Abstract
During anaerobic growth Escherichia coli synthesizes two membrane-associated hydrogen-oxidizing [NiFe]-hydrogenases, termed hydrogenase 1 and hydrogenase 2. Each enzyme comprises a catalytic subunit containing the [NiFe] cofactor, an electron-transferring small subunit with a particular complement of [Fe-S] (iron-sulfur) clusters and a membrane-anchor subunit. How the [Fe-S] clusters are delivered to the small subunit of these enzymes is unclear. A-type carrier (ATC) proteins of the Isc (iron-sulfur-cluster) and Suf (sulfur mobilization) [Fe-S] cluster biogenesis pathways are proposed to traffic pre-formed [Fe-S] clusters to apoprotein targets. Mutants that could not synthesize SufA had active hydrogenase 1 and hydrogenase 2 enzymes, thus demonstrating that the Suf machinery is not required for hydrogenase maturation. In contrast, mutants devoid of the IscA, ErpA or IscU proteins of the Isc machinery had no detectable hydrogenase 1 or 2 activities. Lack of activity of both enzymes correlated with the absence of the respective [Fe-S]-cluster-containing small subunit, which was apparently rapidly degraded. During biosynthesis the hydrogenase large subunits receive their [NiFe] cofactor from the Hyp maturation machinery. Subsequent to cofactor insertion a specific C-terminal processing step occurs before association of the large subunit with the small subunit. This processing step is independent of small subunit maturation. Using western blotting experiments it could be shown that although the amount of each hydrogenase large subunit was strongly reduced in the iscA and erpA mutants, some maturation of the large subunit still occurred. Moreover, in contrast to the situation in Isc-proficient strains, these processed large subunits were not membrane-associated. Taken together, our findings demonstrate that both IscA and ErpA are required for [Fe-S] cluster delivery to the small subunits of the hydrogen-oxidizing hydrogenases; however, delivery of the Fe atom to the active site might have different requirements.
Introduction
Iron-sulfur ([Fe-S]) clusters are ubiquitous prosthetic groups of many metalloenzymes in almost all life-forms and they have a variety of functions in diverse cellular processes. Generation of [Fe-S] clusters does not occur spontaneously but requires dedicated machineries that orchestrate their assembly and subsequent transfer to apoprotein substrates (for reviews see [1]–[3]. There are at least three different [Fe-S] biosynthetic systems known and they are referred to as Nif (nitrogen fixation-associated), Isc (iron sulfur cluster) and Suf (sulfur mobilization). The initial discovery of the specialized NifUS proteins for the generation of [Fe-S] clusters in the nitrogenase enzyme of the nitrogen-fixing bacterium Azotobacter vinelandii [4] made it immediately clear that further generalized [Fe-S] machineries in bacteria must exist and these are represented by the Isc and Suf systems in many microbes [5], [6].
The protein components of the Isc and Suf biogenesis systems can be roughly divided into those proteins dedicated to [Fe-S] assembly and those proposed to be involved in the subsequent trafficking of the pre-formed cluster to the ultimate apoprotein acceptor [3]. The proteins involved in transfer or trafficking of [Fe-S] are referred to as A-type carrier (ATC) proteins and the bacterium Escherichia coli has three of these, which are phylogenetically related [7], and are termed IscA, SufA and ErpA [8]. Current evidence is consistent with a role in cluster transfer between the Isc or Suf scaffold machinery and apoprotein substrates [3], [9], [10]; however, it has also been proposed that the ATC proteins deliver iron to the scaffold proteins [11].
An erpA mutation severely impairs aerobic growth of E. coli while single mutations in the sufA and iscA genes are viable [8]. Individual knock-out mutations in the iscA, erpA or sufA genes have a limited effect on anaerobic growth [8], suggesting redundancy of function for [Fe-S] cluster insertion into key iron-sulfur proteins. This contrasts with iscA sufA double null mutants, which generally are non-viable aerobically unless another means of [Fe-S] cluster assembly is present, such as heterologously expressed nifUS, or introduction of the eukaryal isoprenoid biosynthetic pathway [3], [7], [12]. Similarly, iscA erpA double mutants also require introduction of the eukaryal isoprenoid biosynthetic pathway together with supplementation of mevalonate to restore growth [7], [8]. Taken together these genetic studies suggest that IscA and SufA are functionally redundant during aerobic growth of E. coli, while IscA and ErpA show redundancy even anaerobically. Little is known, however, about which apoproteins these putative [Fe-S] cluster-delivery proteins have as substrates.
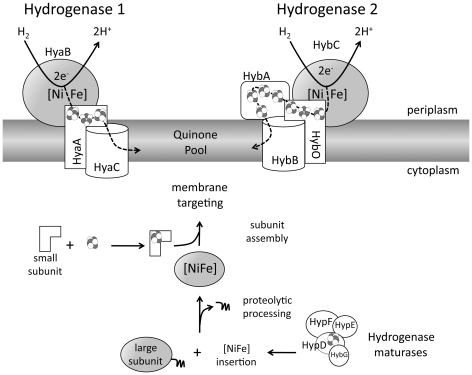
[NiFe]-hydrogenases are evolutionarily ancient [Fe-S] cluster-containing proteins that catalyze the reduction of protons to molecular hydrogen or the oxidation of hydrogen to protons and electrons [13], [14]. The genome of E. coli encodes four membrane-associated [NiFe]-hydrogenases, only three of which are synthesized under anaerobic growth conditions. Hydrogenase 1 and hydrogenase 2 have their respective active site located in the periplasm and they both catalyze hydrogen oxidation (Fig. 1). Hydrogenase 3 forms part of the multi-subunit, hydrogen-evolving formate hydrogenlyase (FHL) complex [15], which disproportionates formic acid into CO2 and H2 during fermentation.
Figure 1. Schematic representation of the iron-sulphur cluster-containing proteins involved in the hydrogen oxidation of E. coli.
The two anaerobic, membrane-associated [NiFe]-hydrogenases 1 and 2 are schematically represented with their associated subunits. The iron-sulphur clusters are shown as groups of spheres. No distinction is made between [3Fe-4S], [4Fe-4S] or [4Fe-3S] clusters. The ‚squiggle' attached to the apoprotein form of the hydrogenase catalytic subunit (bottom of the Figure) represents the C-terminal peptide that is removed subsequent to insertion of the [NiFe] cofactor. The dotted arrows indicate electron flow within the modular enzymes or to the quinone pool.
The two hydrogen-oxidizing hydrogenases comprise a large catalytic subunit (HyaB and HybC for hydrogenase 1 and 2, respectively; see Fig. 1), which lacks a [Fe-S] cluster but has a [NiFe] cofactor that catalyzes hydrogen activation, a small, electron-transfer subunit with three [Fe-S] clusters (HyaA and HybO for hydrogenase 1 and 2, respectively), as well as a membrane-anchor subunit that carries menaquinone-binding sites (HyaC and HybB, for hydrogenase 1 and 2, respectively) [15]–[18]. Hydrogenase 2 has an additional [Fe-S] cluster subunit, HybA, which is not a typical small subunit, but has been shown to be required for growth on hydrogen and fumarate [19] (Fig. 1).
Synthesis and assembly of these modular, cofactor-containing hydrogen-oxidizing hydrogenases require the coordinated involvement of a number of ancilliary proteins. For example, specific hydrogenase maturases, termed Hyp proteins, are required to synthesize and insert the [NiFe] cofactor into the apo-form of the large subunit [13], [15]. Once synthesis and insertion of the [NiFe] cofactor has been completed, the large subunit is processed at its C-terminus by a hydrogenase-specific endopeptidase. Only after this step can the mature large subunit associate with the mature, [Fe-S] cluster-containing small subunit. The respective small subunits of hydrogenases 1 and 2 have an N-terminal Tat (twin-arginine transport) signal sequence, which directs the dimeric protein to the membrane where it is transported across the cytoplasmic membrane by the Tat-translocon [20]. After transport the dimer of the large and small subunits associates with the respective membrane-integral anchor subunit to generate an active hydrogen-oxidizing hydrogenase.
The involvement of [Fe-S] clusters in the biosynthesis and activity of the hydrogen-oxidizing [NiFe]-hydrogenases is extensive and is summarized in Fig. 1. While a considerable amount of information is available concerning the biosynthesis of the [NiFe]-center and the roles of the Hyp maturases (for reviews see [13], [15]), as well as the route of nickel incorporation [21], virtually nothing is known about either the routes of incorporation of the [Fe-S] clusters into the small subunits or where the iron atom in the active site originates. In this study, we have examined the biosynthesis of the hydrogen-oxidizing [NiFe]-hydrogenases of E. coli with respect to the potential involvement of the three ATC paralogues IscA, SufA and ErpA. Our results reveal that IscA and ErpA are both essential for the assembly of active hydrogen-oxidizing hydrogenases.
Results
The iron-sulphur cluster trafficking proteins IscA and ErpA are both required for hydrogen-oxidizing enzyme function
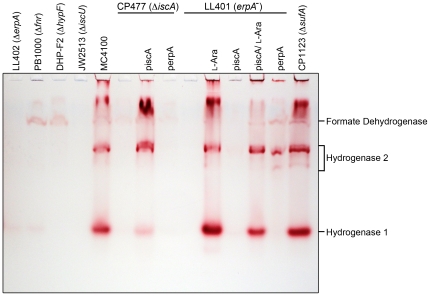
Initially we wanted to examine the effects of deleting the genes encoding the ATC proteins IscA, SufA and ErpA on hydrogen-oxidizing enzyme function in anaerobic E. coli cells. The activities of hydrogenases 1 and 2 can be readily identified after separation in native PAGE followed by specific hydrogenase activity-staining, thus providing a facile method of exclusively examining the consequences of mutations on hydrogen-oxidizing enzyme activity [22], [23]; hydrogenase 3 enzyme activity is labile under these conditions and therefore does not interfere with this analysis. Extracts derived from a sufA mutant revealed hydrogenase 1 and hydrogenase 2 activity profiles similar to the hydrogenase wild type strain MC4100 (Fig. 2). In contrast, extracts derived from the iscA and erpA mutants were devoid of both activities. Both strains retained a slowly migrating hydrogen: benzyl viologen oxidoreductase activity that has been shown to be due to a side reaction of the respiratory formate dehydrogenases [24], [25]. As a further control, we analyzed the hydrogenase 1 and hydrogenase 2 activity profiles in the iscU mutant JW2513 and observed no activity of either enzyme (Fig. 2). In general, this is a similar phenotype to that of the ΔhypF mutant DHP-F2, which cannot synthesize the [NiFe] cofactor and therefore lacks all hydrogenase enzyme activities [26]. This phenotype is also similar to that observed in a mutant lacking the transcriptional regulator Fnr (Fig. 2), which lacks hydrogenase 1 and 2 activities [23], [27].
Figure 2. Hydrogenases 1 and 2 are inactive in iscA and erpA mutants.
Aliquots of crude extracts (25 µg protein) derived from the bacterial stains shown were separated by non-denaturing PAGE (7.5% w/v polacrylamide) and subsequently stained for hydrogenase enzyme activity. Hydrogenase 1 migrates as a single active enzyme species while hydrogenase 2 shows multiple active forms, which are designated on the right of the panel. The weak hydrogen: benzyl viologen oxidoreductase activity that is independent of the [NiFe]-hydrogenases, and which is associated with formate dehydrogenase [25], is also designated.
Quantitative determination of total hydrogenase enzyme activity in the crude extracts from these mutants revealed that when compared with extracts derived from the hydrogenase strain MC4100, the activity was reduced by more than 95% in the iscA and erpA mutants and was abolished in the iscU mutant (MC4100, 3.00±0.59 U mg protein−1; CP477 (iscA), 0.12±0.07 U mg protein−1; LL401 (erpA), 0.13±0.07 U mg protein−1; JW2513 (iscU), <0.01 U mg protein−1). The residual hydrogenase enzyme activity measured in the iscA and erpA mutants was due to hydrogenase 3 (data not shown). Taken together, these data demonstrate that the hydrogen-oxidizing hydrogenases 1 and 2 depend on both ErpA and IscA for enzyme activity. Furthermore, the Isc machinery, but not the Suf machinery, is required for hydrogen oxidation in E. coli.
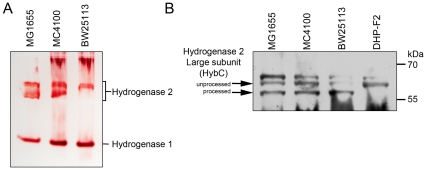
It should be noted that various E. coli K-12 wild type strains, including MC4100, MG1655 and BW25113 and mutants thereof, shared the same hydrogen-oxidizing phenotype and are therefore directly comparable (Fig. 3). The total hydrogenase activities were 2.80±0.48 U mg protein−1 for MG1655 and 2.74±0.43 U mg protein−1 for BW25113.
Figure 3. Comparative analysis of hydrogen-uptake hydrogenases in different E. coli K-12 derivatives.
A. Aliquots of crude extracts (25 µg protein) derived from the bacterial strains shown were separated by non-denaturing PAGE (7.5% w/v polyacrylamide) and subsequently stained for hydrogenase enzyme activity. The locations of hydrogenase 1 and hydrogenase 2 activity bands are shown. B. Western blot analysis of the unprocessed and processed forms of the HybC large subunit of hydrogenase 2 was performed using crude extracts (25 µg protein) derived from the bacterial strains indicated. Strain DHP-F2 is a derivative of MC4100 carrying a deletion in the hypF gene.
Complementation analysis
Introduction of the iscA gene on a multicopy plasmid (piscA) into the iscA mutant CP477 restored total hydrogenase enzyme activity (3.59±0.19 U mg protein−1) to levels similar to those in MC4100. Similarly, introduction of the erpA gene on plasmid perpA restored total hydrogenase activity to the conditional erpA mutant LL401 (3.37±1.45 U mg protein−1). Because the erpA gene in LL401 is under the control of the araP promoter [8], growth of the strain in the presence of arabinose induces erpA expression and restored hydrogenase activity (5.34±1.02 U mg protein−1). The reason why the activity in the presence of arabinose was significantly higher than in the wild type is currently unclear.
Analysis of extracts derived from CP477 and LL401 transformed with either piscA or perpA revealed that only multicopy iscA restored hydrogenase 1 and hydrogenase 2 activities to CP477, while only growth in the presence of arabinose (or less effectively perpA) restored hydrogenase 1 and hydrogenase 2 activities to LL401 (Fig. 2); addition of L-arabinose to strain LL401 carrying plasmid piscA was performed as a control to demonstrate that multicopy iscA did not interfere with complementation by induced erpA expression. The erpA gene when supplied in trans on a plasmid was less effective at restoring hydrogenase 1 activity to strain LL401 and the reason for this is unclear; however, it suggests that hydrogenase 1 and hydrogenase 2 might be differentially responsive to the cellular levels of ErpA. The recovery of total hydrogenase by plasmid perpA in LL401 can be explained by the fact that under the growth conditions analyzed here hydrogenase 3 comprises the bulk of the measureable activity with a limited overall contribution by hydrogenase 1 [23], [24].
Low levels of the processed hydrogenase catalytic subunit in iscA and erpA mutants
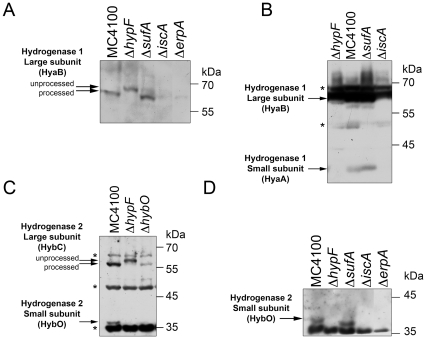
It is possible to distinguish two forms of the hydrogenase catalytic subunits using western blot analysis after SDS-PAGE [13]. The two forms of the approximately 65 kDa large subunit represent an unprocessed polypeptide and a processed form of the polypeptide. The processed species lacks a short C-terminal peptide that results from a specific endoproteolytic cleavage event [13], [28] and which only occurs after insertion of the [NiFe] cofactor has been completed [29]. Western blot analysis of the catalytic subunit (HyaB) of hydrogenase 1 in crude extracts derived from the strain MC4100 revealed mainly the faster-migrating, processed polypeptide (Fig. 4A). In contrast, a mutant unable to make the HypF maturase, which provides the cyanide ligands to the iron atom of the [NiFe] cofactor [26], showed only the unprocessed form of the polypeptide (Fig. 4A) because the [NiFe] cofactor cannot be synthesized. Analysis of the hydrogenase 1 large subunit in a crude extract derived from the sufA mutant CP1223 revealed mainly the processed form at a level similar to that observed for MC4100 (Fig. 4A). This is consistent with the wild type level of hydrogenase 1 activity seen in the sufA mutant (see Fig. 2). Analysis of extracts derived from the iscA and erpA mutants revealed that there were very low amounts of the processed form of the polypeptide in the mutants (Fig. 4A). This finding shows that, despite the reduced level of the protein, possibly due to enhanced degradation, the reason for the lack of detectable hydrogenase 1 enzyme activity in Fig. 2 was not because the catalytic subunit could not receive the completed [NiFe] cofactor. Similar observations were made for the catalytic subunit (HybC) of hydrogenase 2 (see below and Fig. 4).
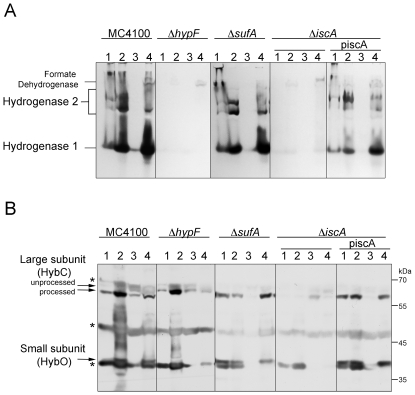
Figure 4. Immunological analysis reveals maturation of the catalytic subunit and absence of the small subunit of the hydrogen-uptake enzymes in erpA and iscA mutant.
Samples of crude extracts (25–50 µg protein) derived from the strains (genotypes are shown above each lane) indicated were separated in 10% (w/v) SDS-PAGE, transferred to nitrocellulose membranes and probed with antibodies against the hydrogenase 1 large subunit (HyaB) (A) or the small subunit (B). The locations of the processed and unprocessed forms of the large subunit polypeptide are shown on the left of the panel and the migration position of molecular mass size markers are shown on the right. Results of a similar experiment showing western blots of the large (HybC) and small (HybO) subunits of hydrogenase 2 are shown in C and D. Unspecific cross-reacting polypeptides that were used as internal loading controls are marked with an asterisk. MC4100, wild type; DHP-F2 (ΔhypF); CP1223 (ΔsufA); CP477 (ΔiscA); LL402 (ΔerpA); CP795 (ΔhyaB ΔhybO ΔhycE) was used in the lane labelled ΔhybO.
The respective [Fe-S] cluster-containing small subunit of both hydrogen-uptake hydrogenases is absent in a hypF mutant
The antibodies raised against hydrogenase 1 and hydrogenase 2 also recognize the respective [Fe-S] cluster-containing small subunit. Western blot analysis of crude extracts derived from MC4100 grown under fermentative conditions using antiserum raised against hydrogenase 1 (Fig. 4B) or hydrogenase 2 (Fig. 4C) identified the small subunits HyaA and HybO, respectively, each with a molecular mass of approximately 35 kDa [30], [31]. As a control, no hydrogenase 2 small subunit (HybO) could be detected in extracts of the hybO mutant CP795 (Fig. 4C). Notably, the level of the large subunit (HybC) was significantly reduced in CP795 compared with extracts of MC4100, which can synthesize an active hydrogenase 2. This suggests that in the absence of the small subunit, the processed large subunit is more rapidly degraded.
In extracts derived from the ΔhypF strain DHP-F2 only the unprocessed form of the large subunits of hydrogenases 1 and 2 were detected (Fig. 4A and C). Surprisingly, however, the respective HyaA and HybO small subunits could not be detected (Fig. 4B, C). This suggests that in the absence of the processed form of the catalytic subunit, the small subunit is subject to rapid degradation. This can be concluded because the genes encoding the respective small subunits are the first genes in the multicistronic hya and hyb operons [17], [18], thus ruling out a transcriptional effect.
The respective [Fe-S] cluster-containing small subunit of both hydrogen-uptake hydrogenases is absent in iscA and erpA mutants
Recent studies have demonstrated that the [Fe-S] cluster-containing small subunit is essential to allow measurement of hydrogen-dependent benzyl viologen (BV) reduction of the hydrogenase 1, hydrogenase 2 and hydrogenase 3 enzymes [24]. Western blot analysis of extracts derived from the iscA mutant CP477 and the erpA mutant LL402 [8] also lacked the small subunits of hydrogenase 2 (Fig. 4D) and hydrogenase 1 (Fig. 4B and data not shown). In contrast, extracts derived from the sufA mutant CP1223 showed wild type levels of both subunits (Fig. 4B, D). These data indicate that the reason no hydrogenase 1 or hydrogenase 2 activity could be detected in the iscA or erpA mutants (see Fig. 2) was due to the absence of the small subunit. Moreover, the data suggest that in the ATC mutants the small subunits of both hydrogenases are more rapidly degraded, possibly because they lack a full complement of [Fe-S] clusters.
The processed catalytic HybC subunit of hydrogenase 2 is not membrane-associated in erpA and iscA mutants
Convincing evidence has been presented to indicate that the hydrogenase small subunit associates with the large subunit only after the [NiFe] cofactor has been inserted [13], [20]. As the small subunits HyaA and HybO of hydrogenases 1 and 2, respectively, bear the signal peptide for recognition and membrane transport to the periplasmic side of the membrane by the Tat translocon, membrane translocation can only take place after complex formation between the mature catalytic subunit and the mature small subunit has occurred. In order to determine how the mutations in the genes encoding the ATC proteins affected the subcellular localization of the catalytic subunits of the hydrogen-oxidizing enzymes and whether they could indeed still be detected, we examined hydrogenase 2 distribution in the soluble and membrane fractions. Crude extracts of the respective mutants were fractionated into soluble and membrane fractions and analyzed by native PAGE, with subsequent activity-staining, and by western blotting with anti-hydrogenase 2 antiserum (Fig. 5). In extracts derived from MC4100, both hydrogenase 1 and hydrogenase 2 activities were associated with the membrane fraction (Fig. 5A), which was expected and served as a positive control [22], [23], [31]. Strain DHP-F2 (hypF) lacks hydrogenase activity [26] and acted as a negative control (Fig. 5A). The weak, slowly migrating hydrogen: benzyl viologen oxidoreductase activity observed in the membrane fraction of strain DHP-F2 was due to the side-activity of formate dehydrogenase [25].
Figure 5. Subcellular localization of hydrogenase 2 in iscA, sufA and erpA mutants.
Aliquots (25 µg protein) derived from whole cells (1), crude extracts (2), soluble fractions (3) or membrane fractions (4) from MC4100, DHP-F2 (ΔhypF), CP1223 (ΔsufA), CP477 (ΔiscA) and CP477+piscA were separated either by native-PAGE (A) (7.5% w/v polyacrylamide) and stained for hydrogenase enzyme activity, or by 10% SDS-PAGE (B) and subjected to Western blotting using anti-hydrogenase 2 antiserum. On the left side of panel A, the migration positions of hydrogenase 1, hydrogenase 2, and of the hydrogenase-independent formate dehydrogenase hydrogen: BV oxidoreductase activity are indicated. In panel B the migration positions of the unprocessed and processed forms of the catalytic subunit HybC and the small subunit HybO are shown. The asterisks indicate unspecifically cross-reacting polypeptides of unknown identity, which acted as internal loading controls. The migration positions of the molecular mass standards (in kDa) are indicated on the right of the Figure.
Western blot analysis demonstrated that the processed form of the hydrogenase 2 large subunit, HybC was also found associated with the membrane fraction (Fig. 5B). In contrast, after fractionation of extracts from CP477 (ΔiscA), the low amount of processed HybC polypeptide still detectable was primarily found in the soluble fraction (Fig. 5B) and no hydrogenase 2 activity was associated with this material (Fig. 5A). Fractionated extracts derived from the sufA mutant CP1223 showed a similar distribution of enzyme activity (Fig. 5A) and the catalytic subunit, HybC, (Fig. 5B) to that in extracts of MC4100. While a clear membrane association of the HybO small subunit of hydrogenase 2 was observed in fractionated extracts of MC4100 and the sufA mutant (Fig. 5B), no HybO could be detected in any sub-cellular fraction, or in whole cells of the iscA mutant CP477 or indeed the hypF mutant DHP-F2.
Introduction of the iscA gene on plasmid piscA into CP477 restored the activities of both hydrogen-oxidizing enzymes, their association with the membrane fraction (Fig. 5A), as well as the appearance of the membrane-associated, processed HybC large subunit and the small subunit HybO (Fig. 5B).
Similar results to those observed for CP477 (ΔiscA) were also observed when the sub-cellular fractions of the erpA mutant LL401 were analyzed (data not shown). Taken together, these results demonstrate that in the absence of the ATC proteins IscA or ErpA the small subunit of hydrogenase 2 could not be detected in any sub-cellular fraction. The lack of a small subunit results, presumably, in more rapid degradation of the processed, matured large subunit and the remaining detectable polypeptide was mainly soluble.
The [Fe-S] cluster-containing maturase HypD is present in erpA and iscA mutants but not in a strain lacking the scaffold protein IscU
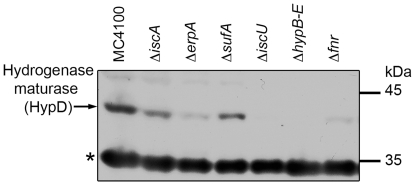
The [4Fe-4S] cluster-containing HypD maturase, together with a number of other maturase enzymes, is required for the biosynthesis of the [NiFe] cofactor in the large subunit of [NiFe]-hydrogenases [32]–[34]. It was reported [13], [33] that amino acid exchanges in a quartet of the C-terminally localized Cys residues in HypD, which coordinate the [4Fe-4S] cluster [34], destabilize the protein. Cell-free, crude extracts derived from MC4100 and the [Fe-S] cluster-trafficking mutants were separated by SDS-PAGE and subjected to western blot analysis with anti-HypD antibodies (Fig. 6). HypD migrated as an approximately 40 kDa polypeptide in extracts from MC4100. An extract derived from the hyp operon deletion mutant BEF314 [35] showed no polypeptide that migrated at this position or that reacted with the anti-HypD antibodies (Fig. 6). Expression of the hyp operon is regulated by the [Fe-S]-containing regulator Fnr [36] and extracts of the fnr mutant PB1000 [27] showed reduced levels of the Fnr HypD protein (Fig. 6). While extracts from CP477 (ΔiscA) and CP1223 (ΔsufA) had levels of HypD essentially similar to those in MC4100, an extract derived from an erpA mutant showed a HypD polypeptide of reduced intensity. In contrast, extracts from the iscU mutant JW2513 showed no polypeptide, a result which would be consistent with a lack of the [Fe-S] cluster in HypD causing rapid turnover of the protein (see [13]). This result demonstrates that the Isc machinery is also required for insertion of the [Fe-S] cluster into HypD, but that in contrast to the hydrogenase small subunits, IscA and ErpA show redundancy in cluster delivery to HypD.
Figure 6. Identification of the HypD maturase in mutants defective in iron-sulphur cluster biogenesis.
Aliquots (25 µg protein) derived from MC4100, CP477 (ΔiscA), LL402 (ΔerpA), CP1223 (ΔsufA), JW2513 (ΔiscU), BEF314 (ΔhypB-E) and PB1000 (Δfnr) were separated by 10% (w/v polyacrylamide) SDS-PAGE and subjected to western blotting using anti-HypD antiserum. The asterisk indicates an unidentified cross-reacting polypeptide that served as an internal loading control. The migration positions of the molecular mass standards (in kDa) are indicated on the right of the figure.
Discussion
Deficiences in anaerobic modular [Fe-S] cluster enzyme assembly
In this study we have shown that a mutant lacking the A-type carrier proteins ErpA and IscA cannot oxidize molecular hydrogen. A mutant unable to synthesize the [Fe-S] cluster scaffold protein IscU of the Isc machinery [3] was also deficient in [NiFe]-hydrogenases 1 and 2. Both IscA and ErpA are therefore essential for synthesis of functional hydrogen-oxidizing hydrogenases. The severe reduction in overall hydrogenase enzyme activity, measured as hydrogen-dependent BV reduction, and the complete loss of hydrogenase 1 and hydrogenase 2 activities, suggests that IscA and ErpA are also probably important for hydrogen-evolution by E. coli, which is catalyzed by hydrogenase 3 [23], [37]. The lack of an effect of a sufA mutation on hydrogenases 1 and 2 rules out any direct involvement of the SufA protein in anaerobic hydrogen oxidation and underscores and extends the genetic evidence indicating that IscA and ErpA are solely involved in the biogenesis of [Fe-S] clusters during anaerobic respiratory metabolism [3]. The findings of the current study are also supported by the recent demonstration that the modular anaerobic respiratory enzymes formate dehydrogenase and nitrate reductase are inactive in erpA mutants and severely reduced in iscA mutants [38]. Taken together these data indicate that the Isc machinery is crucial for the biosynthesis and assembly of functional modular anaerobic oxidoreductases that depend on [Fe-S] clusters for electron transfer processes.
Absence of the small subunit is the reason for the lack of hydrogenase enzyme activity
The lack of hydrogenase 1 and 2 enzyme activity (measured as benzyl viologen: oxidoreduction) in the individual ATC mutants proved to be due to the absence of the small, electron-transferring subunit, which relays electrons between the catalytic site in the large subunit and the quinone pool [15], [39], [40]. In the respiratory hydrogenase 1 and 2 enzymes each small subunit has three [Fe-S] clusters with the medial one being a [3Fe-4S] cluster flanked by [4Fe-4S] clusters in the case of hydrogenase 2 and by a [4Fe-4S] and a [4Fe-3S] cluster in the case of hydrogenase 1 [40], [41]. We believe it is valid to assume that if the [Fe-S] clusters cannot be inserted into these proteins they are rapidly degraded. This is strongly supported by the apparent instability of the [Fe-S] cluster-containing [NiFe]-hydrogenase maturase HypD observed previously, which was caused by substitution of the Cys residues that coordinate the cluster [33] and the complete absence of HypD in an iscU mutant shown in this study (see Fig. 6). Our recent demonstration that in the absence of the small subunit hydrogen-dependent benzyl viologen reduction is abolished [24] explains why enzyme activity could not be detected in the ATC mutants.
Because the HyaA and HybO small subunits of hydrogenases 1 and 2, respectively, carry Tat signal peptides [42], if they are rapidly degraded then they are unavailable to associate with the mature catalytic subunit and thus the HyaA-HyaB and HybO-HybC dimeric complexes cannot be translocated by the Tat translocon. Our ability to detect the mature forms of the HyaB and HybC large subunits, albeit in drastically lower amounts compared with MC4100, supports the evidence [20], [42] that the matured large subunits of hydrogenase 1 and hydrogenase 2 become trapped in the cytoplasmic compartment. Moreover, because the processed form of the HybC large subunit of hydrogenase 2 could be detected in the soluble fraction this indicates that biosynthesis of the [NiFe] cofactor was not compromised in the ATC mutants. This provides futher evidence that the large subunit is matured independently of the small subunit. The findings also indicate that both proteins form a complex only after maturation of each has been completed.
The detection of processed, mature forms of the catalytic subunits of hydrogenases 1 and 2 in the ATC mutants indicates that delivery of the iron for biosynthesis of the [NiFe] cofactor is either independent of the [Fe-S] cluster biogenesis machinery, or that the IscA and ErpA proteins show redundancy in this function. Because the [Fe-S] cluster-containing HypD maturase could be detected in both ATC mutants but not in an iscU mutant this indicates that the [Fe-S] cluster in HypD relies upon the Isc machinery and IscA and ErpA exhibit redundancy in this insertion process. Unfortunately, the question of whether the ATC proteins might be involved in Fe delivery for [NiFe] cofactor biosynthesis cannot be resolved by the findings presented in this study because without HypD it is not possible to synthesize and introduce the [NiFe] cofactor into the hydrogenase catalytic subunits. This problem will require the development of an in vitro system [43] to address the origin of the iron atom in the [NiFe] cofactor.
Do ErpA and IscA deliver different [FeS] clusters to apoprotein targets?
The dependence on both IscA and ErpA for [Fe-S] cluster insertion into the small subunits of hydrogenases 1 and 2 in E. coli suggests different roles for these proteins in cluster insertion. This conclusion has also been reached for certain [Fe-S] cluster-containing enzymes involved in aerobic growth [3], [8]. Although the approximately 110 amino acid ErpA and IscA proteins are phylogenetically related, they share only 40% amino acid sequence identity (57% similarity), which would be consistent with them having different apoprotein substrates or cluster assembly functions. Py and Barras have proposed that in aerobically growing E. coli IscA accepts a pre-formed [Fe-S] cluster from IscU and transfers this cluster via ErpA to the apo-protein substrate [3]. This model would potentially be consistent with the findings observed for hydrogenases 1 and 2; however, [Fe-S] cluster transfer from IscA to ErpA cannot explain the phenotype of the iscA and erpA mutations with respect to HypD stability, which suggests some redundancy between the proteins. Rather, it is possible that ErpA might preferentially transfer [4Fe-4S] clusters while IscA might transfer either [4Fe-4S], [3Fe-4S] or [4Fe-3S] clusters. The recent exciting discoveries of a new type of [4Fe-3S] cluster in the small subunit of the oxygen-tolerant, membrane-associated respiratory [NiFe]-hydrogenase in Ralstonia eutropha [44], Hydrogenovibrio marinus [45] and hydrogenase 1 of E. coli [41], together with the findings presented here, clearly indicate that either IscA or ErpA performs this function.
The paradoxical phenotype of a hypF mutant and the spatio-temporal assembly of hydrogenases
HypF is central to the maturation of [NiFe]-hydrogenases because it synthesizes the CN− ligands to the Fe atom, which is present in the [NiFe] cofactor of the catalytic subunit of [NiFe]-hydrogenases [13]. The consequence of deleting the hypF gene is that the catalytic subunits do not receive the [NiFe] cofactor, consequently remain unprocessed and are enzymatically inactive. The results of this study demonstrate that the small subunits of hydrogenase 1 and hydrogenase 2 cannot be detected in a hypF mutant, which is a similar phenotype to that observed in Isc− mutants. While in iscA and erpA mutants the residual large subunit is processed and mature, in the hypF mutant the large subunit remains unprocessed. Surprisingly, if the large subunit cannot be matured the small subunit appears to be rapidly degraded. Equally, if the small subunit does not receive its complement of [Fe-S] clusters then it is rapidly degraded and the processed large subunit is then also degraded, albeit more slowly. The apparent paradox lies in the observation that the unprocessed large subunit is clearly comparatively stable in a hypF mutant but the processed species is not stable in an iscA or erpA mutant (see Fig. 4). This suggests that the unprocessed catalytic subunit is somehow stabilized against degradation. The molecular basis underlying this stability is currently unclear.
Based on the findings presented here and those of previous studies from other groups, the temporal order of events along the maturation pathway of the hydrogen-oxidizing hydrogenases can be summarized as follows. First, IscA or ErpA delivers the [Fe-S] cluster to apo-HypD and together with the other Hyp maturases [13] these proteins synthesize the [NiFe] cofactor and insert it into the precursors of the HybC and HyaB large subunits. Whether the Fe atom in the active site cofactor is derived from the Isc machinery or is delivered by another route remains to be elucidated. Subsequent to processing of the catalytic subunit by removal of the C-terminal peptide [13], maturation of the large subunit is completed. In a presumably independent, but temporally orchestrated, reaction the apo-HybO and apo-HyaA small subunits receive their complement of [Fe-S] clusters from the combined actions of IscA and ErpA. Whether further specific chaperones are involved in this process, as has been suggested from studies on the small subunit of the [NiFe]-hydrogenase in the nitrogen-fixing symbiotic bacterium Rhizobium leguminosarum [46], also remains to be established. Once maturation has been completed the holo-form of the small subunit can associate with the mature large subunit to form the holo-dimer that is a proficient substrate for the Tat translocon. If the [Fe-S] clusters cannot be inserted into the small subunit it is directed to the protein degradation machinery.
Methods
Strains, plasmids and growth conditions
All bacterial strains and plasmids used in this study are listed in Table 1.
Table 1. Strains and plasmids used in this study.
| Strains/plasmids | Genotypea | Reference/Source |
| MC4100 | F− araD139 Δ(argF-lac)U169 ptsF25 deoC1 relA1 flbB5301 rspL150 − | [53] |
| BEF314 | MC4100 ΔhypB-hypE2 (hyp:: cat pACYC184) | [35] |
| DHP-F2 | MC4100 ΔhypF 59-629AA; ECK2707 | [26] |
| PB1000 | MC4100 ΔpurT ΔpurU ΔinsH4-fnr | [27] |
| CP795 | MC4100 ΔhyaB hybO hycE | [24] |
| CP477 | As MC4100 but ΔiscA::KanR; ECK2525 | [38] |
| CP1223 | As MC4100 but ΔsufA::CmR; ECK1680 | [38] |
| LL401 | MG1655 conditional araP::erpA | [8] |
| LL402 | MG1655 ΔerpA::CmR | [8] |
| JW2513 | BW25113 ΔiscU::KanR; ECK2526 | + |
| Plasmids | ||
| pCP20 | FLP +, λcI857+, λp R Repts, AmpR, CmR | [49] |
| perpA | pBluescript SK(+) containing erpA in BamHI and EcoRI site; AmpR | [38] |
| piscA | pBluescript SK(+) containing iscA in BamHI and EcoRI site; AmpR | [38] |
National BioResources Project (NIG, Japan): E. coli.
Allele numbers are given for single gene mutants and refer to the K-12 nomenclature.
E. coli was grown aerobically in Erlenmeyer flasks filled to maximally 10% of their volume with TGYEP medium [47]. Cultures were incubated on a rotary shaker (250 rpm) and at 37°C. Anaerobic growths were performed at 37°C in sealed bottles filled with anaerobic TGYEP medium under a nitrogen gas atmosphere. When required, the growth medium was solidified with 1.5% (w/v) agar. All growth media were supplemented with 0.1% (v/v) SLA trace element solution, including 7.5 µM iron chloride [48]. The antibiotics chloramphenicol, kanamycin and ampicillin, when required, were added to the medium at the final concentrations of 12.5 µg ml−1, 50 µg ml−1 and 100 µg ml−1, respectively. Where indicated, L-arabinose was added to the growth medium to 0.2% (w/v).
When required, the KanR cassette of certain mutants was removed by transforming the strain in question with pCP20 encoding a Flp-recombinase [49]. Mutants were subsequently tested for sensitivity to kanamycin.
Preparation of cell extracts and determination of enzyme activities
Anaerobic cultures were harvested at an OD600 nm of approximately 0.8. Cells from cultures were harvested by centrifugation at 4000× g for 10 min at 4°C, resuspended in 2–3 ml of MOPS buffer pH 7.0 and lysed on ice by sonication (30 W power for 5 minutes with 0.5 sec pulses). Unbroken cells and cell debris were removed by centrifugation for 15 min at 10 000× g at 4°C and the supernatant was used as the crude cell extract. Membrane and soluble fractions were prepared as described [22]. Samples designated as cells in the figures indicates that whole cell samples were collected by centrifugation and the cell pellets were either resuspended directly in SDS sample buffer for western blot analysis or they were treated with 4% (v/v) Triton X-100 for 30 min on ice prior to being loaded directly onto non-denaturing polyacrylamide gels in 100 µl of the respective buffer at an optical density OD600 nm equivalent to 1. Protein concentration of crude extracts was determined [50] with bovine serum albumin as standard. Hydrogenase activity was measured according to [22] except that the buffer used was 50 mM MOPS, pH 7.0. The wavelength used in the hydrogenase enzyme assay was 578 nm and an EM value of 8,600 M−1 cm−1 was assumed for reduced benzyl viologen. One unit of activity corresponded to the oxidation of 1 µmol of hydrogen per min. Experiments were performed minimally three times and each time enzyme assays were perfomed in triplicate. Data are presented as standard deviation of the mean.
Polyacrylamide gel electrophoresis and immunoblotting
Aliquots of 25–50 µg of protein from the indicated sub-cellular fractions were separated by SDS-polyacrylamide gel electrophoresis (PAGE) using 10% (w/v) polyacrylamide [51] and transferred to nitrocellulose membranes as described [52]. Antibodies raised against hydrogenase 1 (1∶ 10000; [31]), hydrogenase 2 (1∶20000; a kind gift from F. Sargent) and HypD (1∶3000; a kind gift from A. Böck) were used. Secondary antibody conjugated to horseradish peroxidase was obtained from Bio-Rad. Visualisation was done by the enhanced chemiluminescent reaction (Stratagene).
Non-denaturating PAGE was performed using 5% (w/v) polyacrylamide gels pH 8.5 and included 0.1% (w/v) Triton X-100 in the gels and running buffer [22]. Samples (25 µg of protein) were incubated with 5% (w/v) Triton X-100 prior to application to the gels. Hydrogenase activity-staining was done as described in [22] except that the buffer used was 50 mM MOPS pH 7.0.
Acknowledgments
We thank Frédéric Barras for supplying strains and for discussion. We are also indebted to Frank Sargent and August Böck for supplying antibodies. The “National BioResources Project (NIG, Japan): E. coli” is thanked for providing E. coli mutants.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grant Sa 494/3-1 548 from the Deutsche Forschungsgemeinschaft. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ayala-Castro C, Saini A, Outten FW. Fe-S cluster assembly pathways in bacteria. Microbiol Mol Biol Rev. 2008;72:110–125. doi: 10.1128/MMBR.00034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 3.Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8:436–446. doi: 10.1038/nrmicro2356. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson MR, Cash VL, Weiss MC, Laird NF, Newton WE, et al. Biochemical and genetic analysis of the nifUSVWZM cluster from Azotobacter vinelandii. Mol Gen Genet. 1989;219:49–57. doi: 10.1007/BF00261156. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi Y, Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J Biol Chem. 2002;277:28380–28383. doi: 10.1074/jbc.C200365200. [DOI] [PubMed] [Google Scholar]
- 6.Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 7.Vinella D, Brochier-Armanet C, Loiseau L, Talla E, Barras F. Iron-sulfur (Fe/S) protein biogenesis: phylogenomic and genetic studies of A-type carriers. PLoS Genet. 2009;5:e1000497. doi: 10.1371/journal.pgen.1000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loiseau L, Gerez C, Bekker M, Ollagnier-de Choudens S, Py B, et al. ErpA, an iron sulfur (Fe S) protein of the A-type essential for respiratory metabolism in Escherichia coli. Proc Natl Acad Sci USA. 2007;104:13626–13631. doi: 10.1073/pnas.0705829104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ollagnier-de Choudens S, Sanakis Y, Fontecave M. SufA/IscA: reactivity studies of a class of scaffold proteins involved in [Fe-S] cluster assembly. J Biol Inorg Chem. 2004;9:828–838. doi: 10.1007/s00775-004-0581-9. [DOI] [PubMed] [Google Scholar]
- 10.Chahal HK, Dai Y, Saini A, Ayala-Castro C, Outten FW. The SufBCD Fe–S Scaffold Complex Interacts with SufA for Fe–S Cluster Transfer. Biochemistry. 2009;48:10644–10653. doi: 10.1021/bi901518y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Bitoun JP, Ding H. Interplay of IscA and IscU in biogenesis of iron-sulfur clusters. J Biol Chem. 2006;281:27956–27963. doi: 10.1074/jbc.M601356200. [DOI] [PubMed] [Google Scholar]
- 12.Tokumoto U, Kitamura S, Fukuyama K, Takahashi Y. Interchangeability and distinct properties of bacterial Fe-S cluster assembly systems: functional replacement of the isc and suf operons in Escherichia coli with the nifSU-like operon from Helicobacter pylori. J Biochem. 2004;136:199–209. doi: 10.1093/jb/mvh104. [DOI] [PubMed] [Google Scholar]
- 13.Böck A, King P, Blokesch M, Posewitz M. Maturation of hydrogenases. Adv Microb Physiol. 2006;51:1–71. doi: 10.1016/s0065-2911(06)51001-x. [DOI] [PubMed] [Google Scholar]
- 14.Vignais PM, Billoud B. Occurrence, classification, and biological function of hydrogenases: an overview. Chem Rev. 2007;107:4206–4272. doi: 10.1021/cr050196r. [DOI] [PubMed] [Google Scholar]
- 15.Forzi L, Sawers RG. Maturation of [NiFe]-hydrogenases in Escherichia coli. Biometals. 2007;20:565–578. doi: 10.1007/s10534-006-9048-5. [DOI] [PubMed] [Google Scholar]
- 16.Menon NK, Robbins J, Wendt J, Shanmugam K, Przybyla A. Mutational analysis and characterization of the Escherichia coli hya operon, which encodes [NiFe] hydrogenase 1. J Bacteriol. 1991;173:4851–4861. doi: 10.1128/jb.173.15.4851-4861.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon NK, Chatelus CY, Dervartanian M, Wendt JC, Shanmugam KT, et al. Cloning, sequencing, and mutational analysis of the hyb operon encoding Escherichia coli hydrogenase 2. J Bacteriol. 1994;176:4416–4423. doi: 10.1128/jb.176.14.4416-4423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sargent F, Ballantine S, Rugman P, Palmer T, Boxer D. Reassignment of the gene encoding the Escherichia coli hydrogenase 2 small subunit-identification of a soluble precursor of the small subunit in a hypB mutant. Eur J Biochem. 1998;255:746–754. doi: 10.1046/j.1432-1327.1998.2550746.x. [DOI] [PubMed] [Google Scholar]
- 19.Dubini A, Pye R, Jack R, Palmer T, Sargent F. How bacteria get energy from hydrogen: a genetic analysis of periplasmic hydrogen oxidation in Escherichia coli. International Journal of Hydrogen Energy. 2002;27:1413–1420. [Google Scholar]
- 20.Jack RL, Buchanan G, Dubini A, Hatzixanthis K, Palmer T, et al. Coordinating assembly and export of complex bacterial proteins. EMBO J. 2004;23:3962–3972. doi: 10.1038/sj.emboj.7600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eitinger T, Mandrand-Berthelot MA. Nickel transport systems in microorganisms. Arch Microbiol. 2000;173:1–9. doi: 10.1007/s002030050001. [DOI] [PubMed] [Google Scholar]
- 22.Ballantine S, Boxer D. Nickel-containing hydrogenase isoenzymes from anaerobically grown Escherichia coli K-12. J Bacteriol. 1985;163:454–459. doi: 10.1128/jb.163.2.454-459.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawers RG, Ballantine S, Boxer D. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J Bacteriol. 1985;164:1324–1331. doi: 10.1128/jb.164.3.1324-1331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinske C, Krüger S, Soboh B, Ihling C, Kuhns M, et al. Efficient electron transfer from hydrogen to benzyl viologen by the [NiFe]-hydrogenases of Escherichia coli is dependent on the coexpression of the iron-sulfur cluster-containing small subunit. Arch Microbiol. 2011;193:893–903. doi: 10.1007/s00203-011-0726-5. [DOI] [PubMed] [Google Scholar]
- 25.Soboh B, Pinske C, Kuhns M, Waclawek M, Ihling C, et al. The respiratory molybdo-selenoprotein formate dehydrogenases of Escherichia coli have hydrogen: benzyl viologen oxidoreductase activity. BMC Microbiol. 2011;11:173. doi: 10.1186/1471-2180-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paschos A, Bauer A, Zimmermann A, Zehelein E, Böck A. HypF, a carbamoyl phosphate-converting enzyme involved in [NiFe] hydrogenase maturation. J Biol Chem. 2002;277:49945–49951. doi: 10.1074/jbc.M204601200. [DOI] [PubMed] [Google Scholar]
- 27.Pinske C, Bönn M, Krüger S, Lindenstrauß U, Sawers RG. Metabolic deficiences revealed in the biotechnologically important model bacterium Escherichia coli BL21(DE3). PLoS ONE. 2011;6:e22830. doi: 10.1371/journal.pone.0022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossmann R, Sauter M, Lottspeich F, Böck A. Maturation of the large subunit (HYCE) of Escherichia coli hydrogenase 3 requires nickel incorporation followed by C-terminal processing at Arg537. Eur J Biochem. 1994;220:377–384. doi: 10.1111/j.1432-1033.1994.tb18634.x. [DOI] [PubMed] [Google Scholar]
- 29.Magalon A, Böck A. Dissection of the maturation reactions of the [NiFe] hydrogenase 3 from Escherichia coli taking place after nickel incorporation. FEBS Lett. 2000;473:254–258. doi: 10.1016/s0014-5793(00)01542-8. [DOI] [PubMed] [Google Scholar]
- 30.Ballantine S, Boxer D. Isolation and characterisation of a soluble active fragment of hydrogenase isoenzyme 2 from the membranes of anaerobically grown Escherichia coli. Eur J Biochem. 1986;156:277–284. doi: 10.1111/j.1432-1033.1986.tb09578.x. [DOI] [PubMed] [Google Scholar]
- 31.Sawers RG, Boxer D. Purification and properties of membrane-bound hydrogenase isoenzyme 1 from anaerobically grown Escherichia coli K12. Eur J Biochem. 1986;156:265–275. doi: 10.1111/j.1432-1033.1986.tb09577.x. [DOI] [PubMed] [Google Scholar]
- 32.Blokesch M, Albracht SPJ, Matzanke BF, Drapal NM, Jacobi A, et al. The complex between hydrogenase-maturation proteins HypC and HypD is an intermediate in the supply of cyanide to the active site iron of [NiFe]-hydrogenases. J Mol Biol. 2004;344:155–167. doi: 10.1016/j.jmb.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 33.Blokesch M, Böck A. Properties of the [NiFe]-hydrogenase maturation protein HypD. FEBS Lett. 2006;580:4065–4068. doi: 10.1016/j.febslet.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe S, Matsumi R, Arai T, Atomi H, Imanaka T, et al. Crystal structures of [NiFe] hydrogenase maturation proteins HypC, HypD, and HypE: insights into cyanation reaction by thiol redox signaling. Mol. Cell. 2007;27:29–40. doi: 10.1016/j.molcel.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 35.Jacobi A, Rossmann R, Böck A. The hyp operon gene products are required for the maturation of catalytically active hydrogenase isoenzymes in Escherichia coli. Arch Microbiol. 1992;158:444–451. doi: 10.1007/BF00276307. [DOI] [PubMed] [Google Scholar]
- 36.Lutz S, Jacobi A, Schlensog V, Böhm R, Sawers RG, et al. Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol. 1991;5:123–135. doi: 10.1111/j.1365-2958.1991.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 37.Sauter M, Böhm R, Böck A. Mutational analysis of the operon (hyc) determining hydrogenase 3 formation in Escherichia coli. Mol Microbiol. 1992;6:1523–1532. doi: 10.1111/j.1365-2958.1992.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 38.Pinske C, Sawers RG. The A-type carrier protein ErpA is essential for formation of an active formate-nitrate respiratory pathway in Escherichia coli K-12. J Bacteriol. 2012 doi: 10.1128/JB.06024-11. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawers RG. The hydrogenases and formate dehydrogenases of Escherichia coli. Antonie Van Leeuwenhoek. 1994;66:57–88. doi: 10.1007/BF00871633. [DOI] [PubMed] [Google Scholar]
- 40.Volbeda A, Charon M, Piras C, Hatchikian E, Frey M, et al. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature. 1995;373:580–587. doi: 10.1038/373580a0. [DOI] [PubMed] [Google Scholar]
- 41.Lukey MJ, Roessler MM, Parkin A, Evans RM, Davies RA, et al. Oxygen-tolerant [NiFe]-hydrogenases: the individual and collective importance of supernumerary cysteines at the proximal Fe-S cluster. J Am Chem Soc. 2011;133:16881–16892. doi: 10.1021/ja205393w. [DOI] [PubMed] [Google Scholar]
- 42.Sargent F. Constructing the wonders of the bacterial world: biosynthesis of complex enzymes. Microbiology. 2007;153:633–651. doi: 10.1099/mic.0.2006/004762-0. [DOI] [PubMed] [Google Scholar]
- 43.Soboh B, Krüger S, Kuhns M, Pinske C, Lehmann A, et al. Development of a cell-free system reveals an oxygen-labile step in the maturation of [NiFe]-hydrogenase 2 of Escherichia coli. FEBS Lett. 2010;584:4109–4114. doi: 10.1016/j.febslet.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 44.Fritsch J, Scheerer P, Frielingsdorf S, Kroschinsky S, Friedrich B, et al. The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur centre. Nature. 2011;479:249–252. doi: 10.1038/nature10505. [DOI] [PubMed] [Google Scholar]
- 45.Shomura Y, Yoon K-S, Nishihara H, Higuchi Y. Structural basis for a [4Fe-3S] cluster in the oxygen-tolerant membrane-bound [NiFe]-hydrogenase. Nature. 2011;479:253–256. doi: 10.1038/nature10504. [DOI] [PubMed] [Google Scholar]
- 46.Manyani H, Rey L, Palacios JM, Imperial J, Ruiz-Argüeso T. Gene products of the hupGHIJ operon are involved in maturation of the iron-sulfur subunit of the [NiFe] hydrogenase from Rhizobium leguminosarum bv. viciae. J Bacteriol. 2005;187:7018–7026. doi: 10.1128/JB.187.20.7018-7026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begg Y, Whyte J, Haddock B. The identification of mutants of Escherichia coli deficient in formate dehydrogenase and nitrate reductase activities using dye indicator plates. FEMS Microbiol Lett. 1977;2:47–50. [Google Scholar]
- 48.Hormann K, Andreesen J. Reductive cleavage of sarcosine and betaine by Eubaterium acidaminophilum via enzyme systems different from glycine reductase. Arch Microbiol. 1989;153:50–59. [Google Scholar]
- 49.Cherepanov P, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158:9–14. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 50.Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 51.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 52.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casadaban MJ. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]