Abstract
Background
The Brown Marmorated Stink Bug (BMSB), Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), native to Asia, is becoming an invasive species with a rapidly expanding range in North America and Europe. In the US, it is a household pest and also caused unprecedented damage to agriculture crops. Exploring its climatic limits and estimating its potential geographic distribution can provide critical information for management strategies.
Methodology/Principals
We used direct climate comparisons to explore the climatic niche occupied by native and invasive populations of BMSB. Ecological niche modelings based on the native range were used to anticipate the potential distribution of BMSB worldwide. Conversely, niche models based on the introduced range were used to locate the original invasive propagates in Asia. Areas with high invasion potential were identified by two niche modeling algorithms (i.e., Maxent and GARP).
Conclusions/Significance
Reduced dimensionality of environmental space improves native model transferability in the invade area. Projecting models from invasive population back to native distributional areas offers valuable information on the potential source regions of the invasive populations. Our models anticipated successfully the current disjunct distribution of BMSB in the US. The original propagates are hypothesized to have come from northern Japan or western Korea. High climate suitable areas at risk of invasion include latitudes between 30°–50° including northern Europe, northeastern North America, southern Australia and the North Island of New Zealand. Angola in Africa and Uruguay in South America also showed high climate suitability.
Introduction
The rapid spread of invasive species that has accompanied globalization has threatened native biodiversity worldwide, and has also resulted in great economic losses [1]. As international trade increases, numbers of both accidental and intentional exotic introductions are increasing. Indeed, biological invasions have become the second most important cause of current biodiversity loss, after habitat destruction [2]. Identification of areas environmentally suitable for invasive species can offer great opportunities for preventing or slowing invasions. Recently, ecological niche modeling (ENM) has been widely used to identify the potential distributions of species [1], [3]–[5]. Based on occurrence data and environmental data sets, the ENM seeks to characterize environmental conditions suitable for the species, and then identify where those suitable environments are distributed spatially [6].
ENM analyses must be designed carefully, to reflect the fact that species' distribution manifests a complex interplay of abiotic factors, biotic factors, and dispersal constraints, that together determine distributional limits [7]–[9]. The ecological niche of a species as used herein is the set of environmental conditions under which the species can maintain populations without immigrational subsidy [10], [11]. Some recent studies have questioned the key assumption of niche conservatism during species' invasion (e.g., [12]–[14]). However, such conclusion appear artifactual [15], as they confuse differential representation of portions of the ecological niche (i.e., different “existing fundamental ecological niches” in different landscapes [16]) with genuine, evolved ecological niche difference [17]. When analyses are designed with these caveats in mind, coincidence between reciprocal prediction among native and introduced distributional areas improves markedly [17].
The Brown Marmorated Stink Bug (BMSB) Halyomorpha halys (Stål, 1855) (Hemiptera: Pentatomidae), is native to North and South Korea, Japan, and China. This species is becoming an invasive species showing rapid spreading in North America and Europe. The first record in America was reported in Allentown, Pennsylvania in 1996 [18]. Since then, the species has expanded its range dramatically in east America [19]. In 2005, it was reported in Vallejo, Solano County, California, facilitated by a new resident who had relocated from Pennsylvania [19]. In 2008, the species was first reported in Europe near Zürich, Switzerland [20]–[22]. By 2010, an individual was found in South Dunedin, New Zealand, apparently introduced via a used vehicle shipped from Tokyo, Japan [23]. Recently, researchers have focused on the spreading, life history and phenology, and possible control strategies for BMSB in North America [24]–[30].
In its native range, BMSB is a fruit-piercing stink bug that causes extensive damage to various fruits and soybeans, it has recently become a serious pest of apples in Japan [31]. In the US, BMSB not only represents a household pest, where it seeks winter retreats and releases unpleasant smells from stink glands when disturbed, but also has become a pest of almost unprecedented importance to agriculture, particularly in the mid-Atlantic region. Crops affected include orchard crops, vegetables, grapes, other small fruits, row crops, ornamentals, and nursery crops [32].
In this study, we explored several methods that were applied in recent studies on invasive species: climate space comparisons [33], [34], modified component space comparisons [12]–[14] and niche modeling [35]. Recent studies have suggested using pooled native and introduced distributional data to produce a consensus model of distributional potential [36]. However, this approach does not allow any independent test of model robustness, and if the ecological niche has shifted or expanded during the invasion, the pooled niche model would be overly broad to predict the distributional potential [17]. Here, we first compared niche space occupied by native and invasive BMSB populations, then evaluated native niche model transferability based two variable sets in the invade region. In the end, niche model based on the introduced range were used to locate the source region of invasion, classical niche modeling approach (i.e., Native-to-introduced ecological niche modeling) were used to explore areas of potential invasion [37].
Methods
Occurrence data
We assembled 552 occurrence localities of BMSB, including, localities from mainland China from the literatures and specimens records in the Institute of Entomology at Nankai University, localities from Taiwan were obtained from the Taiwan e-Learning & Digital Archives Program (http://culture.teldap.tw/culture/), localities from European from Wermelinger et al. and Wyniger and Kment [20], [22], localities from Japan and South Korea from Global Biodiversity Information Facility (http://www.gbif.org/). All of these occurrences were manifested as points of latitude and longitude. In the US, however, data were counties of known occurrence from the U. S. Department of Agriculture Animal and Plant Health Inspection Service [24], we converted these records to points by digitizing the centroid of each positive county in Arc GIS 9.2 [38] following recent suggestions [13], [14]. Localities lacking geographic coordinates were georeferenced using Google Maps, Gazetteer of China [39] or BioGeomancer (http://bg.berkeley.edu/latest/). Records with unspecified or unknown localities were deleted. The native range points covered the full known geographic range of BMSB except for North Korea due to inaccessibility of distributional data.
The native 383 occurrence points varied in spatial density due to variable sampling intensity over geography. As a result, and to avoid overemphasizing heavily on sampled area, we selected points for model calibration using a subsampling regime to reduce sampling bias and spatial autocorrelation. Following Nuñez and Medley [40], we generated models using all available occurrence points and measured spatial autocorrelation among model pseudo-residuals (1 – probability of occurrence generated by model) by calculating Moran's I at multiple distance classes using SAM v4.0 [41]. Significance was determined using permutation tests. A minimum distance of 335 km was detected, so we created a grid with cell dimensions of 3×3° and selected the occurrence point that close to the centroid of each grid cell. This procedure reduced the number of occurrences to 95 points used for model calibration, leaving the remaining points used for model testing. The procedure greatly reduced sampling bias and spatial autocorrelation, resulting in evenly distributed occurrence points across space [40].
Environmental variables
Environmental dimensions in which to characterize ecological niches were selected by considering the climate, topography, habitats, and human impacts that might potentially affect BMSB distribution [31], [42]–[44]. We chose bioclimatic variables representing annual trends and extreme or limiting conditions, because many taxa are limited by environmental extremes. Variables that were highly correlated were excluded from our selection, leaving six variables summarizing aspect of temperature and precipitation that were derived from the WorldClim database [45] and three variables summarizing aspect of solar from the CliMod [46]. Topography variable represented by elevation data were also obtained from the WorldClim database. All dimensions were set at a spatial resolution of 2.5 arc-min for analysis.
Previous studies have demonstrated that using simpler and less dimensional environmental data sets improves model projections among major distributional areas [15], [17], [33]. The GLC and NDVI are global land cover types and normalized difference vegetation index respectively, these variables might have a relationship with BMSB distributions. The human footprint index is a composite summary of human influence on land surfaces, and is well known to facilitate species invasions [1]. We considered protocols of Liu et al. [1] and initially incorporated these variables into our model, however, although their incorporation improved model prediction on the native range, it did not improve model projections onto other regions. In the end, we used two sets of bioclimatic variables only, to show the reduced dimensionality effect on spatial predictions for BMSB. We first used ten bioclimatic variables representing the annual trends and extreme environmental factors of temperature, precipitation and sunshine that might impact the distribution of BMSB (Table 1). Since temperature and sunshine are two major factors that impact BMSB's distribution [31], [42], [43], and the sunshine can be related as another mean of temperature, we reduced the dimension by excluding the BIO 13, 14 and BIO 20, 21 (Table 1).
Table 1. Principal components analysis (PCA) of bioclimatic variables associated with occurrence of BMSB.
| Factor Loading | ||||
| Variables | Description | PC-1 | PC-2 | PC-3 |
| *BIO1 | Annual mean temperature | 0.93 | 0.14 | 0.22 |
| *BIO5 | Maximum temperature of warmest month | 0.45 | 0.48 | 0.56 |
| *BIO6 | Minimum temperature of coldest month | 0.92 | −0.19 | 0.12 |
| *BIO12 | Annual precipitation | 0.66 | −0.51 | 0.18 |
| BIO13 | Precipitation of wettest month | 0.70 | 0.07 | −0.12 |
| BIO14 | Precipitation of driest month | −0.08 | −0.69 | 0.56 |
| *BIO20 | Annual mean radiation | −0.10 | 0.88 | 0.11 |
| BIO21 | Highest weekly radiation | −0.74 | 0.35 | 0.35 |
| BIO22 | Lowest weekly radiation | 0.65 | 0.66 | −0.24 |
| *DEM | Elevation | 0.14 | −0.15 | −0.92 |
| Eigenvalue | 3.84 | 2.38 | 1.76 | |
| Percentage variance | 38.42 | 23.79 | 17.64 | |
| Cumulative percentage variance | 38.42 | 62.20 | 79.84 | |
*indicate the variables used in the final model construction.
Eigenvalues for the most important variables (>0.8) in PCA are in bold.
Direct climate comparisons and Principal component analysis (PCA)
We compared climate space occupied by native and introduced populations using direct climate comparisons and principal component analysis (PCA) before ecological niche modeling, as these methods allows for quick assessment of the relative positions of populations in climate space [33], [34]. We superimposed occurrence data on the bioclimatic grids, and extracted the climate value for each occurrence using ArcGIS 9.2 [38]. The ten variables occupied by native and introduced populations were compared visually in boxplots, and statistically tested using independent sample test in SPSS 19. PCA on the correlation matrix was used to reduce dimensionality further. To facilitate data visualization among continents, occurrence points were pooled for the native Asian region (383 points), the invaded region in North America (161 points) and the invaded region in Europe (8 points).
Modeling approach
All models were developed using a maximum entropy algorithm implemented in Maxent (version 3. 3. 3a) [47]–[49]. In exploring areas of potential invasion, another algorithm (i.e., GARP) was used (see below). Maximum entropy is a machine-learning technique that predicts species' distribution by integrating detailed environmental variables with species occurrence data. It follows the principle of maximum entropy and spreads out probability as uniformly as possible, but subject the caveat that they must match empirical information such as known presence [48]. The models were developed using the linear, quadratic, and hinge functions to avoid problem of over-fitting [49], [50]. A jack-knife procedure was used to evaluate the relative importance of each predictor variable in the model [51].
Two variable sets comparison
To evaluate niche model predictability based on two variable sets, our models were built on native range by using occurrence points and environmental data clipped to the appropriate size then transferring them onto the US (not include Hawaii and Alaska). We used the reduced native occurrence points with an enforced distance from one another to calibrate model, leaving the remaining native occurrence data for native model evaluation. When projecting onto the US, the invasive records in the US were used for model transferability evaluation. To better exhibit the result, the logistic output of Maxent with suitability values ranging from 0 (unsuitable habitat) to 1 (optimal habitat) was used. Logistic output gives an estimate of probability of presence, it estimates probability of presence assuming that the sampling design is such that typical presence localities have probability of presence of about 0.5 [48], [49].
We used the Area Under Curve (AUC) of Receiver Operating Characteristic (ROC) plot and binary omission rate for our model comparison and evaluation. AUC is a composite measure of model performance and weights the omission error (predicted absence in areas of actual presence) and commission error (predicted presence in areas of actual absence) equally. AUC values range from 0 to 1, where 1 is a perfect fit. Useful models produce AUC values of 0.7–0.9, and models with ‘good discriminating ability’ produce AUC values above 0.9 [52]. The “area under the curve” (AUC) of the ROC plot is a threshold-independent measure of model accuracy, which juxtaposes correct and incorrect predictions over a range of thresholds. Omission rates weights mainly on omission error, our binary omission rates were calculated by the proportion of test points that were not predicted at a threshold. We plotted omission rate across the threshold spectrum of Maxent, specifically, we calculated omission rate at the increasing rate of 0.05 degrees against the total 1.0 logistic output.
Locating source region of invasion
Although BMSB is expanding its range in the US and is far from equilibrium in Europe (i.e., not inhabiting the entire habitable area), we tentatively used “retro ecological niche modeling” approach to search the matching climate spaces occupied both by native and introduced populations and to predict its spatial distribution in Asia. These models were effectively built using invasive occurrence records and transferred onto the potential native area allow to hypothesize source regions for the invasion. We set aside 25% of these points for binary omission rates test, the remaining were used to run a ten cross-validation replicates to get a more robust result in Maxent. Six variable data set was used (Table 1). Data splitting outside (25/75) and then inside (50/50) Maxent reduced sample bias and spatial autocorrelation greatly. Cross-validation also has one big advantage over using a single training/test split that it uses all of the data for the validation [48], [49]. The cumulative output of Maxent with suitability values ranging from 0 (unsuitable habitat) to 100 (optimal habitat) was used. Cumulative output gives a prediction of suitable condition for the species above a threshold, depending on the level of predicted omission that is acceptable [48], [49]. We used the standard deviation of AUC values in ten replicates and omission rates at threshold of M10 for model evaluation. The M10 threshold assumed that a grid cell was suitable if its suitability score was more than 10, which has been suggested as an appropriate threshold [51]. We also calculated the omission rate of native occurrence to evaluate the retro model transferability.
Exploring areas of potential invasion
To explore areas of potential invasion globally, the six variable data set was used (Table 1). We calibrated models based on native range, and transferred their prediction onto the other continents. Considering that the record in the US does not characterize the actual distribution, and the sample bias in native Asia, we used 95 occurrences of the reduced native sample for model calibration. Maxent model was first run using logistic output then rerun using cumulative output. For model evaluation, we calculated binary omission rate of the remaining occurrence at the threshold of M10. Although Maxent has appeared superior to GARP in some previous studies [4], carefully assessments of model quality showed no significant differences between the two [50]. Recent studies suggested using multiple algorithms to infer a consensus estimate of niche dimensions [51], [53]–[56]. Hence, we further used the Genetic Algorithm for Rule-set Prediction (GARP, [57]) to explore areas of potential invasion (Text S1).
Results
Direct climate comparisons
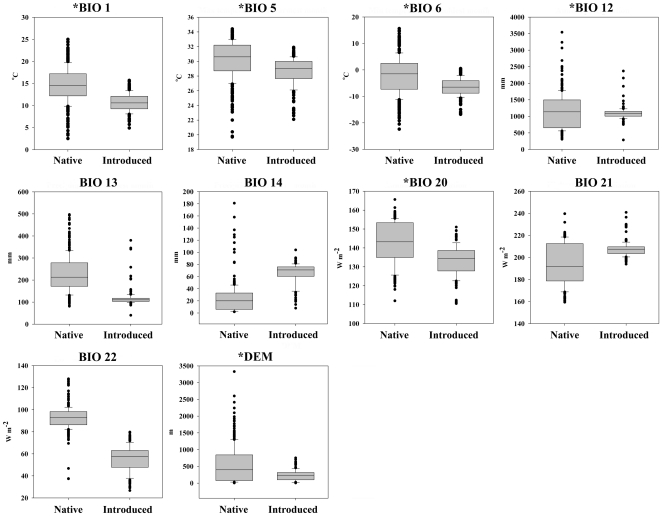
Figure 1 summarized 10 climatic dimensions and their ranges among native and invasive populations of BMSB. The extreme values for the invasive population fell well within that of the native population, with the exception of precipitation in wettest month (BIO 13) and lowest weekly radiation (BIO 22), for which some invasive records fell beyond the lowest values observed on the corresponding native range (Figure 1). Introduced population occurred in areas with lower annual mean temperatures (BIO 1), lower maximum temperature of warmest month (BIO 5), lower minimum temperature of coldest month (BIO 6), lower precipitation of wettest month, lower annual mean radiation (BIO 20), lower lowest weekly radiation (BIO 22), lower elevation (DEM), and higher precipitation of driest month (BIO 14) and higher highest weekly radiation (BIO 21) (p<0.001). The mean annual precipitation (BIO 12) was nearly equal between native and invasive populations (Figure 1). Since BMSB is still expanding its range, incorporation of newly established populations in invaded regions might change the pattern of their distribution in climate space.
Figure 1. Direct comparison of BMSB occurrence-associated variables between native and introduced distributional areas.
Asterisk (*) indicate variables used in the final model calibration.
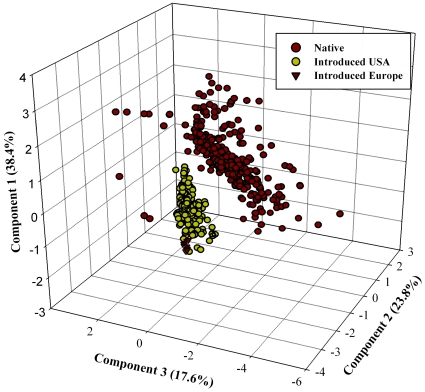
Principal component analysis of the climatic data defined a climate space of reduced dimensionality that allows investigation of niche conservatism and differentiation (Figure 2). The first three components of the PCA were significant, and together explained 79.8% of the overall variance. The first component (PC-1) was closely associated with temperature while the second (PC-2) and third components (PC-3) were associated with radiation and elevation respectively (Table 1). The climate space occupied by invasive records departed from that occupied by native records with respect to component 1 and 2, but not component 3 (Figure 2). The climate space occupied by US records shifted principally along component 1, while the European records shifted along both components 1 and 2 (Figure 2). The shifting positions of invasive records in climate space might suggest that the species is undergoing change in tolerance or even niche differentiation during the invasion process. However, many alternative explanations exist: in particular, the full dimension of ecological niches may not be observed on a given range, such that these niche “differences” may rather reflect the different portions of the scare fundamental niche that are manifested on native-range versus invasive-range areas. In addition, the importance of environmental conditions can vary greatly across short distances, suggesting that the resolution of existing global environmental data sets may be too coarse to accurately describe the species' ecological niche [34].
Figure 2. Principal component analysis of 10 variables associated with occurrences of BMSB.
Symbols represent BMSB occurrences in native areas in Asia and introduced areas in the US and Europe.
Environmental data sets and model comparisons
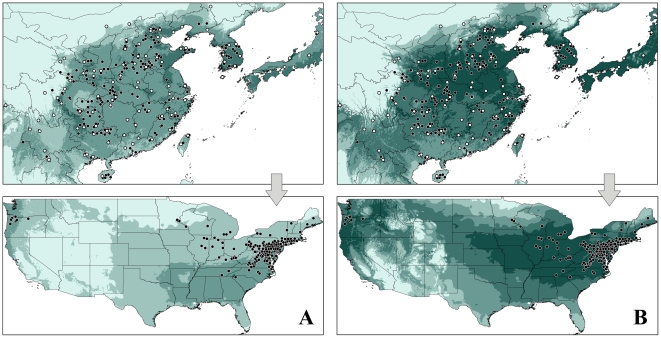
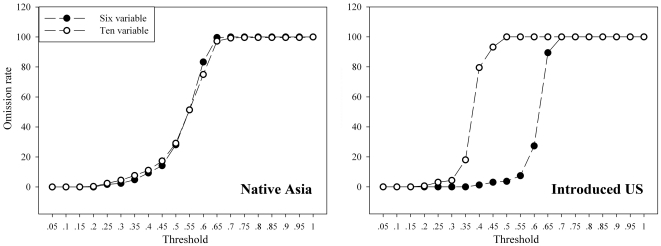
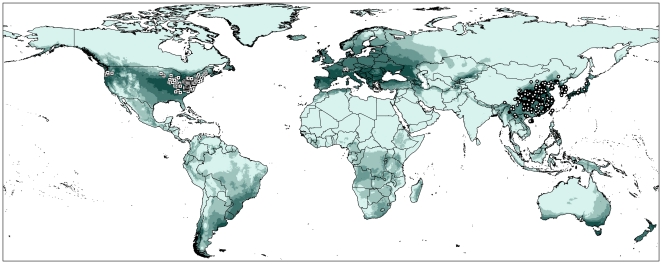
Comparing the two environmental data sets, one highly dimensional and the other simple, the simpler data set showed greatly improved model projection onto North America (Six variable: AUC = 0.894 VS Ten variables: AUC = 0.791, Figure 3), although some detail in anticipating the native range was sacrificed (Six variable: AUC = 0.765 VS Ten variables: AUC = 0.782, Figure 3). In omission rate test, no difference was observed in native model prediction based on six and ten variables, however, when transferring onto the US, model based on six variables with omission rate decreased at threshold of 0.15 to 0.7 comparing to that based on ten variables (Figure 4). Indeed, models based on both environmental data sets showed good discriminating ability compared to random prediction (Figure 3). Both model transferrings also successfully identified the current disjunct distribution of BMSM in the US, which suggests the western areas of Oregon, California and Washington state possess a similar climate space with northeast America. In six variables based model, the three west states, the northeast states, and the middle states including Kansas, Nebraska, Iowa, Missouri, Illinois, Indiana, Kentucky, Tennessee, Wisconsin, Ohio, West Virginia showed high suitability for BMSB. Preventing strategy should pay more attention to these areas to slow down the current rapid spreading.
Figure 3. Niche models based on reduced native records and projected onto the US using Maxent.
Dark green color represents high suitability, light green indicates low suitability. A: using 10 variables, B: using 6 variables, white and black dots represent the 95 occurrences for model calibration and the remaining for model evaluation.
Figure 4. Omission rate comparison between the six- and ten-variable based models.
Omission rates were plotted in native Asia models and their transferring in the US across the threshold spectrum of Maxent.
Locating source regions
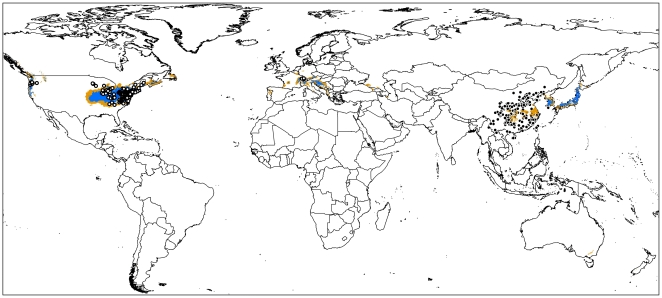
The standard deviation of 0.003 across ten crossvalidate replicates in Maxent suggests high coindence among replicate models. The omission rate at M10 was 4.65% in invaded range suggesting good model calibration, when transferring onto native areas and using native records as test data, the corresponding omission rate reached 88.68% indicating poor model transferability. However, projection of the model onto Asia identified areas of matching climate space (Figure 5): parts of Honshu in Japan, western South Korea showed high suitability of climate space that matching the introduced population. Coincidence between phylogenetic study and ecological niche modeling would provided richer evidence, but this pattern is at least interesting [13], [55], [58], [59]. The low retro model transferability also suggests the invasive population covers a portion of the climate space occupied by native population, suggesting that the invasive population is not in distributional equilibrium, or that the two distributional areas hold distinct subset of the fundamental niche space.
Figure 5. Niche model based on invasive records and transferred worldwide using Maxent.
White and black dots represent occurrences of BMSB in introduced and native areas repectively, yellow areas indicate predictive probability under M10 threshold, blue areas with probability above M10 in Asia indicate the source region of invasion.
Areas of potential invasion
Maxent model based on the reduced 95 native points omitted 3.06% of the independent test points (total 457 points), suggesting good model performance. Projection of GARP is a little conservative comparing to Maxent (Figure 6, Figure S1). Outside of native-range areas, high suitable climate space identified by both modeling algorithms include the northeastern areas along the Pacific coast and east central states in the US in North America. Elsewhere include Uruguay and areas in southern Brazil and northern Argentina in South America, and areas around the Black Sea and the areas west to its same latitudinal range in Europe. Maxent also identified northern Europe as suitable. Northern Angola and adjacent areas of Congo and Zambia in Africa, the southeastern and southwestern Australia, and much of New Zealand also showed high climate suitability. All the areas mentioned above should pay attention to quarantine and inspection when engaging in interchanges with East Asia.
Figure 6. Niche model based on reduced native records and transferred worldwide using Maxent.
Dark green color represents high suitability, light green indicates low suitability. White circles indicate the 95 occurrences used for model calibration, black dots and white squares represent the remaining native and invasive records used for model evaluation.
Discussion
Niche differentiation
The classical approach to estimating species' invasive potential calibrates models based on native range and then transferring these data onto the invade region [37]. Through assessment of species' ecological requirements and comparisons of climatic properties of native and invasive populations, we can infer the degree of niche conservatism prior to prediction [33], although not without complications. That is, the observed niche shifts may result from genuine shifts in the fundamental niche [60], or the realized niche is a subset of the fundamental niche [61], [62], observed niche difference are only interpretable as reflecting change in the fundamental niche under restrictive circumstance [33]. Specifically, the portions of the fundamental niche that are represented an actual landscape must be interpreted with considerable care.
Our results comparing niche spaces between native and invasive populations can be considered as comparing realized niches. In the US and Europe, BMSB is rapidly spreading and is far from equilibrium, while in the native China, BMSB coexists with predators and competitors, which may impact its distribution to some extent. Certainly, climate features also play a role, as well. Erthesina fullo (Thunberg) and Dolycoris baccarum (L.) are two other stinkbugs that are often reported as serious pests along with BMSB in orchards [63]–[69]. However, BMSB usually acts as the dominant species in the orchard pest community, for example, in gardens in northern Henan Province, BMSB represents about 73% of individuals, while E. fullo and D. baccarum account to about 21% and 7%, respectively [65]. Among natural enemies of BMSB in the Beijing area are 6 parasitoids and 3 predators [70], [71], the dominant natural enemy is the parasitoid Trissolcus halyomorpha Yang, which showed parasitism rates reaching 20%–70% (average 50%), and has been studied as a potential biological control agent [71]. However, T. halyomorpha does not appear to have constrained the native distribution of BMSB, as it parasitizes other stink bugs [70] and competes with other parasites [72]. So far, no effective natural enemy is available for BMSB control in Asia and the US, although many efforts explored possibilities [31].
Dimensionality and model projections
Selection of environmental variables is very important for model calibration. Apart from biological importance that may restrict species' distributions, the resolution, extent of study range [73], and correlation among variables [37] have to be taken into consideration. Comparison of the climatic envelopes occupied by native and invasive populations offers useful information for variable selection prior to the prediction, since niches may be conserved along some environmental axes but not along others [13], [33]. We initially incorporated the GLC, NDVI, and the human footprint index into model calibration. We found that incorporation of these variables indeed improved native model prediction, however, in transferring the model onto the invaded region, the predictive ability varied with dimensionality of the environmental space. We demonstrated statistically this point by using two sets of bioclimatic variables. The reduced dimensionality improved model predictions in invaded areas greatly, although detail was lost in the native range predictions. Hence, we recommend prediction of native actual distributions using more dimensional environmental data sets, but transferring among regions using simpler models, similar recommendations have made by Peterson and Nakazawa [15], Rödder and Lötters [33], and Peterson [17].
Source regions
Invasive species undergoing range expansion are not appropriate for testing niche conservatism by the retro modeling approach, since they haven't reach their equilibrium. However, the retro modeling can identify areas of matching climate space occupied by invasive populations in the native distributional area and potentially help us to identify the source region of invasion. The possible source region identified in our study was in accordance with the North America interception records. During 1973–1987, two interceptions of BMSB were recorded by U. S. Department of Agriculture. One was intercepted in an aircraft from Japan in 1983, and the other in baggage coming from Korea in 1984 (the original identification as Halyomorpha picus F. is a misidentification, as populations from Korea, Japan and China are assignable to H. halys, which is BMSB, H. picus occurs only in tropical south and southeast Asia, see Josifov & Kerzhner [74]). During 1989–1998, USDA listed eight interception records from China, Korea and Japan [18], although the details of province within countries were not known. Population genetic studies could complement these results with lineage information to identify source region much more precision [13], [55], [58], [59].
Areas of potential invasion
Prior to inferring areas of potential invasion, one must keep in mind that the ENMs seek to identify suitable climate space for species, but without consideration of biotic interactions or dispersal ability. Many factors influence successful establishment of non-indigenous species into a community depends on existing species composition and richness, competitors, predators, food availability, human footprint, and climatic similarity, compared with the source areas [33]. Although the area predicted as suitable for a species does not mean that it can necessarily establish populations there, it does offer useful information for detecting areas of potential invasion and spread.
Many invasive species in the US have similar distribution patterns: that is populations in the northeast and a disjunct population in the northwest. Examples include the Japanese beetle (Popillia japonica Newman) and the Europe Chafer (Amphimallon majale (Razoumowsky)) [24]. This pattern reflects the fact that the northwest possesses a climate space similar to the northeast in North America. Our models successfully identified the current disjunct distribution pattern of BMSB in the US (Figures 3, 6), including successful anticipation of the specific counties from Oregon, California and Washington. Because some central states also showed high suitability for BMSB, populations may be able to form a continuous distribution in the US.
The region between latitudes 40° and 50°N in Europe showed high climate suitability, supported both by Maxent and GARP. The result of Maxent is somewhat more liberal compared to GARP, with suitable space extending north to latitude 60°N. The newly established BMSB population in Switzerland must be monitored carefully as a result. Much of New Zealand also showed high climate suitability for BMSB, although BMSB individuals newly discovered in South Dunedin have not as yet established population [23]. Attention should be paid to the high-suitability areas around the world, especially in developed areas with intensive trade activity with Japan, Korea or China must take strict quarantine inspection since commercial interchange might facilitate new invasions.
Supporting Information
Niche model based on reduced native records and transferred worldwide using GARP. Dark green color represents high suitability, light green indicates low suitability. White circles indicate the 95 occurrences used for model calibration, black dots and white squares represent the remaining native and the invasive records used for model evaluation.
(TIF)
GARP Protocol in exploring area of potential invasion.
(DOCX)
Acknowledgments
We thank Prof. Jose Alexandre Felizola Diniz-Filho (Universidade Federal de Goiás, Brasil) for assistance with spatial autocorrelation analysis, Dr. Jin-Tang Dong (Emory University, USA) and Dr. David Redei (Hungarian Natural History Museum, Hungary) for reviewing the manuscript. Special thanks to Prof. A. Townsend Peterson (University of Kansas, USA) for valuable comments on model protocols and linguistic writing.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by a postdoctoral fellowship award to GZ in Nankai University and by the National Education Project in Basic Science for Special Subjects (No. J0630963) and National Natural Science Foundation of China (No. 30725005). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liu X, Guo Z, Ke Z, Wang S, Li Y. Increasing potential risk of a global aquatic invader in Europe in contrast to other continents under future climate change. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018429. doi: 10.1371/journal.pone.0018429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution. 2002;17:164–170. [Google Scholar]
- 3.Araújo MB, Guisan A. Five (or so) challenges for species distribution modelling. Journal of Biogeography. 2006;33:1677–1688. [Google Scholar]
- 4.Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, et al. Novel methods improve prediction of species distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 5.Papes M, Gaubert P. Modelling ecological niches from low numbers of occurrences: assessment of the conservation status of poorly known viverrids (Mammalia: Carnivora) across two continents. Diversity and Distributions. 2007;13:890–902. [Google Scholar]
- 6.Pearson RG. Species' Distribution Modeling for Conservation Educators and Practitioners. Synthesis. 2007. American Museum of Natural History, Available at http://ncep.amnh.org. Accessed 2010 October 10.
- 7.Peterson AT, Soberón J, Sánchez-Cordero V. Conservatism of ecological niches in evolutionary time. Science. 1999;285:1265–1267. doi: 10.1126/science.285.5431.1265. [DOI] [PubMed] [Google Scholar]
- 8.Peterson AT, Ortega-Huerta MA, Bartley J, Sanchez-Cordero V, Soberon J, et al. Future projections for Mexican faunas under global climate change scenarios. Nature. 2002;416:626–629. doi: 10.1038/416626a. [DOI] [PubMed] [Google Scholar]
- 9.Soberón J, Peterson AT. Interpretation of models of fundamental ecological niches and species' distributional areas. Biodiversity Informatics. 2005;2:1–10. [Google Scholar]
- 10.Grinnell J. The niche-relationships of the California thrasher. Auk. 1917;34:427–433. [Google Scholar]
- 11.Grinnell J. Geography and evolution. Ecology. 1924;5:225–229. [Google Scholar]
- 12.Broennimann O, Treier UA, Muller-Scharer H, Thuiller W, Peterson AT, et al. Evidence of climatic niche shift during biological invasion. Ecology Letters. 2007;10:701–709. doi: 10.1111/j.1461-0248.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick MC, Weltzin JF, Sanders NJ, Dunn RR. The biogeography of prediction error: why does the introduced range of the fire ant over-predict its native range? Global Ecology and Biogeography. 2007;16:24–33. [Google Scholar]
- 14.Medley KA. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Global Ecology and Biogeography. 2010;19:122–133. [Google Scholar]
- 15.Peterson AT, Nakazawa Y. Environmental data sets matter in ecological niche modelling: an example with Solenopsis invicta and Solenopsis richteri. Global Ecology and Biogeography. 2008;17:135–144. [Google Scholar]
- 16.Peterson AT, Soberón J, Anderson RP, Pearson RG, Martínez-Meyer E, et al. Ecological niches and geographic distributions: A modeling perspective. 2012. Princeton University Press, Princeton, NJ, USA.
- 17.Peterson AT. Ecological niche conservatism: a time-structured review of evidence. Journal of Biogeography. 2011;38:817–828. [Google Scholar]
- 18.Hoebeke ER, Carter ME. Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proceedings of the Entomological Society of Washington. 2003;105:225–237. [Google Scholar]
- 19.Jones JR, Lambin PL. New county and state records for Tennessee of an exotic pest, Halyomorpha halys (Hemiptera: Pentatomidae), with potential economic and ecological implications. Florida Entomologist. 2009;92:177–179. [Google Scholar]
- 20.Wermelinger B, Wyniger D, Forster B. First records of an invasive bug in Europe: Halyomorpha halys (Stål) (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Mitteilungen der Schweizerischen Entomologischen Gesellschaft. 2008;81:1–8. [Google Scholar]
- 21.Rabitsch W. Alien true bugs of Europe (Insecta: Hemiptera: Heteroptera). Zootaxa. 2008;1827:1–44. [Google Scholar]
- 22.Wyniger D, Kment P. Key for the separation of Halyomorpha halys (Stål) from similar-appearing pentatomids (Insecta: Heteroptera: Pentatomidae) occurring in Central Europe, with new Swiss records. Mitteilungen der Schweizerischen Entomologischen Gesellschaft. 2010;83:261–270. [Google Scholar]
- 23.Harris AC. Halyomorpha halys (Hemiptera: Pentatomidae) and Protaetia brevitarsis (Coleoptera: Scarabaeidae: Cetoniinae) intercepted in Dunedin. The Weta. 2010;40:42–44. [Google Scholar]
- 24.APHIS. Pest Tracker: a public website of the NAPIS/CAPS database. 2004. http://www.ceris.purdue.edu/napis. Accessed 2011 June 6.
- 25.Nielsen AL, Hamilton GC, Matadha D. Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environmental Entomology. 2008;37:348–355. doi: 10.1603/0046-225x(2008)37[348:drealt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen AL, Shearer PW, Hamilton GC. Toxicity of insecticides to Halyomorpha halys (Hemiptera: Pentatomidae) using glass-vial bioassays. Journal of Economic Entomology. 2008;101:1439–1442. doi: 10.1603/0022-0493(2008)101[1439:toithh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen AL, Hamilton GC. Seasonal occurrence and impact of Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in north America. Journal of Economic Entomology. 2009;102:1133–1140. doi: 10.1603/029.102.0335. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen AL, Hamilton GC. Life history of the invasive species Halyomorpha halys (Hemiptera: Pentatomidae) in Northeastern United States. Annals of the Entomological Society of America. 2009;102:608–616. [Google Scholar]
- 29.Aldrich JR, Khrimian A, Chen X, Camp MJ. Semiochemically based monitoring of the invasion of the brown marmorated stink bug and unexpected attraction of the native green stink bug (Heteroptera: Pentatomidae) in Maryland. Florida Entomologist. 2009;92:483–491. [Google Scholar]
- 30.Khrimian A, Shearer PW, Zhang A, Hamilton GC, Aldrich JR. Field trapping of the invasive Brown Marmorated Stink Bug, Halyomorpha halys, with geometric isomers of methyl 2,4,6-decatrienoate. Journal of Agricultural and Food Chemistry. 2008;56:197–203. doi: 10.1021/jf072087e. [DOI] [PubMed] [Google Scholar]
- 31.Toyama M, Ihara F, Yaginuma K. Photo-response of the brown marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae), and its role in the hiding behavior. Applied Entomology and Zoology. 2011;46:37–40. [Google Scholar]
- 32.ESA. 2011. Entomology Society of America, Eastern Branch Meeting, Plague of the Brown Marmorated Stink Bug, March 2011, http://www.northeastipm.org/. Accessed 2011 June 6.
- 33.Rödder D, Lötters S. Niche shift versus niche conservatism? Climatic characteristics of the native and invasive ranges of the Mediterranean house gecko (Hemidactylus turcicus). Global Ecology and Biogeography. 2009;18:674–687. [Google Scholar]
- 34.Mandle L, Warren DL, Hoffmann MH, Peterson AT, Schmitt J, et al. Conclusions about niche expansion in introduced Impatiens walleriana populations depend on method of analysis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015297. doi: 10.1371/journal.pone.0015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson AT. Predicting the geography of species' invasions via ecological niche modeling. Quarterly Review of Biology. 2003;78:419–433. doi: 10.1086/378926. [DOI] [PubMed] [Google Scholar]
- 36.Broennimann O, Guisan A. Predicting current and future biological invasions: both native and invaded ranges matter. Biology Letters. 2008;4:585–589. doi: 10.1098/rsbl.2008.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiménez-Valverde A, Peterson AT, Soberón J, Overton JM, Aragón P, et al. Use of niche models in invasive species risk assessments. Biological Invasions. 2011 doi: 10.1007/s10530-011-9963-4. [Google Scholar]
- 38.ESRI. ArcGIS 9.2. 2006. Environmental Systems Research Institute, Redlands, CA, USA.
- 39.Gazetteer of China. 1997. Sinomaps Press, Beijing, China.
- 40.Nuñez MA, Medley KA. Pine invasions: climate predicts invasion success; something else predicts failure. Diversity and Distributions. 2011;17:703–713. [Google Scholar]
- 41.Rangel TF, Diniz-Filho JAF, Bini LM. Towards an integrated computational tool for spatial analysis in macroecology and biogeography. Global Ecology and Biogeography. 2006;15:321–327. [Google Scholar]
- 42.Niva CC, Takeda M. Effects of photoperiod, temperature and melatonin on nymphal development, polyphenism and reproduction in Halyomorpha halys (Heteroptera: Pentatomidae). Zoological Science. 2003;20:963–970. doi: 10.2108/zsj.20.963. [DOI] [PubMed] [Google Scholar]
- 43.Li ZX, Liu YS. Effect of temperature on development of egg parasitoid Trissolcus halyomorphae and the eggs of its host, Halyomorpha halys. Chinese Journal of Biological Control. 2004;20:64–6. [Google Scholar]
- 44.Capinha C, Anastácio P. Assessing the environmental requirements of invaders using ensembles of distribution models. Diversity and Distributions. 2010;17:13–24. [Google Scholar]
- 45.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- 46.Kriticos DJ, Webber BL, Leriche A, Ota N, Macadam I, et al. CliMond: global high resolution historical and future scenario climate surfaces for bioclimatic modeling. Methods in Ecology and Evolution. 2011 doi: 10.1111/j.2041-210X.2011.00134.x. [Google Scholar]
- 47.Phillips SJ, Dudik M, Schapire RE. A maximum entropy approach to species distribution modeling. Proceedings of the Twenty-First International Conference on Machine Learning. 2004:655–662. [Google Scholar]
- 48.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. [Google Scholar]
- 49.Phillips SJ, Dudik M. Modelling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161–175. [Google Scholar]
- 50.Peterson AT, Papes M, Eaton M. Transferability and model evaluation in ecological niche modeling: a comparison of GARP and Maxent. Ecography. 2007;30:550–560. [Google Scholar]
- 51.Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. Journal of Biogeography. 2007;34:102–117. [Google Scholar]
- 52.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 53.Araújo MB, Whittaker RJ, Ladle RJ, Erhard M. Reducing uncertainty in projections of extinction risk from climate change. Global Ecology and Biogeography. 2005;14:529–538. [Google Scholar]
- 54.Pearson RG, Thuiller W, Araújo MB, Martínez-Meyer E, Brotons L, et al. Model-based uncertainty in species range prediction. Journal of Biogeography. 2006;33:1704–1711. [Google Scholar]
- 55.Waltari E, Hijmans RJ, Peterson AT, Nyári ÁS, Perkins SL, et al. Locating Pleistocene refugia: comparing phylogeographic and ecological niche model predictions. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000563. doi: 10.1371/journal.pone.0000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waltari E, Guralnick RP. Ecological niche modelling of montane mammals in the Great Basin, North America: examining past and present connectivity of species across basins and ranges. Journal of Biogeography. 2009;36:148–161. [Google Scholar]
- 57.Stockwell DRB, Peters DP. The GARP modeling system: problems and solutions to automated spatial prediction. International Journal of Geographical Information Systems. 1999;13:143–158. [Google Scholar]
- 58.Flanders J, Wei L, Rossiter SJ, Zhang S. Identifying the effects of the Pleistocene on the greater horseshoe bat, Rhinolophus ferrumequinum, in East Asia using ecological niche modelling and phylogenetic analyses. Journal of Biogeography. 2011;38:439–452. [Google Scholar]
- 59.Hawlitschek O, Porch N, Hendrich L, Balke M. Ecological niche modelling and nDNA sequencing support a new, morphologically cryptic beetle species unveiled by DNA barcoding. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016662. doi: 10.1371/journal.pone.0016662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearman PB, Guisan A, Broennimann O, Randin CF. Niche dynamics in space and time. Trends in Ecology & Evolution. 2008;23:149–158. doi: 10.1016/j.tree.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Hutchinson GE. Concluding remarks. Cold Spring Harbour Symposia on Quantitative Biology. 1957;22:415–427. [Google Scholar]
- 62.Hutchinson GE. An introduction to population ecology. 1978. Yale University Press, New Haven, CT, USA.
- 63.Yu CL, Jin XF, Liu XQ, Zhao HY, Jin CC, et al. Study on the damage of Halyomorpha halys and Dolycoris baccarum to the pear and its control strategy. China Fruits. 2002;2:5–7. [Google Scholar]
- 64.Zhang CT, Li DL, Su HF, Xu GL. A study on the biological characteristics of Halyomorpha halys and Erthesina fullo. Froest Research. 1993;6:271–275. [Google Scholar]
- 65.Li QC, Cheng AY, Wang HS, Zhang WY. Control techniques on the Halyomorpha halys and Erthesina fullo. Plant Doctor. 1998;11:17–18. [Google Scholar]
- 66.Yang SY, Wang DY, Chen GM, Li H, Bian XR. Study on the phenology and the synthetical prevention of Halyomorpha halys and Erthesina fullo. Shanxi Fruits. 2006;3:10–11. [Google Scholar]
- 67.Ji SL, Li J, Mu XH, Zhang YQ, Bian XR. Study on the synthetical control of Erthesina fullo and Halyomorpha halys. Northern Fruits. 2006;5:25–26. [Google Scholar]
- 68.Zhang YQ, Mu XH, Ji SL, Li J, Bian XR. Biological characteristics and control strategy of Erthesina fullo and Halyomorpha halys. Deciduous Fruits. 2006;3:42–44. [Google Scholar]
- 69.Song HW, Wang CM. Study on the damage of Erthesina fullo and Halyomorpha halys to jujube tree and its control strategy. Entomological Knowledge. 1993;4:225–228. [Google Scholar]
- 70.Qiu LF. Phd dissertation, Studies on biology of the brown marmorated stink bug Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), an important pest for pome trees in China and its biological control. 2007. Chinese Academy of Froestry, October.
- 71.Qiu LF. Nature enemy species of Halyomorpha halys and control effects of the Parasitoids in Beijing. Northern Horticulture. 2010;9:181–183. [Google Scholar]
- 72.Qiu LF, Yang ZQ. Population dynamics and interspecific competition between Trissolcus halyomorphae Yang and Anastatus sp. in Parasitizing Halyomorpha halys Eggs. Chinese Agricultural Science Bulletin. 2010;26:211–225. [Google Scholar]
- 73.Barve N, Barve V, Jiménez-Valverdea A, Lira-Noriega A, Maher SP, et al. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecological Modelling. 2011;222:1810–1819. [Google Scholar]
- 74.Josifov MV, Kerzhner IM. Heteroptera aus Korea. II. Teil (Aradidae, Berytidae, Lygaeidae, Pyrrhocoridae, Rhopalidae, Alydidae, Coreidae, Urostylidae, Acanthosomatidae, Scutelleridae, Pentatomidae, Cydnidae, Plataspidae). Fragmenta Faunistica. 1978;23:137–196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Niche model based on reduced native records and transferred worldwide using GARP. Dark green color represents high suitability, light green indicates low suitability. White circles indicate the 95 occurrences used for model calibration, black dots and white squares represent the remaining native and the invasive records used for model evaluation.
(TIF)
GARP Protocol in exploring area of potential invasion.
(DOCX)