Abstract
Two case reports discussing Korean ginseng-induced allergic reactions have been published; both were inhalation-induced respiratory allergies in occupational settings. In this report we discuss the first case of anaphylaxis that developed after an oral intake of ginseng, confirmed by an open oral challenge, a skin prick test (SPT), and a basophil activation test (BAT). A 44-year-old man experienced rhinorrhea and nasal stiffness, followed by respiratory difficulty with wheeze and abdominal pain 10 minutes after oral intake of fresh ginseng. He had suffered from episodes of allergic rhinitis during the spring season for several years. Upon presentation, a physical examination, chest radiograph, and routine laboratory tests were unremarkable. Total serum IgE level was 41 IU/mL. The SPT results showed strong positive responses to alder, birch pollens, and ginseng extracts (1:500 w/v). The methacholine bronchial challenge test revealed a positive result at PC20 of 5.83 mg/mL. The open oral challenge was performed using 50 g of fresh ginseng and showed immediate onset of facial flushing, cough, respiratory difficulty with wheeze, and abdominal pain combined with a significant decrease in FEV1 levels (54% from the baseline). Serum-specific IgE and IgG4 antibodies were not detectable by enzyme-linked immunosorbent assay. BAT showed a remarkable increase in the expression of CD203c and CD63 with the addition of ginseng extract in a dose-dependent manner, while no changes were noted in the controls. In conclusion, oral intake of Korean ginseng could induce anaphylaxis, which is mediated by non-IgE-dependent direct activation of basophil/mast cells.
Keywords: Anaphylaxis, basophil, flow cytometry, Panax
INTRODUCTION
Ginseng has long been used in the Far East as a herbal medicine. The most widely used ginseng is Panax ginseng (Korean ginseng). In Korea ginseng products are commercially available in various formats such as root, powder, tablet, capsule, liquid extract, and tea.1 Ginseng is one of the most widely used medicinal plants; however, there have been two case reports of Korean ginseng-induced allergic reactions.2,3 Both cases were inhalation-induced respiratory allergies developed in occupational settings. Here, we present the first case of an anaphylactic reaction developed after oral intake of ginseng, confirmed by open oral challenge, skin prick test (SPT), and basophil activation test (BAT).
CASE REPORT
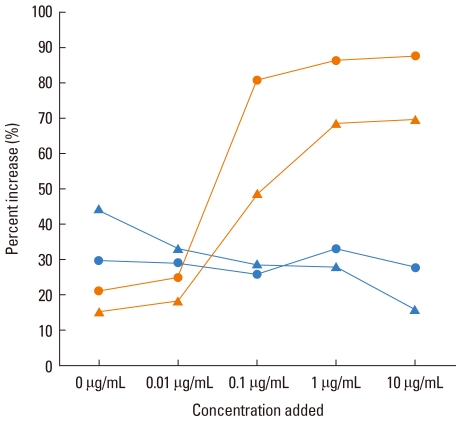
A 44-year-old man developed anaphylaxis after an oral intake of fresh ginseng. He complained of rhinorrhea and nasal stiffness, followed by respiratory difficulty and abdominal pain 10 minutes after ingestion. He had suffered from seasonal allergic rhinitis during the spring season for several years. Upon presentation, a physical examination, chest radiography, and routine laboratory tests were unremarkable, with normal serum IgE levels. To confirm the causal relationship, ginseng extracts were prepared and used for SPT, as well as for enzyme-linked immunosorbent assay (ELISA) and BAT. The SPT results (A/H ratio) showed positive responses to ginseng extract (2+ with 1:500 w/v) and fresh ginseng (3+) as well as to alder pollens (4+) and birch pollens (4+). The methacholine bronchial challenge test produced a positive result at 5.83 mg/mL. The open oral challenge was performed using 50 g of fresh ginseng, and the patient showed immediate onset of facial flushing, cough, respiratory difficulty with wheeze, and abdominal pain. The patient's blood pressure was 140/90 mmHg, respiration rate was 22 breaths/minute, pulse rate was 66/minute, and body temperature was 36.6℃. His oxygen saturation decreased to 90% from a baseline value of 96%, with a significant decrease of FEV1 (54% from baseline). The patient was treated with nasal oxygen, IV steroids and salbutamol nebulization leading to a rapid recovery. Serum-specific antibodies were detected by ELISA, and BAT was performed using flow cytometry, according to previously described methods.2,4 Serum-specific IgE and IgG4 were not detected, but a higher level of serum-specific IgG1 was noted in the patient samples, as compared to the control samples. BAT showed a dose-dependent increase in the expression of CD203c and CD63 on the basophils of the patient in response to ginseng extracts, while no changes were observed in the controls (Figure).
Figure.
Increase in the expression of CD203c and CD63 on basophils after incubation with Ginseng extracts in the patient [CD203c ( ), CD63 (
), CD63 ( )] and an atopic control [CD203c (
)] and an atopic control [CD203c ( ) CD63 (
) CD63 ( )].
)].
DISCUSSION
We believe this is the first reported case of ginseng-induced anaphylaxis, wherein sensitisation and induction was via the oral route in a non-occupational setting; both the previously reported cases of occupational asthma were induced by the inhalation of ginseng dust in the workplace.2,3 Although these two cases were confirmed by specific bronchial allergen tests, only one had high levels of serum-specific IgE antibodies,3 suggesting the involvement of a non-IgE-mediated response in its pathogenic mechanism.2 In the present study, serum-specific IgE was not detected by ELISA, although the patient had a positive SPT result. He had a high level of serum-specific IgG1 against ginseng, which may indicate that exposure to ginseng did not have a pathogenic role. The results of BAT showed a significant dose-dependent increase in the expression of the basophil activation markers, namely, CD203c and CD63, due to exposure to ginseng extracts. BAT, using these two markers is considered to be a reliable and useful tool for the diagnosis of basophil involvement in allergic diseases,5,6 suggesting that oral exposure to ginseng could directly activate basophil/mast cells, and induce anaphylaxis in the sensitised subjects. Another possible explanation for the mechanism is the cross reactivity between ginseng and tree pollen. However, we believe the possibility of this to be low, considering our wealth of experience of specific IgE detection.
In conclusion, we report the first case of anaphylaxis induced by oral exposure to Korean ginseng, which may be mediated by non-IgE dependent direct activation of basophil/mast cells. Further studies are needed to investigate other immunological and non-immunological mechanisms underlying this ginseng-induced anaphylaxis in a larger cohort of individuals with ginseng allergy.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Science and Engineering Foundation (KOSEF), funded by the Korea Government (MEST, 2009-0078646).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16(Suppl):S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JY, Lee YD, Bahn JW, Park HS. A case of occupational asthma and rhinitis caused by Sanyak and Korean ginseng dusts. Allergy. 2006;61:392–393. doi: 10.1111/j.1398-9995.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim KM, Kwon HS, Jeon SG, Park CH, Sohn SW, Kim DI, Kim SS, Chang YS, Kim YK, Cho SH, Min KU, Kim YY. Korean ginseng-induced occupational asthma and determination of IgE binding components. J Korean Med Sci. 2008;23:232–235. doi: 10.3346/jkms.2008.23.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JH, An S, Kim JE, Choi GS, Ye YM, Park HS. Beef-induced anaphylaxis confirmed by the basophil activation test. Allergy Asthma Immunol Res. 2010;2:206–208. doi: 10.4168/aair.2010.2.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bühring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol. 2004;133:317–329. doi: 10.1159/000077351. [DOI] [PubMed] [Google Scholar]
- 6.Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry B Clin Cytom. 2008;74:201–210. doi: 10.1002/cyto.b.20419. [DOI] [PubMed] [Google Scholar]