven neurocognitive tests: VIQ (t=−2.58; P<.01; b=−.35), PIQ (t=−2.76; P<.01; b=−.31), SOP (t=−2.99; P<.01; b=−.29), and CAS (t=−2.60; P<.01; b=−.29). The one significant result for the growth parameters and ADHD was that the age 11 ADHD score, but not the age 15 score, was associated with the intercept of physical aggression (t=2.64; P<.01; b=.38). ADHD symptoms at age 15 were no longer significantly associated to the neurocognitive tests, above variance attributable to age 11 ADHD, physical aggression and theft. This suggests that the ADHD score at age 11 captures persisting and unmeasured ADHD problems (included in age 15 ADHD) which remain related to neurocognitive function at age 20.
DISCUSSION
The results of this study suggest that not only does the adolescence development of physical aggression (decrease in levels) and theft (increase in levels) differ, but these behaviors also relate differently to neurocognitive capacities and to levels of ADHD. Specifically, physical aggression associated to poor verbal IQ and low verbal short-term memory skills. Previous research [Walsh, 1987] had shown similar effects of general IQ with property crime, but without information on the specific neurocognitive functions involved. In this study, the neurocognitive profile for theft was opposite to that for aggression; verbal abilities and verbal short-term memory were positively linked to initial levels of theft. However, increases in theft over time were negatively related to conditional associative learning, an important regulatory ability that is likely to reflect trial-and-error learning abilities.
In general, there is support for neurocognitive impairments being characteristic of CD and antisocial behavior even after controlling for ADHD [Giancola et al., 1998; Raine et al., 2005; Séguin et al., 2004]. We suggest that this may be specifically for physically aggressive CD behaviors; that is, ADHD symptoms shared variance with only half of the neurocognitive tests that had previously related to physical aggression, but not to theft. This contrasts somewhat with one study that found a relation of ADHD with both aggressive and nonaggressive CD in girls [Giancola et al., 1998]. However, it was unclear in that study if ADHD was related to theft among nonaggressive CD behaviors as theft had not been specifically examined.
The negative relation of CAS with the average increase in theft across adolescence is consistent with the idea that brain maturation of top-down control systems during adolescence is related to increased regulation over the limbic reward systems that give rise to impulsivity and impaired decision making [Casey et al., 2008; Hare et al., 2008]. In other words, the more one is likely to show increases in theft across adolescence, the less one may have developed an ability to learn from trial and error as measured at age 20. The observation that decreases in the slope for theft were even more strongly associated with success on the CAS after controlling for impulsivity via our ADHD measure lends further support to that hypothesis.
The commonalities and distinctions between physical aggression, ADHD symptoms and theft may be further elucidated by psycho-social developmental phenotypes. A conceptual framework offered by Fontaine [2006] is considered here. According to Fontaine, certain types of physical aggression and theft can be separated on the basis of (1) the nature of the motivation underlying the conduct problem and (2) the intra- vs. inter-personal origin of the conduct problem. For example, covert antisocial behavior can be characterized as a goal driven behavior (e.g., monetary gain) with an intra-individual origin (internally generated). In contrast, noninstrumental physical aggression, though perhaps sometimes goal-based (e.g., elimination of perceived threat), is not goal driven (e.g., the premeditated elimination of perceived threat). In this example, physical aggression is described as interpersonal or social in nature—an impulsive reaction to (at least the perception of) extra-personal social cues. Hence, certain thefts may be more instrumental in nature and characterized by low levels of neurocognitive impairment. By contrast, noninstrumental physical aggression may be more impulsive and person-oriented, with relatively higher levels of neurocognitive impairment and ADHD. Research on the distinction between instrumental and reactive aggression is consistent with this framework. For example, in one study reactive aggression was found to be negatively related to executive control [Giancola et al., 1996]. More recently another study revealed a moderating effect of hostile attributional bias on the relation between executive function and proactive and reactive aggression [Ellis et al., 2009].
The present results should be interpreted in the context of seven main limitations. First, our results do not speak to the joint development of neurocognition and behavior problems, but to the neurocognitive function of young adults and their histories of physical aggression and theft. To understand these developmental processes, future research should include assessments that begin earlier in the life course [Séguin et al., 2009] and that are collected repeatedly, along with the assessments of aggression and theft. Such a design can better assess the reciprocal influences of neurocognition and behaviors across development. Second, although ADHD served as a proxy control for impulsivity and reward sensitivity, tasks sensitive to limbic reward systems need to be specifically contrasted with tasks sensitive to top-down control systems. A more comprehensive test battery could therefore include affectively sensitive regulatory tasks [Nigg, 2005; Séguin et al., 2007a,b], as well as measures of top-down inhibitory control, planning, and nonverbal short term-memory. Third, imaging studies of adolescents engaging in problem solving are needed to validate the actual neural circuitry related to physical aggression, theft and ADHD. Fourth, like others [Martel et al., 2007], we have used a total symptom score for ADHD. Some studies find that the inattentive, hyperactive and impulsive subtypes may show different neurocognitive profiles [Nigg, 2005; Solanto et al., 2007]. Thus, a research design examining simultaneously CD and ADHD subtypes in clinical samples may be warranted. A symptom count, which we found to be highly sensitive to neurocognition, is likely appropriate for a nonclinical sample because it may best reflect the ADHD combined subtype which is thought to be the most sensitive to neurocognition [Willcutt et al., 2005]. Fifth, although CD girls show neurocognitive impairments [Pajer et al., 2008], it is unclear if our findings for theft could be generalized across genders [Fontaine et al., 2009]. However, in a study of conduct disordered adolescent females, executive control (based on tests sensitive to frontal cortical lesions) was specifically related to aggressive CD but not to nonaggressive CD symptoms, even after controlling for ADHD symptoms [Giancola et al., 1998]. Although that test battery might have been sensitive to theft, executive control tasks were aggregated, and theft scores were aggregated within nonaggressive CD behaviors. Sixth, although the present sample consisted of at-risk males from low SES neighborhoods, the findings appear to generalize across SES levels [Barker et al., 2007], and across clinical and nonclinical populations [Walsh, 1987]. Seventh, the measures of physical aggression and theft were based on self reports, raising the possibility of shared method variance. Future studies should incorporate multiple informants in the assessment of these two types of ASB.
In summary, there is growing evidence that histories of physical aggression and theft relate differently to neurocognitive abilities. Although ADHD accounted for some of the relationships between physical aggression and neurocognition, the relationships between theft and neurocognition were unaffected. These findings have implications for research and clinical practice. With regard to research, developmental continuity of theft needs closer examination, particularly for youth without neurocognitive impairment. For such youth, the adult expression of ASB and related cost burden to society and individuals is largely unknown. From a practical standpoint, such a research question is not without difficulty; childhood assessments of theft (as opposed to adolescent or adult) rely largely on mother and teacher reports, and children with sophisticated neurocognition may be adept at escaping detection [Fontaine et al., 2008]. Hence more deceptive children may be underrepresented at assessment. With regard to clinical practice, the present results suggest that diagnostic or screening devices based on aggregate histories of different CD behaviors may confuse youth who may require different types of interventions [Tremblay, 2000]. Experimental preventive and corrective interventions should examine if youth who engage in different CD subtypes respond differentially to targeted interventions. Interventions that target the motivation and origin underlying the expression of ASB (e.g., both impulsive and instrumental) may be worth investigating [Phillips and Lochman, 2003].
Acknowledgments
Support for this research was provided partly by the Research Grants Committee at the University of Alabama to EDB, by research scientist awards from the Canadian Institutes for Health Research, the Fonds de Recherche en Santé du Québec (FRSQ), and the Molson Foundation, Montréal, Québec, to JRS, by operating grants from the American National Science Foundation (NSF grant SES-9911370), the National Consortium on Violence Research (NCOVR is supported under grant SBR-9513040 from the NSF), and Human Resources and Development Canada, by infrastructure grants from the FRSQ, the Fonds de Recherche sur la Société et la Culture du Québec (2002-RS-79238), the Social Sciences and Humanities Research Council of Canada (412-2000-1003), and the Medical Research Council of the United Kingdom (MRC-G0500953). We express thanks to the boys, their families, and teachers for their long-term commitment to this project; to Hélène Beaumont and the many research assistants; to Mathieu Pilon and Anna Neumann for comments on a previous version of the manuscript; and the Research Unit on Children’s Psychosocial Maladjustment staff. These results were presented in a poster at the Biennial Meeting of the Society for Research in Child Development, April 3rd, 2009, Denver, Colorado.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Quadratic terms were not included in the model because the variance of the latent quadratic terms for physical aggression (Qslope=.001, SE=.001, t=1.12) and theft (Qslope=.001, SE=.003, t=0.40) failed to reach significance. A model with an intercept (baseline levels) and a linear slope term (increases/decreases with age) was used. In Step 2 of the analysis, we regressed the seven neurocognitive tests on the intercepts and slopes of both physical aggression and theft.
References
- Alpherts WCJ, Vermeulen J, van Rijen PC, da Silva FL, van Veelen CWM. Standard versus tailored left temporal lobe resections: Differences in cognitive outcome? Neuropsychologia. 2008;46:455–460. doi: 10.1016/j.neuropsychologia.2007.08.022. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders—Text Revision (DSM-IV-TR) 4. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- Baker SF, Ireland JL. The link between dyslexic traits, executive functioning, impulsivity and social self-esteem among an offender and non-offender sample. International J Law Psychiatry. 2007;30:492–503. doi: 10.1016/j.ijlp.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Barker ED, Séguin JR, White HR, Bates ME, Lacourse É, Carbonneau R, Tremblay RE. Developmental trajectories of physical violence and theft: Relation to neuro-cognitive performance. Arch Gen Psychiatry. 2007;64:592–599. doi: 10.1001/archpsyc.64.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker ED, Larsson H, Viding E, Maughan B, Rijsdijk F, Fontaine N, Plomin R. Common genetic but specific environmental influences for aggressive and deceitful behaviors in preadolescent males. J Psychopathol Behav Assess. 2009;31:299–308. [Google Scholar]
- Bentler PM, Bonett DG. Significance Tests and Goodness of Fit in the Analysis of Covariance-Structures. Psychol Bull. 1980;88:588–606. [Google Scholar]
- Bongers IL, Koot HM, van der Ende J, Verhulst FC. Developmental trajectories of externalizing behaviors in childhood and adolescence. Child Dev. 2004;75:1523–1537. doi: 10.1111/j.1467-8624.2004.00755.x. [DOI] [PubMed] [Google Scholar]
- Breton JJ, Bergeron L, Valla JP, Berthiaume C, St-Georges M. Diagnostic interview schedule for children (DISC-2.25) in Quebec: Reliability findings in light of the MECA study. J Am Acad Child Adolesc Psychiatry. 1998;37:1167–1174. doi: 10.1097/00004583-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newburry Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Burt C. The Young Delinquent. 4. London, England: University of London Press; 1944. [Google Scholar]
- Burt SA. Are there meaningful etiological differences within antisocial behavior? Results of a meta-analysis. Clin Psychol Rev. 2009a;29:163–178. doi: 10.1016/j.cpr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: A meta-analysis of shared environmental influences. Psychol Bull. 2009b;135:608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier JM. É preuve Individuelle d’Habileté Mentale [Individual Tasks of Mental Ability] Montréal, Canada: Institut de Recherches Psychologiques; 1989. [Google Scholar]
- Colom R, Jung RE, Haier RJ. Distributed brain sites for the g-factor of intelligence. Neuroimage. 2006;31:1359–1365. doi: 10.1016/j.neuroimage.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Craig WM, Vitaro F, Gagnon C, Tremblay RE. The road to gang membership: Characteristics of male gang and nongang members from ages 10 to 14. Soc Dev. 2002;11:53–68. [Google Scholar]
- Déry M, Toupin J, Pauzé R, Mercier H, Fortin L. Neuropsychological characteristics of adolescents with conduct disorder: Association with Attention-Deficit-Hyperactivity and aggression. J Abnorm Child Psychol. 1999;27:225–236. doi: 10.1023/a:1021904523912. [DOI] [PubMed] [Google Scholar]
- Diamantopoulou S, Rydell AM, Thorell LB, Bohlin G. Impact of executive functioning and symptoms of attention deficit hyperactivity disorder on children’s peer relations and school performance. Dev Neuropsychol. 2007;32:521–542. doi: 10.1080/87565640701360981. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Elmslie H. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Ellis ML, Weiss B, Lochman JE. Executive functions in children: Associations with aggressive behavior and appraisal processing. J Abnorm Child Psychol. 2009;37:945–956. doi: 10.1007/s10802-009-9321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SHM, Stollery SJ, Aitken MRF, Savage J, Moore SC, Goodyer IM. Decision making and executive function in male adolescents with early-onset or adolescence-onset Conduct Disorder and control subjects. Biol Psychiatry. 2009;66:162–168. doi: 10.1016/j.biopsych.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine RG. Evaluative behavioral judgments and instrumental antisocial behaviors in children and adolescents. Clin Psychol Rev. 2006;26:956–967. doi: 10.1016/j.cpr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Fontaine N, Barker ED, Salekin RT, Viding E. Dimensions of psychopathy and their relationships to cognitive functioning in children. J Clin Child Adolesc Psychol. 2008;37:690–696. doi: 10.1080/15374410802148111. [DOI] [PubMed] [Google Scholar]
- Fontaine N, Carbonneau R, Vitaro F, Barker ED, Tremblay RE. Research review: A critical review of studies on the developmental trajectories of antisocial behavior in females. J Child Psychol Psychiatry. 2009;50:363–385. doi: 10.1111/j.1469-7610.2008.01949.x. [DOI] [PubMed] [Google Scholar]
- Gath D, Tennent G. High intelligence and delinquency: A review. Br J Criminol. 1972;12:174–181. [Google Scholar]
- Giancola PR, Moss HB, Martin CS, Kirisci L, Tarter RE. Executive cognitive functioning predicts reactive aggression in boys at high risk for substance abuse: A prospective study. Alcohol Clin Exp Res. 1996;20:740–744. doi: 10.1111/j.1530-0277.1996.tb01680.x. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, Tarter RE. Executive cognitive functioning, temperament, and antisocial behavior in conduct-disordered adolescent females. J Abnorm Psychol. 1998;107:629–641. doi: 10.1037//0021-843x.107.4.629. [DOI] [PubMed] [Google Scholar]
- Hancock M, Tapscott JL, Hoaken PNS. Role of executive dysfunction in predicting frequency and severity of violence. Aggr Behav. 2010;36:338–349. doi: 10.1002/ab.20353. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoaken PNS, Allaby DB, Earle J. Executive cognitive functioning and the recognition of facial expressions of emotion in incarcerated violent offenders, non-violent offenders, and controls. Aggr Behav. 2007;33:412–421. doi: 10.1002/ab.20194. [DOI] [PubMed] [Google Scholar]
- Lacourse É, Côté S, Nagin DS, Vitaro F, Brendgen M, Tremblay RE. A longitudinal-experimental approach to testing theories of antisocial behavior development. Dev Psychopathol. 2002;14:911–926. doi: 10.1017/s0954579402004121. [DOI] [PubMed] [Google Scholar]
- LeBlanc M, Fréchette M. Male Criminal Activity From Childhood Through Youth: Multilevel and Developmental Perspective. New York, NY: Spinger; 1989. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annu Rev Psychol. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Loeber R, Stouthamer-Loeber M. Development of juvenile aggression and violence: Some common misconceptions and controversies. Am Psychol. 1998;53:242–259. doi: 10.1037//0003-066x.53.2.242. [DOI] [PubMed] [Google Scholar]
- Loeber R, Wung P, Keenan K, Giroux B, Stouthamer-Loeber M, Van Kammen WB, Maughan B. Developmental pathways in disruptive child behavior. Dev Psychopathol. 1993;5:103–133. [Google Scholar]
- Loeber R, Burke JD, Lahey BB, Winters A, Zera M. Oppositional defiant and conduct disorder: A review of the past 10 years, Part I. J Am Acad Child Adolesc Psychiatry. 2000;39:1468–1484. doi: 10.1097/00004583-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Martel M, Nikolas M, Nigg JT. Executive function in adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2007;46:1437–1444. doi: 10.1097/chi.0b013e31814cf953. [DOI] [PubMed] [Google Scholar]
- McCord W, McCord J, Zola IK. Origins of Crime: A New Evaluation of the Cambridge-Somerville Youth Study. New York: Columbia University Press; 1959. [Google Scholar]
- Merrill MA. Problems of Child Delinquency. London: Harrap & Co; 1947. [Google Scholar]
- Moffitt TE. The neuropsychology of conduct disorder. Dev Psychopathol. 1993;5:135–151. [Google Scholar]
- Moffitt TE, Caspi A, Harrington HL, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: Follow-up at age 26 years. Dev Psychopathol. 2002;14:179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Jaffee SR, Kim-Cohen J, Koenen KC, Odgers CL, Slutske WS, Viding E. Research Review: DSM-V conduct disorder: Research needs for an evidence base. J Child Psychol Psychiatry. 2008;49:3–33. doi: 10.1111/j.1469-7610.2007.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuteaux MC, Biederman J, Doyle AE, Mick E, Faraone SV. Genetic risk for conduct disorder symptom subtypes in an ADHD sample: Specificity to aggressive symptoms. J Am Acad Child Adolesc Psychiatry. 2009;48:757–764. doi: 10.1097/CHI.0b013e3181a5661b. [DOI] [PubMed] [Google Scholar]
- Moore PM, Baker GA. Validation of the Wechsler memory scale-revised in a sample of people with intractable temporal lobe epilepsy. Epilepsia. 1996;37:1215–1220. doi: 10.1111/j.1528-1157.1996.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clin Psychol Rev. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24:882–891. [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: The state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Corina DP, Corrigan N, Schoenfield-McNeill J, Poliakov A, Zamora L, Zanos S. Neuronal correlates of functional magnetic resonance imaging in human temporal cortex. Brain. 2010;133:46–59. doi: 10.1093/brain/awp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajer K, Chung J, Leininger L, Wang W, Gardner W, Yeates K. Neuropsychological function in adolescent girls with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:416–425. doi: 10.1097/CHI.0b013e3181640828. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Deficits on conditional associative-learning tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1985;23:601–614. doi: 10.1016/0028-3932(85)90062-4. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci USA. 1993a;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA. 1993b;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proc Natl Acad Sci USA. 1995;92:5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips NC, Lochman JE. Experimentally manipulated change in children’s proactive and reactive aggressive behavior. Aggr Behav. 2003;29:215–227. [Google Scholar]
- Raine A, Moffitt TE, Caspi A, Loeber R, Stouthamer-Loeber M, Lynam DR. Neurocognitive impairments in boys on the life-course persistent antisocial path. J Abnorm Psychol. 2005;114:38–49. doi: 10.1037/0021-843X.114.1.38. [DOI] [PubMed] [Google Scholar]
- Romano E, Baillargeon RH, Wu HX, Zoccolillo M, Vitaro F, Tremblay RE. A new look at inter-informant agreement on conduct disorder using a latent class approach. Psychiatry Res. 2004;129:75–89. doi: 10.1016/j.psychres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Satorra A. Scaled and adjusted restricted tests in multi-sample analysis of moment structures. In: Heijmans RDH, Pollock DSG, Satorra A, editors. Innovations in Multivariate Statistical Analysis. London: Kluwer Academic Publishers; 2000. pp. 233–247. [Google Scholar]
- Séguin JR, Nagin DS, Assaad JM, Tremblay RE. Cognitive-neuropsychological function in chronic physical aggression and hyperactivity. J Abnorm Psychol. 2004;113:603–613. doi: 10.1037/0021-843X.113.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguin JR, Arseneault L, Tremblay RE. The contribution of “Cool” and “Hot” components of executive function to problem solving in adolescence: Implications for developmental psychopathology. Cogn Dev. 2007a;22:530–543. [Google Scholar]
- Séguin JR, Sylvers P, Lilienfeld SO. The neuropsychology of violence. In: Waldman ID, Flannery DJ, Vazsonyi AT, editors. The Cambridge Handbook of Violent Behavior and Aggression. New York: Cambridge University Press; 2007b. pp. 187–214. [Google Scholar]
- Séguin JR, Parent S, Tremblay RE, Zelazo PD. Different neurocognitive functions regulate physical aggression and hyperactivity in early childhood. J Child Psychol Psychiatry. 2009;50:679–687. doi: 10.1111/j.1469-7610.2008.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Gilbert SN, Raj A, Zhu J, Pope-Boyd S, Stepak B, Vail L, Newcorn JH. Neurocognitive functioning in AD/HD, predominantly inattentive and combined subtypes. J Abnorm Child Psychol. 2007;35:729–744. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL, Krueger RF, Iacono WG, McGue M. Symptombased subfactors of DSM-defined conduct disorder: Evidence for etiologic distinctions. J Abnorm Psychol. 2005;114:483–487. doi: 10.1037/0021-843X.114.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennent G, Gath D. Bright delinquents: A three-year follow-up study. Br J Criminol. 1975;15:386–390. [Google Scholar]
- Tremblay RE. The development of aggressive behaviour during childhood: What have we learned in the past century? Int J Behav Dev. 2000;24:129–141. [Google Scholar]
- Tremblay RE. Developmental origins of disruptive behaviour problems: The “original sin” hypothesis, epigenetics and their consequences for prevention. J Child Psychol Psychiatry. 2010;51:341–367. doi: 10.1111/j.1469-7610.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- van Lier PAC, Vitaro F, Barker ED, Koot HM, Tremblay RE. Developmental links between trajectories of physical violence, vandalism, theft, and substance use from childhood to adolescence. J Abnorm Child Psychol. 2009;37:481–492. doi: 10.1007/s10802-008-9289-6. [DOI] [PubMed] [Google Scholar]
- Walsh A. Cognitive functioning and delinquency: Property versus violent offenses. Int J Offender Ther Comp Criminol. 1987;31:285–289. [Google Scholar]
- Waschbusch DA. A meta-analytic examination of comorbid hyperactive—impulsive—problems and conduct problems. Psychol Bull. 2002;128:118–150. doi: 10.1037/0033-2909.128.1.118. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-III. Toronto: The Psychological Corporation: Harcourt, Brace & Company; 1997. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]