Abstract
Peritonitis caused by Staphylococcus aureus is a serious complication of peritoneal dialysis (PD), which is associated with poor outcome and high PD failure rates. We reviewed the records of 62 S. aureus peritonitis episodes that occurred between 1996 and 2010 in the dialysis unit of a single university hospital and evaluated the host and bacterial factors influencing peritonitis outcome. Peritonitis incidence was calculated for three subsequent 5-year periods and compared using a Poisson regression model. The production of biofilm, enzymes, and toxins was evaluated. Oxacillin resistance was evaluated based on minimum inhibitory concentration and presence of the mecA gene. Logistic regression was used for the analysis of demographic, clinical, and microbiological factors influencing peritonitis outcome. Resolution and death rates were compared with 117 contemporary coagulase-negative staphylococcus (CoNS) episodes. The incidence of S. aureus peritonitis declined significantly over time from 0.13 in 1996–2000 to 0.04 episodes/patient/year in 2006–2010 (p = 0.03). The oxacillin resistance rate was 11.3%. Toxin and enzyme production was expressive, except for enterotoxin D. Biofilm production was positive in 88.7% of strains. The presence of the mecA gene was associated with a higher frequency of fever and abdominal pain. The logistic regression model showed that diabetes mellitus (p = 0.009) and β-hemolysin production (p = 0.006) were independent predictors of non-resolution of infection. The probability of resolution was higher among patients aged 41 to 60 years than among those >60 years (p = 0.02). A trend to higher death rate was observed for S. aureus episodes (9.7%) compared to CoNS episodes (2.5%), (p = 0.08), whereas resolution rates were similar. Despite the decline in incidence, S. aureus peritonitis remains a serious complication of PD that is associated with a high death rate. The outcome of this infection is negatively influenced by host factors such as age and diabetes mellitus. In addition, β-hemolysin production is predictive of non-resolution of infection, suggesting a pathogenic role of this factor in PD-related S. aureus peritonitis.
Introduction
Peritonitis is a serious complication of peritoneal dialysis (PD) and is responsible for a high rate of technique failure and death in PD patients [1]. Gram-positive cocci are the main etiological agents of peritonitis in the world, with coagulase-negative staphylococci (CoNS) being the most common microbial agents, whereas Staphylococcus aureus is associated with more severe episodes, a higher risk of hospitalization, catheter removal, and death [1], [2]. Although S. aureus is responsible for a small proportion of peritonitis episodes in most countries, it continues to be the leading cause of this infection in some Latin American countries, particularly in Brazil [3].
A poor prognosis of PD-related S. aureus peritonitis has been frequently reported [2], [4], [5], but there are only two reports [6], [7] that specifically describe the clinical outcome and predictors of treatment response in this infection. In the largest series, Govindarajulu et al. [6] showed that methicillin-resistant S. aureus (MRSA) peritonitis was independently predictive of an increased risk of permanent hemodialysis transfer and tended to be associated with a high risk of hospitalization. Szeto et al. [7] reported a lower primary response rate and complete cure rate for episodes caused by MRSA compared to episodes due to other S. aureus. In both cases the clinical outcome of S. aureus peritonitis was not encouraging. The rates of relapse, catheter removal and hospitalization were 20%, 23% and 67%, respectively, in the study of Govindarajulu et al. [6]. In the series of Szeto et al. [7], only 51% of patients with methicillin-susceptible S. aureus peritonitis and 46% with MRSA peritonitis presented complete cure without relapse, recurrent or repeat episodes, or need for catheter removal.
In addition to antibiotic resistance, the severity of S. aureus infections is associated with virulence factors produced by this bacterium, such as enzymes (coagulase, lipase, and nucleases) and multiple toxins with diverse activities. One family of protein toxins are the staphylococcal enterotoxins and the related toxic shock syndrome toxin-1 (TSST-1) that act as superantigens [8]. The biofilm produced by most S. aureus strains facilitates bacterial adhesion to catheters and colonization and simultaneously worsens the response to infection, protecting bacterial cells from the host's natural defense mechanisms and from the action of antibiotics [8], [9]. Although these products may influence clinical outcome, their role in PD-related S. aureus peritonitis is still not fully defined. Data published by Haslinger-Löffler et al. [10] suggest that α-hemolysin plays a specific role in the pathogenesis of peritonitis. Using cultured human peritoneal mesothelial cells, these authors showed that α-hemolysin produced by S. aureus was able to induce caspase-independent cell death. In a recent study, our group demonstrated that biofilm and α-hemolysin production were the only independent predictors of non-resolution of staphylococcal peritonitis [11]. However, the small number of S. aureus episodes analyzed was an important limitation of that study.
For the last 15 years we have monitored clinical and microbiological characteristics of S. aureus peritonitis in PD patients, including virulence factors produced by this pathogen and presence of the mecA gene that confers resistance to methicillin/oxacillin. The objective of the present study was to describe the experience of a single Brazilian center with PD-related S. aureus peritonitis, focusing on host and bacterial factors that influence peritonitis outcome.
Results
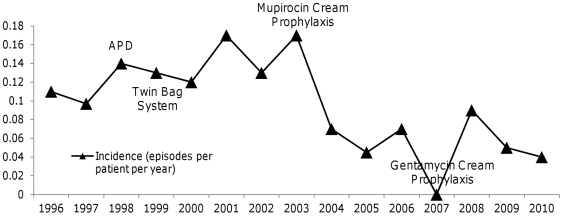
A total of 682 peritonitis episodes were diagnosed in our unit between 1996 and 2010. The overall peritonitis rate was 0.96 episodes per patient per year. Seventy-three (10.7%) episodes were caused by S. aureus. After application of the exclusion criteria, 62 episodes that occurred in 56 patients were analyzed. The demographic and baseline clinical data of the patients are summarized in Table 1. The clinical findings in peritonitis episodes were expressed in Table 2. The incidence of S. aureus peritonitis declined significantly over time and was 0.13 episodes per patient per year in 1996–2000, 0.10 in 2001–2005, and 0.04 in 2006–2010 (p = 0.03). The annual S. aureus peritonitis rate is presented in figure 1; a strong decline of the incidence was observed after 2003. Vancomycin was used in 35 (56.5%) episodes. Overall, 32 (51.6%) episodes were resolved; among cases that were not resolved one (0.16%) relapsed, 18 (29%) required removal of the catheter due to refractory peritonitis, five (8%) were resolved with a second antibiotic regimen, and six (9.7%) progressed to death. Of 117 contemporary CoNS peritonitis episodes, 63 (53.8%) were resolved and three (2.5%) progressed to death. The death rate tended to be lower among episodes caused by CoNS than among S. aureus episodes (p = 0.08), whereas resolution rates were similar (p = 0.16).
Table 1. Summary of Patient Characteristics at Baseline (n = 56).
| Frequency | % | |
| Age (years) | ||
| ≤20 | 4 | 7.2 |
| 21–40 | 12 | 21.4 |
| 41–60 | 20 | 35.7 |
| >60 | 20 | 35.7 |
| Male gender | 23 | 41.1 |
| Presence of diabetes | 28 | 50 |
| Educational Level | ||
| Elementary | 30 | 53.6 |
| Secondary | 8 | 14.3 |
| Higher | 6 | 10.7 |
| Illiterate | 7 | 12.5 |
| Unknown | 5 | 8.9 |
| PD modality | ||
| APD | 9 | 16.6 |
| CAPD | 47 | 83.3 |
PD, peritoneal dialysis; APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis.
Table 2. Clinical findings in S. aureus peritonitis episodes.
| Sign or symptom | N | % |
| Cloudy Dialysis Effluent | 60 | 96.8 |
| Abdominal pain | 42 | 67.7 |
| Nausea or vomiting | 26 | 41.9 |
| Fever | 18 | 29.0 |
| Hypotension | 12 | 19.3 |
Figure 1. Annual Rate (episodes/patient/year) of Staphylococcus aureus Peritonitis from January 1996 to December 2010.
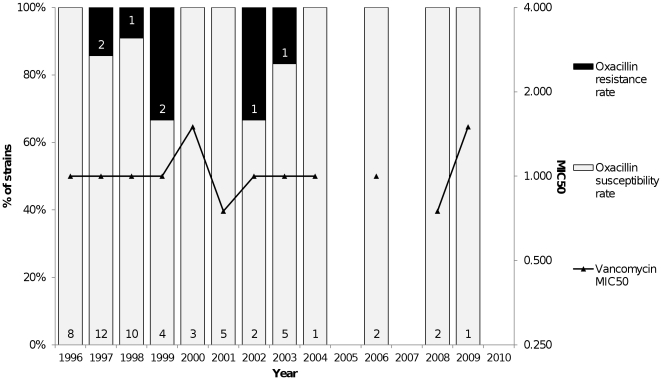
All strains were susceptible to vancomycin (MIC≤3 µg/ml) and seven (11.3%) were resistant to oxacillin (MIC≥4 µg/ml). The vancomycin MIC or proportion of oxacillin-resistant isolates did not change significantly over time (Figure 2). The mecA gene was detected in seven (11.3%) strains.
Figure 2. Vancomycin Minimum Inhibitory Concentration (MIC50) and Proportion of Oxacillin-Resistant Staphylococcus aureus Strains Isolated from Peritoneal Dialysis Patients with Peritonitis between 1996 and 2010.
The rates of toxin and enzyme production by S. aureus are shown in Table 3. No associations were observed between the production of virulence factors and the frequency of initial clinical findings. However, fever was observed in 83.3% of episodes caused by bacteria expressing the mecA gene, whereas this clinical symptom was present in only 24% of episodes due to mecA gene-negative strains (p = 0.03). In addition, there was a trend towards a higher rate of abdominal pain (100%) among strains expressing the mecA gene compared to mecA gene-negative isolates (64.8%) (p = 0.08). The production of virulence factors and presence of the mecA gene were not associated with catheter removal, hospitalization, or death rate.
Table 3. Production Rates of Pathogenic Factors by S. aureus strains Isolated from 62 Peritonitis Episodes.
| N | % | |
| Enzymes | ||
| α-Hemolysin | 27 | 43.5 |
| β-Hemolysin | 24 | 38.7 |
| Lipase | 52 | 83.9 |
| Lecithinase | 57 | 91.9 |
| Deoxyribonuclease | 58 | 93.5 |
| Thermonuclease | 56 | 90.3 |
| Toxins | ||
| SEA | 7 | 11.3 |
| SEB | 17 | 27.4 |
| SEC | 12 | 19.4 |
| TSST-1 | 17 | 27.4 |
| Biofilm | 55 | 88.7 |
SEA, SEB, SEC, enterotoxins A, B and C, respectively; TSST-1, toxic shock syndrome toxin-1.
Gender, age, vancomycin use, presence of diabetes, production of virulence factors (β-hemolysin, lecithinase, deoxyribonuclease, SEC, and TSST-1), presence of the mecA gene, and dialysis vintage were associated with a higher chance of non-resolution in univariate analysis (Table 4), and were therefore included in the multivariate logistic regression model. Multivariate analysis showed that the presence of diabetes and β-hemolysin production were factors independently associated with a higher odds ratio of non-resolution of peritonitis episodes. In contrast, age of 41–60 years was associated with a lower chance of non-resolution when compared to age >60 years. No significant association with peritonitis outcome was observed for the other variables (Table 4).
Table 4. Odds Comparison of Peritonitis Resolution by Logistic Regression Analysis.
| Factor | p value(univariate) | p value(multivariate) | OR | 95% CI |
| Gender (female) | 0.109 | 0.143 | 4.551 | 0.599–34.611 |
| Caucasian race | 0.642 | |||
| Age (years) | 0.042 | |||
| ≤20 | 0.999 | Exp(3.849) | 0.000- | |
| 21–40 | 0.871 | 0.845 | 0.111–6.466 | |
| 41–60 | 0.020 | 0.091 | 0.012–0.684 | |
| >60 (reference) | ||||
| Educational level | 0.934 | |||
| Elementary | ||||
| Secondary | ||||
| Higher | ||||
| Illiteracy | ||||
| Vancomycin use | 0.037 | 0.242 | 0.325 | 0.049–2.140 |
| Presence of diabetes | 0.042 | 0.009 | 14.682 | 1.960–112.676 |
| Enzyme production | ||||
| α-Hemolysin | 0.632 | |||
| β-Hemolysin | 0.077 | 0.006 | 16.597 | 2.246–122.615 |
| Lipase | 0.204 | |||
| Lecithinase | 0.185 | 0.697 | 2.248 | 0.038–131.697 |
| Deoxyribonuclease | 0.033 | 0.999 | Exp(6.296) | 0.000- |
| Thermonuclease | 0.934 | |||
| Presence of mec A gene | 0.195 | 0.838 | 1.430 | 0.046–44.342 |
| Biofilm production | 0.623 | |||
| Toxin production | ||||
| SEA | 0.265 | |||
| SEB | 0.312 | |||
| SEC | 0.071 | 0.217 | 5.621 | 0.363–87.052 |
| TSST-1 | 0.205 | 0.399 | 0.365 | 0.035–3.805 |
| Dialysis vintage (months) | 0.07 | 0.092 | 0.943 | 0.880–1.010 |
| Dialysis modality (APD vs CAPD) | 0.477 |
SEA, SEB, SEC, enterotoxins A, B and C, respectively; TSST-1: toxic shock syndrome toxin-1; APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis.
Discussion
The present results showed a marked decline in the incidence and proportion of peritonitis episodes caused by S. aureus over the past 15 years, in agreement with other studies [3], [12]. The introduction of safer connection systems and the routine use of prophylactic antibiotics at the catheter exit site possibly contributed to the reduction of the incidence of S. aureus peritonitis; however the strong decline in the incidence observed after the introduction of the prophylaxis with mupirocin reinforces the role of this strategy on S. aureus peritonitis prevention. In addition, in the present series we observed a higher death rate among S. aureus episodes compared to episodes caused by CoNS as previously reported [1].
There are few studies reporting the influence of demographic and clinical factors on the prognosis of S. aureus peritonitis episodes. Szeto et al. [7] observed an association between adjuvant rifampicin treatment and a significantly lower risk of relapse, whereas the complete cure rate was similar for cephalosporin and vancomycin empiric treatment protocols. Govindarajulu et al. [6] found that the presence of peripheral vascular disease and the use of vancomycin compared to cephalosporins were significantly associated with an increased risk of relapse of S. aureus peritonitis. According to these authors, female gender and middle tertile of age were independent predictors of a lower risk of relapse. Similarly, we observed that patient age of 41 to 60 years was associated with a higher chance of peritonitis resolution when compared to older patients. In addition, the presence of diabetes was an independent predictor of non-resolution of peritonitis. It is known that the immune response is dysregulated in diabetic patients, increasing the risk of developing infection. In addition, advanced glycation end-products act on peritoneal mesothelial cells, with a potentially negative impact on the local immune response [13]. Although diabetes has been reported to be a risk factor for peritonitis [14], to our knowledge, there are no studies showing diabetes to be a predictor of poor outcome after a peritonitis episode. In the present series, vancomycin use was not an independent predictor of outcome, in agreement with the study of Szeto et al. [7]. We observed no influence of other demographic or clinical factors on resolution rate. Similar results have been reported by Krishnan et al. [15] in a retrospective series of peritonitis episodes of different causes.
Little is known about the influence of specific virulence factors on peritonitis caused by S. aureus. MRSA peritonitis has been associated with poor outcome in the two largest series of S. aureus peritonitis [6], [7]. In the present series, we found a low oxacillin resistance rate, which was confirmed by determination of the mecA gene. On the other hand, the S. aureus strains studied presented expressive enzyme, toxin, and biofilm production. In contrast to previous studies we found no association between oxacillin resistance and resolution rate; however, in the present series only seven peritonitis episodes were caused by oxacillin-resistant S. aureus, a fact that may have influenced the results. Episodes caused by mecA-positive S. aureus isolates were associated with more severe initial clinical symptoms. Studies investigating the role of the mecA gene in the virulence of S. aureus are scarce in the literature. Fowler Jr et al. [16] found an increasing proportion of MRSA among strains isolated from nasal carriage, uncomplicated bacteremia, and bacteremia with hematogenous complications. Analyzing the same sample later, Gill et al. [17] confirmed a higher frequency of the mecA gene among S. aureus strains isolated from severe infections. In our laboratory [18] analyzing 336 MRSA and 107 MSSA strains, we observed a significantly higher proportion of strains expressing SEA, SEB, SEC and TSST-1 genes among MRSA. Taken together, these findings show that, although the number of strains expressing the mecA gene was small in this series, a role of the mecA gene in S. aureus virulence cannot be ruled out.
Among bacterial factors studied, β-hemolysin production was significantly and independently associated with lower resolution odds. The role of β-hemolysin in the pathogenesis of S. aureus infections has not been previously reported in PD-related peritonitis. Nevertheless, some pathways may be suggested based on the findings of experimental models. β-Hemolysin is one of the toxins produced by S. aureus which acts as a sphingomyelinase, degrading sphingomyelin in the outer layer of cell membranes [19]. Deletion of the catalase and β-toxin genes in S. aureus strains has been shown to cause strong attenuation of virulence in intramammary and subcutaneous experimental infections of ewes and lambs and in a murine skin abscess model [19]. Using a mouse model of lung injury, Hayashida et al. [20] found that animals infected with β-hemolysin-deficient S. aureus presented significantly attenuated lesions compared to those infected with S. aureus expressing this toxin. This experimental disease was characterized by intense neutrophilic inflammation and reduced expression of syndecan-1 in alveolar epithelial cells and could be reproduced by administration of recombinant β-hemolysin, but not of mutant β-hemolysin deficient in sphingomyelinase activity.
Extracellular DNA is a major structural component in the biofilms of pathogenic S. aureus. Huseby et al. [21] showed that β-hemolysin forms covalent cross-links to itself in the presence of DNA, irrespective of sphingomyelinase activity, producing an insoluble nucleoprotein matrix in vitro. Using an infectious endocarditis rabbit model, the authors observed that this toxin stimulates biofilm formation in vivo. β-Hemolysin does not lyse most types of host cells but leaves them vulnerable to a number of other lytic agents, such as α-hemolysin and Panton-Valentine leukocidin [20]. We recently demonstrated that α-hemolysin production predicts poor outcome in peritonitis episodes caused by S. aureus and CoNS [11].
A reservoir of phospholipids exists on the peritoneal surface and the main constituents of peritoneal phospholipids are phosphatidylcholine and sphingomyelin [22]. Indeed, evidence suggests that the phospholipids present on the peritoneal surface are derived from peritoneal mesothelial cells. In this respect, β-hemolysin, a sphingomyelinase, may participate directly in biofilm formation, contributing to a poorer outcome of peritonitis episodes, or may render host peritoneal cells susceptible to other pathogenic factors.
Surprisingly, biofilm production was not a predictor of peritonitis resolution. However, the percentages of non-producers was low, a fact impairing the comparison with biofilm producers; therefore, a role of biofilm production in peritonitis outcome cannot be ruled out. Finally, other S. aureus virulence factors that act in a synergistic and coordinated fashion may play a pathogenic role.
The present study has several limitations, the most important of them is the absence of more accurate tests to assess production of β-hemolysin such as mRNA levels using quantitative real time-PCR or specific detection such as by ELISA or Western Blot. Also, the small number of cases analyzed that may reduce its statistical power, and since it is a single-center study its results cannot be extrapolated. Nevertheless, it is the first Latin American study analyzing a series of S. aureus peritonitis cases. In this respect, S. aureus remains the most frequent PD-related etiology in several Latin American countries, including Brazil [3]. Furthermore, this study focused on the role of virulence factors on the outcome of this infection.
In conclusion, despite a strong reduction in the incidence of S. aureus peritonitis, our results showed a poorer outcome of episodes caused by this bacterium when compared to episodes due to CoNS, particularly a higher death rate. Among demographic factors, older age and diabetes were predictors of a lower resolution rate. These findings highlight the importance of peritonitis as a serious complication of PD, particularly among elderly and diabetic patients. β-Hemolysin production was the only virulence factor that negatively influenced peritonitis outcome; however further studies using specific tests to detect the presence of β-hemolysin are necessary to confirm this result.
Materials and Methods
All episodes of ambulatory PD-related peritonitis caused by Staphylococcus aureus between January 1996 and December 2010 were reviewed. The diagnosis of peritonitis was made when at least two of the following criteria were present: 1) presence of a cloudy peritoneal effluent; 2) abdominal pain; 3) dialysate containing more than 100 white blood cells per µl (at least 50% polymorphonuclear cells), and 4) positive culture of peritoneal effluent [23], [24]. Exclusion criteria were episodes of relapsing S. aureus peritonitis, presence of concomitant exit site or tunnel infections, and incomplete clinical data. Resolution was defined as the disappearance of signs and symptoms within 96 h after the beginning of antibiotic therapy and a negative peritoneal fluid culture at least 28 days after treatment completion [23], [24]. Relapse was defined as an episode due to the same organism, or a negative culture result that occurs within 28 days of completion of antibiotic therapy for a prior S. aureus episode [23], [24]. Death related to peritonitis was defined as death of a patient with active peritonitis, or admitted with peritonitis, or within 2 weeks of a peritonitis episode [23], [24]. Non-resolution was the term used for cases presenting initial non-resolution, relapse, peritoneal catheter removal due to refractory peritonitis, need for a second antibiotic regimen, or death.
The following information was recorded for each case: 1) episode: date, clinical findings, treatment, and outcome (resolution, relapse, catheter removal, or death); 2) presence of diabetes mellitus; 3) demographic data: age, gender and race (Caucasian, non-Caucasian), and dialysis treatment time; 4) dialysis modality (continuous ambulatory peritoneal dialysis or automated peritoneal dialysis); 5) educational level (illiterate, elementary, secondary, and higher).
The study was approved by the Research Ethics Committee of the Faculty of Medicine of Botucatu, Brazil (OF. 028/08-CEP). This study was exempted from the requirement to obtain written informed consent from the participants and/or their legal guardians because the Staphylococcus strains included in the study had already been isolated and stored in the Culture Collection of the Department of Microbiology and Immunology, UNESP, Botucatu, São Paulo, Brazil.
Patients were treated within 24 h of the onset of the first clinical signs or symptoms using contemporary empiric antibiotic recommendations [23]–[25]. From 1996 to 2000 (period 1) empiric antibiotic therapy consisted of intraperitoneal (i.p.) cefazolin plus amikacin. Two protocols were used from 2000 to 2005 (period 2): the first consisted of i.p. cefazolin plus amikacin and the second of i.p. cefazolin plus ceftazidime. After 2005 (period 3) all episodes were first treated with i.p. vancomycin plus amikacin. Therapy was evaluated and adjusted as soon as the culture results were available. The duration of antibiotic therapy was 21 days.
In the period 1 no antibiotic prophylaxis at exit site was prescribed and two connection systems were used: the Y set system and the twin bag system, which was introduced in 1999; automated PD (APD) was used from 1998. In the period 2 no antibiotic prophylaxis at exit site was prescribed until 2003, when daily mupirocin cream application at exit site began to be recommended; the twin bag system or APD were prescribed for all patients. In the period 3 until December 2006 all patients were oriented to daily mupirocin cream application, and from January 2007 daily gentamicin cream application at exit site was prescribed to all incident patients; the twin bag system or APD were prescribed for all patients.
The incidence of S. aureus peritonitis was calculated for the three subsequent periods of 5 years and is expressed as episodes per patient per year.
The initial cultures were performed with the Bactec® Automated System (Becton Dickinson Company, Sparks, Maryland, USA) and then seeded onto blood agar. The isolates were Gram stained to confirm purity and to determine morphology and specific color. After confirmation of these characteristics, tests for identification of the isolates were performed as recommended by Koneman et al. [26]. The isolates were stored in a culture collection.
The in vitro susceptibility of S. aureus to oxacillin and vancomycin was evaluated based on the minimum inhibitory concentration determined by the E-test (AB Biodisk, Solna, Sweden). This quantitative method uses a transparent strip of inert plastic that contains drug concentrations ranging from 0.002 to 256 µg/ml. The proportion of strains susceptible to each drug was defined based on the 2011 CLSI breakpoints [27]. Strains presenting intermediate values were considered to be resistant.
Whole nucleic acids were extracted from S. aureus strains cultured on blood agar, individually inoculated into brain heart infusion (BHI) broth, and incubated at 37°C for 24 h. Nucleic acids were extracted using the illustra blood genomic Prep Mini Spin kit (GE Healthcare, Little Chalfont, Buckinghamshire, UK) according to manufacturer instructions. Staphylococcal cells were first digested with lysozyme (10 mg/ml) and proteinase K (20 mg/ml). Next, 500 µl extraction solution was added to the mixture. After centrifugation at 5000 g for 1 min, the supernatant was transferred to a GFX column and centrifuged at 5000 g for 1 min. The supernatant was discarded and 500 µl extraction solution was added to the column. After centrifugation and disposal of the supernatant, 500 µl wash solution was added to the column. The column was then centrifuged at 14,000 rpm for 3 min and transferred to a 1.5-ml tube. Milli-Q water (200 µl) preheated to 70°C was used for elution. The isolates were centrifuged at 5000 g for 1 min and the GFX column was discarded. Extracted DNA was stored under refrigeration at 4°C.
PCR amplification was performed in 0.5-ml microcentrifuge tubes containing 10 pmol of each primer, 2.0 U Taq DNA polymerase, 100 µM deoxyribonucleotide triphosphates, 10 mM Tris-HCl (pH 8.4), 0.75 mM MgCl2, and 3 µl nucleic acid in a total volume of 25 µl. Gene mecA amplification was carried out in an appropriate thermal cycler using the mecA1 (AAA ATC GAT GGT AAA GGT TGG) and mecA2 (AGT TCT GCA GTA CCG GAT TTG) primers as described by Murakami et al. [28]: 40 cycles of denaturation at 94°C for 30 s, annealing of primers at 55.5°C for 30 s, and extension at 72°C for 1 min. After completion of the 40 cycles, the tubes were incubated at 72°C for 5 min and then cooled to 4°C. The S. aureus ATCC 33591 and ATCC 25923 references strains were included in all reactions as positive and negative controls, respectively.
The efficiency of amplification was monitored by electrophoresis on 1.5% agarose gel prepared in 1× TBE buffer and stained with ethidium bromide. The size of the amplified products was compared with a 100-bp standard and the gels were photographed under UV transillumination.
Biofilm production was evaluated according to Christensen et al. [29]. Colonies isolated on blood agar were inoculated into tubes measuring 12.0×75.0 mm and containing 2.0 ml trypticase soy broth and incubated at 37°C for 48 h. Next, 1.0 ml 0.4% trypan blue or Toluidine blue O solution was added to the tubes. After gentle shaking to guarantee staining of the material adhered to the inner surface of the tubes, the dye was discarded. A positive result was defined as the presence of a layer of stained material adhered to the inner wall of the tube. The presence of a colored ring only at the liquid-air surface was classified as a negative result.
Production of α- and β-hemolysin were determined on plates containing blood agar supplemented with 5% rabbit blood and 5% sheep blood, respectively. The plates were incubated at 37°C for 24 h. The formation of hemolysis zones around the isolated colonies indicated a positive result.
Lipolytic activity was evaluated on plates containing blood agar enriched with 0.01% CaCl2∶2H2O and 1% Tween 80. A positive result was defined as the formation of opacity around the colony after incubation at 37°C for 18 h, followed by incubation at room temperature for 24 h [30]. The production of lecithinase was evaluated using Baird-Parker medium. The formation of an opaque halo around the colony indicated a positive result [31].
Nuclease (DNAse) and thermonuclease (TNAse) were determined by the metachromatic Toluidine blue O agar diffusion-DNA technique according to Lachica et al. [32]. Supernatants obtained by the sac culture method of Donnelly et al. [33] as described below were transferred to the wells of plates containing metachromatic Toluidine blue O agar. The culture supernatant was first heated in a water bath for 20 min for the detection of TNAse. Nuclease (DNAse and TNAse) activity was evaluated by measuring the diameter of pink halos (mm) formed on the medium. Positive results were interpreted by comparing the halos with those obtained for a standard DNAse- and TNAse-positive S. aureus strain (ATCC 25923).
For the evaluation of the production of enterotoxins and TSST-1, the sac culture method [33] was used to determine the toxigenic profile of the strains. Dialysis sacs filled with 50 ml double-concentrated BHI broth were placed in U-shaped Erlenmeyer flasks and autoclaved at 121°C for 15 min. A loopful of organisms was added to 18 ml sterile 0.2 M phosphate buffer in 0.9% NaCl, pH 7.4. After incubation at 37°C for 24 h on a shaker at 200 rpm, the cultures were centrifuged at 8000 g for 10 min at 4°C and the supernatants obtained were stored at −20°C until the time of use. The extracellular products were detected by reverse passive latex agglutination (RPLA) using the SET-RPLA-T900 and TST-RPLA-TD940 kits (Oxoid Diagnostic Reagents, Cambridge, UK) for the detection of enterotoxins A (SEA), B (SEB), C (SEC) and D (SED) and TSST-1, respectively, according to manufacturer instructions. Samples that presented nonspecific reactions after this treatment were filtered through a Millipore membrane (0.22 µm) and, if necessary, diluted 1∶10 with 0.02 M phosphate buffer in 0.9% NaCl, pH 7.4. A positive reaction was classified as (+), (++) and (+++) according to the agglutination pattern described by the manufacturer of the kit. The formation of a rose button was interpreted as a negative result.
Statistical analysis
Peritonitis incidences were compared using the Poisson regression model. The association between microbiological characteristics (oxacillin resistance, presence of mecA gene and production of pathogenic factors) and the frequency of clinical findings at initial presentation (abdominal pain, fever, nausea or vomiting, and arterial hypotension) was analyzed by the chi-square or Fisher's exact test. These tests were also used to compare resolution and death rates between S. aureus peritonitis episodes and 117 contemporary CoNS cases. Multivariate analysis by logistic regression was used to test baseline demographic, clinical, and microbiological factors that independently predicted the outcome of a peritonitis episode. Outcome was classified as two mutually exhausted and exclusive results (resolution or non-resolution). For this purpose, univariate analysis using the chi-square or Fisher's exact test (binary variables) or logistic regression (continuous variables) was first performed to select the variables that would enter the final model, with p>0.20 being used as an elimination criterion. A p value less than 0.05 was considered to be significant. All statistical analyses were performed using the SPSS 16.0 software (SPSS®, Inc.).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a grant from the São Paulo state funding agency Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). The funding agency played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Pérez Fontan M, Rodríguez-Carmona A, García-Naveiro R, Rosales M, Villaverde P, et al. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int. 2005;25:274–284. [PubMed] [Google Scholar]
- 2.Cunha MLRS, Montelli AC, Fioravante AM, Neves Batalha JE, Teixeira Caramori JC, et al. Predictive factors of outcome following staphylococcal peritonitis in continuous ambulatory peritoneal dialysis. Clin Nephrol. 2005;64:378–382. doi: 10.5414/cnp64378. [DOI] [PubMed] [Google Scholar]
- 3.Barretti P, Bastos KA, Dominguez J, Caramori JC. Peritonitis in Latin America. Perit Dial Int. 2007;27:332–339. [PubMed] [Google Scholar]
- 4.Bunke CM, Brier ME, Golper TA. Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int. 1997;52:524–529. doi: 10.1038/ki.1997.363. [DOI] [PubMed] [Google Scholar]
- 5.Peacock SJ, Howe PA, Day NP, Crook DW, Winearls CG, et al. Outcome following staphylococcal peritonitis. Perit Dial Int. 2000;20:215–219. [PubMed] [Google Scholar]
- 6.Govindarajulu S, Hawley CM, McDonald SP, Brown FG, Rosman JB, et al. Staphylococcus aureus peritonitis in Australian peritoneal dialysis patients: predictors, treatment, and outcomes in 503 cases. Perit Dial Int. 2010;30:311–319. doi: 10.3747/pdi.2008.00258. [DOI] [PubMed] [Google Scholar]
- 7.Szeto CC, Chow KM, Kwan BC, Law MC, Chung KY, et al. Staphylococcus aureus peritonitis complicates peritoneal dialysis: review of 245 consecutive cases. Clin J Am Soc Nephrol. 2007;2:245–251. doi: 10.2215/CJN.03180906. [DOI] [PubMed] [Google Scholar]
- 8.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am. 2009;23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander W, Rimland D. Lack of correlation of slime production with pathogenicity in continuous ambulatory peritoneal dialysis peritonitis caused by coagulase negative staphylococci. Diagn Microbiol Infect Dis. 1987;8:215–220. doi: 10.1016/0732-8893(87)90052-6. [DOI] [PubMed] [Google Scholar]
- 10.Haslinger-Löffler B, Wagner B, Brück M, Strangfeld K, Grundmeier M, et al. Staphylococcus aureus induces caspase-independent cell death in human peritoneal mesothelial cells. Kidney Int. 2006;70:1089–1098. doi: 10.1038/sj.ki.5001710. [DOI] [PubMed] [Google Scholar]
- 11.Barretti P, Montelli AC, Batalha JE, Caramori JC, Cunha MLRS. The role of virulence factors in the outcome of staphylococcal peritonitis in CAPD patients. BMC Infect Dis. 2009;9:212. doi: 10.1186/1471-2334-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moraes TP, Pecoits-Filho R, Ribeiro SC, Rigo M, Silva MM, et al. Peritoneal dialysis in Brazil: twenty-five years of experience in a single center. Perit Dial Int. 2009;29:492–498. [PubMed] [Google Scholar]
- 13.Ortiz A, Wieslander A, Linden T, Santamaria B, Sanz A, et al. 3,4-DGE is important for side effects in peritoneal dialysis what about its role in diabetes. Curr Med Chem. 2006;13:2695–2702. doi: 10.2174/092986706778201576. [DOI] [PubMed] [Google Scholar]
- 14.Han SH, Lee SC, Ahn SV, Lee JE, Kim DK, et al. Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant. 2007;22:2653–2658. doi: 10.1093/ndt/gfm242. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan M, Thodis E, Ikonomopoulos D, Vidgen E, Chu M, et al. Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int. 2002;22:573–581. [PubMed] [Google Scholar]
- 16.Fowler VG, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, et al. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis. 2007;196:738–747. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- 17.Gill SR, McIntyre LM, Nelson CL, Remortel B, Rude T, et al. Potential associations between severity of infection and the presence of virulence-associated genes in clinical strains of Staphylococcus aureus. PLoS One. 2011;6:e18673. doi: 10.1371/journal.pone.0018673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pimenta-Rodrigues MV. Epidemiologia molecular e fatores de risco para aquisição de clones endêmicos de Staphylococcus aureus resistente à meticilina (MRSA) em um hospital de ensino. [Tese]. Botucatu: Univ Estadual Paulista; 2011. [Google Scholar]
- 19.Martínez-Pulgarín S, Domínguez-Bernal G, Orden JA, de la Fuente R. Simultaneous lack of catalase and beta-toxin in Staphylococcus aureus leads to increased intracellular survival in macrophages and epithelial cells and to attenuated virulence in murine and ovine models. Microbiology. 2009;155:1505–1515. doi: 10.1099/mic.0.025544-0. [DOI] [PubMed] [Google Scholar]
- 20.Hayashida A, Bartlett AH, Foster TJ, Park PW. Staphylococcus aureus beta-toxin induces lung injury through syndecan-1. Am J Pathol. 2009;174:509–518. doi: 10.2353/ajpath.2009.080394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huseby MJ, Kruse AC, Digre J, Kohler PL, Vocke JA, et al. Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms. Proc Natl Acad Sci U S A. 2010;107:14407–14412. doi: 10.1073/pnas.0911032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong JH, Guo QY, Ye RG, Lindholm B, Wang T. Phospholipids in dialysate and the peritoneal surface layer. Adv Perit Dial. 2000;16:36–41. [PubMed] [Google Scholar]
- 23.Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30:393–423. doi: 10.3747/pdi.2010.00049. [DOI] [PubMed] [Google Scholar]
- 24.Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005;25:107–131. [PubMed] [Google Scholar]
- 25.Keane WF, Bailie GR, Boeschoten E, Gokal R, Golper TA, et al. Adult peritoneal dialysis-related peritonitis treatment recommendations: 2000 update. Perit Dial Int. 2000;20:396–411. [PubMed] [Google Scholar]
- 26.Koneman EW, Allen SD, Janda WM, Scheckenberger PC, Winn WC. Color Atlas and text book of Diagnostic Microbiology. Philadelphia: J. B. Lippincott Company; 1992. [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement M100-S18. 18th ed. Wayne, PA: CLSI; 2008. [Google Scholar]
- 28.Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, et al. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991;29:2240–2244. doi: 10.1128/jcm.29.10.2240-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jessen O, Faber V, Rosendal K, Eriksen KR. Some properties of Staphylococcus aureus, possibly related to pathogenicity. Part 1. A study of 446 strains from different types of human infection. Acta Pathol Microbiol Scand. 1959;47:316–326. [PubMed] [Google Scholar]
- 31.Matos JE, Harmon RJ, Langlois BE. Lecithinase reaction of Staphylococcus aureus strains of different origin on Baird-Parker medium. Lett Appl Microbiol. 1995;21:334–335. doi: 10.1111/j.1472-765x.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 32.Lachica RV, Genigeorgis C, Hoeprich PD. Metachromatic agar-diffusion methods for detecting staphylococcal nuclease activity. Appl Microbiol. 1971;21:585–587. doi: 10.1128/am.21.4.585-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donnelly CB, Leslie JE, Black LA, Lewis KH. Serological identification of enterotoxigenic staphylococci from cheese. Appl Microbiol. 1967;15:1382–1387. doi: 10.1128/am.15.6.1382-1387.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]