Abstract
Background
The long-term results of homografts used in systemic circulation are controversial. We assessed the long-term results of using a cryopreserved homograft for an aortic root or aorta and its branch replacement.
Materials and Methods
From June 1995 to January 2010, 23 patients (male:female=15:8, 45.4±15.6 years) underwent a homograft replacement in the aortic position. The surgical techniques used were aortic root replacement in 15 patients and aortic graft interposition in 8 patients. Indications for the use of a homograft were systemic vasculitis (n=15) and complicated infection (n=8). The duration of clinical follow-up was 65±58 months.
Results
Early mortality occurred in 2 patients (8.7%). Perioperative complications included atrial arrhythmia (n=3), acute renal failure (n=3), and low cardiac output syndrome (n=2). Late mortality occurred in 6 patients (26.1%). The overall survival rates at 5 and 10 years were 66.3% and 59.6%, respectively. Six patients (28.6%) suffered from homograft-related complications.
Conclusion
Early results of homograft replacement in aortic position were favorable. However, close long-term follow-up is required due to the high rate of homograft-related events.
Keywords: Homograft, Endocarditis, Aortic root, Aorta
INTRODUCTION
The cryopreserved homograft has been used as an alternative to the artificial vascular graft or composite valved conduit [1-3]. Its advantages include excellent hemodynamics, no need for anticoagulation, and resistance to infection [4-8]. However, its use has been limited due to the lack of availability of donors and concern about long-term durability [5,9]. The aims of this study were to evaluate the long-term results of surgery performed with a cryopreserved homograft in the aortic position including the aortic root or aorta and its branch, and to compare those according to the indications and surgical techniques used.
MATERIALS AND METHODS
1) Patient characteristics
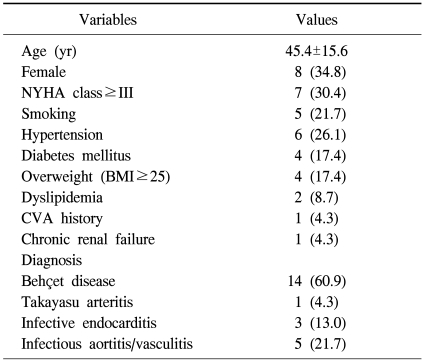
From June 1995 to January 2010, 23 patients underwent homograft replacement in the aortic position. Fifteen of the patients were male, and 8 were female. The mean age at the time of surgery was 45.4±15.6 years. Hypertension (n=6, 26.1%) and diabetes mellitus (n=4, 17.4%) were common comorbidities (Table 1). Indications for the use of a homograft were 1) systemic vasculitis (n=15) such as Behçet disease (n=14) and Takayasu arteritis (n=1) and 2) complicated infection (n=8) involving the aortic root (n=3) or aorta and its branch (n=5). The surgical techniques used were aortic root replacement in 15 patients and aortic graft interposition in 8 patients.
Table 1.
Preoperative characteristics of study patients
Values are presented as mean±standard deviation or number (%).
NYHA=New York Heart Association; BMI=body mass index (kg/m2); CVA=cerebrovascular accident.
2) Surgical procedures
The basic surgical procedures of aortic root replacement (ARR) have been previously described [10]. ARR was performed under standard aortic and bicaval cannulation via a median sternotomy. In addition to moderate systemic hypothermia, direct antegrade cardioplegia and/or retrograde cardioplegia through the coronary sinus were administered. Any root defects induced by vasculitis or infection were obliterated with a commercially available bovine pericardial patch or mitral anterior leaflet tissue of the homograft. All anastomoses were performed with non-absorbable monofilament sutures. Proximal anastomosis was performed using 4-0 interrupted polypropylene sutures and tied after reinforcing the suture line with a bovine pericardial strip. Both coronary artery buttons were attached to the new coronary aortic sinuses using 5-0 polypropylene continuous sutures. No other materials besides bovine pericardium and monofilament suture were used to minimize the risk of infectious complications. The mean cardiopulmonary bypass time and aortic cross clamp time were 300±116 minutes and 192±85 minutes, respectively. Replacement of aorta and its branch was performed via a thoracoabdominal incision (n=4), or median sternotomy with or without an oblique neck incision (n=4). All sutures were placed with polypropylene alone to minimize the risk of infection. The size of the homograft was marked as an inner diameter of the aortic valve level and the mean size of the homograft was 24.2±2.4 mm.
3) Evaluation of clinical outcomes
The patients underwent regular postoperative follow-up through the outpatient clinic at 3 or 4 month intervals, and they were contacted by telephone for confirmation of their condition if the last clinic visit was not conducted at the scheduled time. Clinical follow-up was closed on September 30, 2010. Follow-up was completed in all patients with a mean follow-up duration of 66±59 months (range, 4 to 183 months). Cardiac death was defined as any death related to cardiac events, including sudden death during follow-up. Late homograft-related events were defined as a composite of cardiac death, infection of homograft, thromboembolism, homograft-related reoperation or intervention, homograft valve deterioration causing valve regurgitation, and anastomosis site pseudoaneurysm.
4) Statistical analysis
Statistical analysis was performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean±standard deviation or proportions. Comparison between the 2 groups was performed using the Fisher's exact test for categorical variables and Student's t test for continuous variables. Survival rates were estimated using the Kaplan-Meier method and comparisons between the 2 groups were performed using the log-rank test. A p-value of less than 0.05 was considered statistically significant.
RESULTS
1) Early clinical outcomes
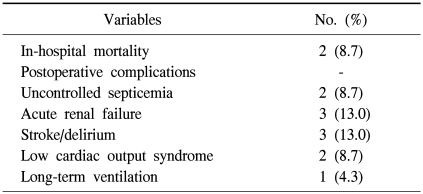
There were 2 early mortalities (8.7%) and both underwent thoracic aorta replacement due to a mycotic aneurysm and died of uncontrolled sepsis. Perioperative complications included uncontrolled sepsis (n=2), acute renal failure (n=3), and low cardiac output syndrome (n=2) (Table 2).
Table 2.
Early clinical results
2) Long-term survival
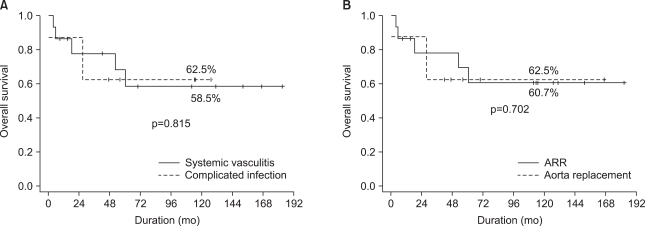
Among the early survivors, late mortality occurred in 6 patients (26.1%) and all were cardiac deaths. The overall survival rates at 5 and 10 years were 66.3% and 59.6%, respectively. Survival rate was not affected by the causative disease (systemic vasculitis versus infection involving the aortic root or aorta and its branch, p=0.815) or the surgical method (ARR vs. aortic graft replacement, p=0.702) (Fig. 1).
Fig. 1.
Log-rank tests demonstrated that overall survival rates were not related to the indication of the use of homograft (A) or the type of operation (B). ARR=aortic root replacement.
3) Freedom from homograft-related events
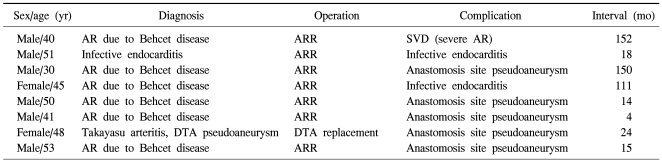
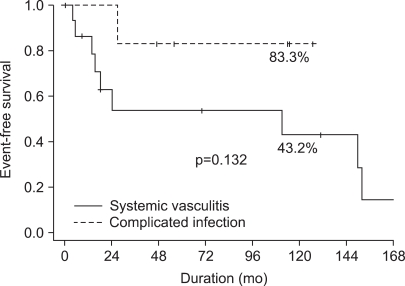
Eight patients suffered from homograft-related events as follows: homograft valve deterioration in one patient, anastomosis site pseudoaneurysm in five patients, and infective endocarditis in two patients (Table 3). The most common event was anastomosis site pseudoaneurysm (n=5). Two of these 8 patients underwent reoperation and another one of the 8 patients underwent a stent graft insertion. Still another one of the 8 patients underwent reoperation and died of sudden cardiac arrest on postoperative day 9. The 5- and 10-year homograft related event-free survival rates were 63.1% and 55.2%, respectively. Event-free survival was lower in patients with systemic vasculitis than in patients with an infectious disease, although the difference was not statistically significant (43.2% vs. 83.3% at 10 years, p=0.132) (Fig. 2). Event-free survival was not related to the surgical method (p=0.295).
Table 3.
Clinical data of patients who experienced homograft-related complications
AR=aortic regurgitation; ARR=aortic root replacement; SVD=structural valve deterioration; DTA=descending thoracic aorta.
Fig. 2.
The difference in homograft-related event-free survival rates according to the indications of homograft replacement.
DISCUSSION
The present study demonstrated two main findings about cryopreserved homograft replacement in the aortic position with acute and chronic inflammation. First, the early results of a cryopreserved homograft used in the aortic position including the aortic root or aorta and its branch were acceptable with an early mortality rate of 8.7%. Second, homograft-related events occurred more frequently in patients who underwent homograft replacement due to systemic vasculitis, although long-term survival was similar regardless of the indications or types of operation.
The cryopreserved homograft has been used as an alternative to the artificial vascular graft or composite valved conduit [1-3]. Its advantages include excellent hemodynamics, avoidance of anticoagulation, and suitability even in the presence of infection [4-8]. For these reasons, the homograft was used in patients with systemic vasculitis, complicated aortic root endocarditis, or mycotic aneurysm [2,8,10-12]. However, long-term structural deterioration was one of the main concerns after the placement of the homografts [5,9,12]. Previous studies have demonstrated that early results of homograft replacement in the aortic position were favorable, despite of the fact that it was usually performed in high risk patients with complicated infections [8,11,12]. In the present study, early mortality occurred in 2 patients and both died of uncontrollable infection that continued from the preoperative period. There were no postoperative homograft-related complications such as anastomosis site bleeding, early dehiscence of graft, or graft infection.
The overall survival rates at postoperative 5 and 10 years were 66.3% and 59.6%, respectively, with no difference in the type or indication of homograft replacement. Previous studies [9,13] have demonstrated that freedom from structural valve deterioration of homografts has ranged from 62% to 80% at 10 years. In the present study, during follow-up up to 183 months, structural valve deterioration was found in only one patient, 13 years after the initial operation. However, anastomosis site pseudoaneurysm was found in 5 patients and infective endocarditis occurred in 2 patients. Overall, the 10-year homograft-related event free survival rate was 55.2%.
Systemic vasculitis involving the aortic root and aorta is a devastating clinical situation. Conventional bioprosthesis, mechanical valve, or valved conduit replacement resulted in poor early and long term outcomes [2,10]. In the present study, 65% of the study patients underwent homograft replacement due to systemic vasculitis. We reported an early favorable outcome without mortality in such patients in a previous report [10]. Anastomosis site pseudoaneurysm is initiated by a small disruption of the anastomosis site that allows blood to leak into the surrounding space. Pseudoaneurysms have the general tendency to grow irrespective of their location, and this could end in rupture [14]. In general, it seldom occurs after homograft replacement [15]. On the contrary, in patients with systemic vasculitis, the delayed complication could occur after a homograft replacement [2,10]. This might be related to poor tissue healing in the anastomosis site due to inflammation in the native aorta and long-term use of steroids. Our surgical strategy was to avoid the use of artificial materials such as pledget or spaghetti to minimize the risk of infectious and inflammatory complications. This might have caused the high occurrence rate of anastomosis site pseudoaneurysm in our study patients. Due to this complication, the event-free survival rate was lower in patients with systemic vasculitis, although it was not statistically significant.
There are limitations to the present study that must be recognized. First, we did not perform a multivariate analysis to find risk factors for mortality or long-term complications because finding any significant risk factor was difficult in such a small patient population. Second, the number of enrolled patients was relatively small for drawing a definite conclusion.
CONCLUSION
Early results of homograft replacement in the aortic position were favorable in patients with systemic vasculitis or complicated aortic infection. However, close long-term follow-up might be necessary due to a high rate of homograft-related events, especially when homografts are used in patients with systemic vasculitis.
Footnotes
This manuscript was presented at the 20th Annual Congress of Association of Thoracic and Cardiovascular Surgeons of Asia.
References
- 1.Ross DN. Homograft replacement of the aortic valve. Lancet. 1962;2:487. doi: 10.1016/s0140-6736(62)90345-8. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Na CY, Oh SS, Lee CH, Baek MJ, Kim CW. Homograft aortic root replacement. Korean J Thorac Cardiovasc Surg. 2005;38:197–203. [Google Scholar]
- 3.Park JW, Park KY, Kim WH, Lee YT. Homograft replacement in prosthetic valve endocarditis (PVE): one case report. Korean J Thorac Cardiovasc Surg. 1997;30:815–818. [Google Scholar]
- 4.Langley SM, McGuirk SP, Chaudhry MA, Livesey SA, Ross JK, Monro JL. Twenty-year follow-up of aortic valve replacement with antibiotic sterilized homografts in 200 patients. Semin Thorac Cardiovasc Surg. 1999;11(4 Suppl 1):28–34. [PubMed] [Google Scholar]
- 5.O'Brien MF, Harrocks S, Stafford EG, et al. The homograft aortic valve: a 29-year, 99.3% follow up of 1,022 valve replacements. J Heart Valve Dis. 2001;10:334–344. [PubMed] [Google Scholar]
- 6.Barratt-Boyes BG, Roche AH, Brandt PW, Smith JC, Lowe JB. Aortic homograft valve replacement: a long-term follow-up of an initial series of 101 patients. Circulation. 1969;40:763–775. [PubMed] [Google Scholar]
- 7.Jin XY, Zhang ZM, Gibson DG, Yacoub MH, Pepper JR. Effects of valve substitute on changes in left ventricular function and hypertrophy after aortic valve replacement. Ann Thorac Surg. 1996;62:683–690. doi: 10.1016/s0003-4975(96)00438-9. [DOI] [PubMed] [Google Scholar]
- 8.Niwaya K, Knott-Craig CJ, Santangelo K, Lane MM, Chandrasekaran K, Elkins RC. Advantage of autograft and homograft valve replacement for complex aortic valve endocarditis. Ann Thorac Surg. 1999;67:1603–1608. doi: 10.1016/s0003-4975(99)00402-6. [DOI] [PubMed] [Google Scholar]
- 9.Lund O, Chandrasekaran V, Grocott-Mason R, et al. Primary aortic valve replacement with allografts over twenty-five years: valve-related and procedure-related determinants of outcome. J Thorac Cardiovasc Surg. 1999;117:77–90. doi: 10.1016/s0022-5223(99)70471-x. [DOI] [PubMed] [Google Scholar]
- 10.Jeong DS, Kim KH, Kim JS, Ahn H. Long-term experience of surgical treatment for aortic regurgitation attributable to Behçet's disease. Ann Thorac Surg. 2009;87:1775–1782. doi: 10.1016/j.athoracsur.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Musci M, Weng Y, Hubler M, et al. Homograft aortic root replacement in native or prosthetic active infective endocarditis: twenty-year single-center experience. J Thorac Cardiovasc Surg. 2010;139:665–673. doi: 10.1016/j.jtcvs.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Pagano D, Guest P, Bonser RS. Homograft replacement of thoraco-abdominal aorta for a leaking mycotic aneurysm. Eur J Cardiothorac Surg. 1996;10:383–385. doi: 10.1016/s1010-7940(96)80099-6. [DOI] [PubMed] [Google Scholar]
- 13.Hasnat K, Birks EJ, Liddicoat J, et al. Patient outcome and valve performance following a second aortic valve homograft replacement. Circulation. 1999;100(19 Suppl):II42–II47. doi: 10.1161/01.cir.100.suppl_2.ii-42. [DOI] [PubMed] [Google Scholar]
- 14.Malvindi PG, van Putte BP, Heijmen RH, Schepens MA, Morshuis WJ. Reoperations for aortic false aneurysms after cardiac surgery. Ann Thorac Surg. 2010;90:1437–1443. doi: 10.1016/j.athoracsur.2010.06.103. [DOI] [PubMed] [Google Scholar]
- 15.Coutinho GF, Antunes MJ. Pseudoaneurysm of an aortic homograft. Ann Thorac Surg. 2006;82:2280–2282. doi: 10.1016/j.athoracsur.2006.04.021. [DOI] [PubMed] [Google Scholar]