Abstract
Purpose
Previous studies have outlined mechanisms by which Mycoplasma pneumonia (M. pneumonia) infection may promote allergic lung inflammation and airway remodeling, and increasing evidence from human studies suggests that atypical bacterial infections contribute to asthma exacerbation, chronic asthma, and disease severity with changes in cytokine expression. The present study evaluated changes in serum levels of vascular endothelial growth factor (VEGF) and interleukin (IL)-5 in atopic children with Mycoplasma pneumoniae pneumonia.
Methods
We recruited a total of 72 children with pneumonia. The patients were divided into 4 groups: atopic children with M. pneumonia pneumonia (group I, n=24), non-atopic children with M. pneumonia pneumonia (group II, n=23), atopic children with viral pneumonia (group III, n=13), and non-atopic children with viral pneumonia (group IV, n=12). Serum levels of IL-5, IL-13, VEGF, and tumor necrosis factor-α were measured at admission and at recovery using enzyme-linked immunosorbent assays.
Results
Serum levels of VEGF and IL-5 were elevated in group I compared with the other groups at both admission phase and clinical recovery phase. In group I, serum levels of VEGF and IL-5 were higher at recovery phase than at admission phase (VEGF: 1,102.2±569.4 vs. 874.9±589.9 pg/mL, respectively; IL-5: 150.5±63.9 vs. 120.2±46.7 pg/mL, respectively).
Conclusions
The serum levels of VEGF and IL-5 were more increased in atopic children with M. pneumonia pneumonia than in the other groups. In this group, the serum levels of VEGF and IL-5 were more increased at recovery phase than at admission phase. The results of this study suggest that increases in VEGF and IL-5 may contribute to the development of hypersensitivity during M. pneumonia infection. These cytokines may act through their respective pro-inflammatory pathways to aggravate the allergic status and induce airway hypersensitivity during M. pneumonia pneumonia in atopic children.
Keywords: Atopy; interleukin-5; Pneumonia, Mycoplasma; vascular endothelial growth factor
INTRODUCTION
Mycoplasma pneumoniae (M. pneumoniae), an atypical microorganism, is a major causative agent of respiratory infection in children1 and is isolated from more than 40% of patients with community-acquired pneumonia.2 M. pneumoniae infection occurs most commonly in children aged 4-5 years, with waves of prevalence every 3-4 years.1
The relationship between M. pneumoniae infection and asthma development has been debated for the past 20 years.3 Previous studies have demonstrated that the incidence of M. pneumoniae infection is higher in patients with chronic stable asthma or acute exacerbation than in control subjects.4-6 In addition, M. pneumoniae infection has been shown to correlate with the induction and aggravation of asthma symptoms. Biscardi et al.7 have demonstrated that the recurrence rate of asthma is higher in atopic children aged 2-15 years whose first attack was accompanied by M. pneumoniae infection, compared with those without. This observation suggests that M. pneumoniae infection is related to asthma attacks. Further studies analyzing the expression of various cytokines are needed to understand the relationship between M. pneumoniae infection and asthma.
Vascular endothelial growth factor (VEGF) is a major mediator that induces airway hypersensitivity to inhalant allergens. VEGF is secreted from endothelial cells and smooth muscle cells as well as inflammatory cells upon inflammatory reactions. It induces angiogenesis, permeability changes, and edema formation in the airway wall; all of these play crucial roles in the pathophysiology of asthma. Hypersecretion of VEGF in the airways induces T cell sensitization to inhalant allergens and stimulates the synthesis of co-stimulatory molecules in lung-resident dendritic cells.8 A previous study has indicated that serum VEGF levels are significantly higher in M. pneumoniae infected-patients with wheezing than in those without wheezing.9
Interleukin (IL)-5 is primarily secreted by Th2 cells and is related to the growth, maturation, differentiation, survival, and activation of eosinophils. Eosinophils accumulate in the lungs of transgenic mice that overexpress IL-5.10,11 Moreover, IL-5 plays a role in the development of asthma. Choi et al.12 have demonstrated that serum levels of VEGF and IL-5 are significantly higher in atopic patients with wheezing than in those without or in control subjects. IL-13, which plays a role in airway remodeling, induces tissue fibrosis through transforming growth factor-β activation.13 In addition, IL-13 has been shown to induce myofibroblast hyperplasia, airway obstruction, and airway hyperresponsiveness.9 In in vitro experiments, Thavagnanam et al.14 showed that IL-13 enhances goblet cell hyperplasia and decreases the number of bronchial epithelial cells detected in samples taken from children, suggesting an IL-13-mediated mechanism for the development of asthma. Interferon (IFN)-γ is released by Th1 cells and suppresses Th2 cells, thus maintaining the balance between Th1 and Th2 cells. Stirling and Chung15 have advocated that serum IFN-γ levels are decreased in atopic patients and that there is a significant relationship between the decrease in IFN-γ and the severity of asthma. Lack et al.16 reported that IL-5 levels and the number of eosinophils are both decreased in bronchoalveolar lavage fluid after intrathecal administration of IFN-γ. Tumor necrosis factor (TNF)-α increases airway responsiveness and promotes the synthesis of various cytokines such as regulated upon activation, normal T-cell expressed and secreted, IL-8, and granulocyte-macrophage stimulating factor, which in turn initiate chronic airway inflammation.15
This study was conducted to investigate the effect of Mycoplasma infection on allergic diseases by measuring the serum levels of VEGF, IL-5, IL-13, IFN-γ, and TNF-α.
MATERIALS AND METHODS
Study subjects
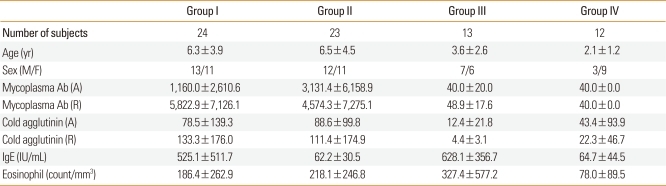
This study enrolled a total of 72 children who were admitted to the Department of Pediatrics at Hanyang University Guri Hospital in Korea, due to pneumonia. This study was performed prospectively from October, 2010 to June, 2011. The mean age of the children was 5.2±3.9 years (range, 2-15 years). All children underwent evaluation for past/family history of allergic diseases, physical examination, routine laboratory tests, and the measurement of total IgE and the number of eosinophils in serum. The children were divided into 4 groups (Table 1): those with M. pneumoniae pneumonia who had increased serum IgE and past/family history of allergic diseases and (group I, n=24); those with M. pneumoniae pneumonia who had normal serum IgE and no past/family history of allergic diseases (group II, n=23); those with viral pneumonia who had increased serum IgE and past/family history of allergic diseases (group III, n=13); and those with viral pneumonia who had normal serum IgE and no past/family history of allergic diseases (group IV, n=12). The protocol and consent forms of this study were approved by the Institutional Review Board at Hanyang University. Informed written consent was obtained from all parents and/or participants.
Table 1.
Characteristics of subject in the study
Diagnosis of M. pneumoniae pneumonia
Pneumonia was diagnosed based on clinical symptoms such as fever, cough, dyspnea, and crackles and radiological findings. M. pneumoniae as a causative organism of pneumonia was identified by anti-mycoplasma antibody titers, which were measured by the microparticle agglutination method using a commercial kit (Serodia-Myco II, Fujirebio, Tokyo, Japan). Anti-mycoplasma antibody titers were regarded as positive when the they were ≥1:160 at admission phase or when the titers at symptomatic recovery phase were more than four-fold those measured at admission phase. The range of duration from admission to symptomatic recovery was 7-10 days.
Diagnosis of viral pneumonia
Viral pneumonia was diagnosed based on respiratory symptoms, positive radiographic findings, and positive results of virus polymerase chain reaction (PCR) analysis of nasopharyngeal secretions. At admission, samples of nasopharyngeal secretions were obtained from the nasopharynx by aspiration through catheters after injecting 5 mL of sterile physiological saline solution into mucus traps. Each specimen was independently screened for the presence of common respiratory viruses, including influenza viruses, parainfluenza viruses, human coronaviruses, human rhinoviruses, human metapneumoviruses, human adenoviruses, and respiratory syncytial viruses, by reverse transcription-PCR using a commercial kit (Seeplex RV 12 ACE Detection, Seegene, Seoul, Korea).
Measurement of serum IgE and number of eosinophils
Serum IgE levels were measured using an IRMA kit (Diagnostic Products Co., Los Angeles, CA, USA). The number of peripheral eosinophils was measured with an automated hematology analyzer (Coulter Counter STKS, Beckman Coulter, Fullerton, CA, USA), using blood samples collected into tubes containing EDTA.
Measurement of serum VEGF, IL-5, IL-13, IFN-γ, and TNF-α
Venous blood was taken from each subject, centrifuged at 1,000×g for 10 minute at 4°, and stored at -70℃. IL-5, IL-13, IFN-γ, and TNF-α levels were measured using commercial enzyme-linked immunosorbent assays (ELISAs) (DBD Inc., San Diego, CA, USA), according to the manufacturer's instructions. The detection limit was 2 pg/mL for IL-5, IL-13, IFN-γ, and TNF-α. VEGF was measured using a human VEGF kit (Biosource, Camarillo, CA, USA). The values measured at admission were compared with those measured at the time of symptomatic recovery.
Statistical analysis
Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The data are expressed as means±SD. Statistical comparisons were made with paired t-tests. A P value of <0.05 indicated statistical significance. Parametric one-way ANOVA with post hoc Tukey correction was used to compare levels of VEGF and IL-5 among the study groups.
RESULTS
Each patient group shows different clinical characteristics, laboratory results, total IgE level, and number of eosinophils (Table 1). In groups I and II, anti-mycoplasma antibody titers were ≥1:160 at admission phase; the titers at the time of symptomatic recovery phase were more than four-fold those at admission phase. In groups III and IV, anti-mycoplasma antibody titers were <1:40 at admission phase. and were not increased at recovery phase. Serum IgE levels were significantly higher in groups I and III compared with groups II and IV. There was no significant difference in the number of eosinophils among the four subject groups.
Serum VEGF levels
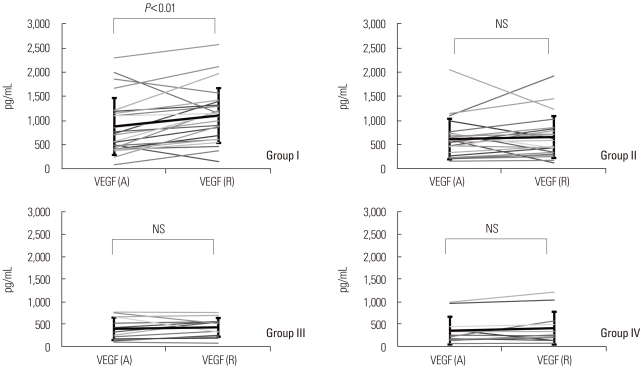
Serum VEGF levels measured at admission phase were significantly higher in group I (874.9±589.9 pg/mL) than in groups III and IV (396.9±251.5 and 356.9±310.6 pg/mL, respectively; P<0.01). At the time of symptomatic recovery phase, serum VEGF levels were significantly higher in group I (1,102.2±569.4 pg/mL) than in groups II, III and IV (659.4±434.8, 431.3±210.6, and 411.7±367.6 pg/mL, respectively) (Fig. 1; Table 2). Serum VEGF levels tended to be higher at recovery phase than at admission phase in all four groups, but only group I showed significantly increased VEGF levels at the time of symptomatic recovery phase (1,102.2±569.4 vs. 874.9±589.9 pg/mL, recovery phase vs. admission phase; P<0.05).
Fig. 1.
Comparison of serum VEGF levels at admission phase (A) and at clinical recovery phase (R) in the four subject groups. The mean serum VEGF level was higher at clinical recovery phase than at admission phase in group I, whereas the mean serum VEGF levels did not differ significantly between recovery phase and admission phase in the other groups.
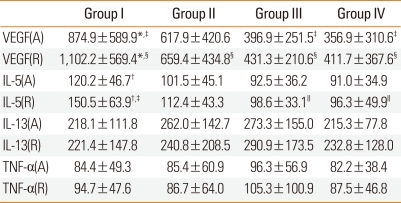
Table 2.
The comparison of serum VEGF, IL-5, IL-13, and TNF-α level in the study
*VEGF(A) vs VEGF(R): P<0.01, †IL-5(A) vs IL-5(R): P=0.01, ‡VEGF(A) group I vs group III, group I vs group IV: P<0.01, §VEGF(R) group I vs group II, group I vs group III, group I vs group IV: P<0.01, ∥IL-5(R) group I vs group III, group I vs group IV: P<0.05, Scale for cytokine level: pg/mL.
VEGF, vascular endothelial growth factor; IL, interleukin; TNF, Tumor necrosis factor.
Serum levels of IL-5, IL-13, and TNF-α
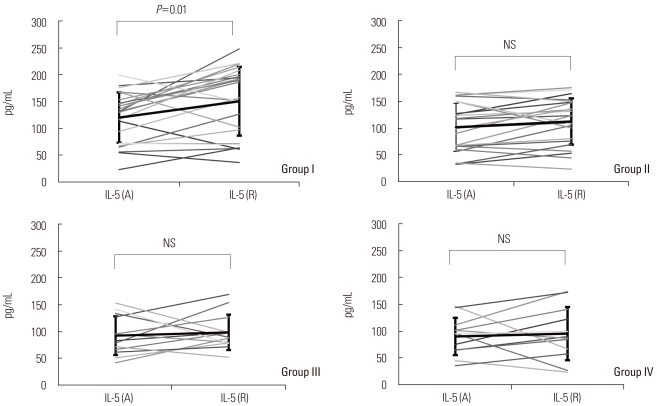
Serum IL-5 levels were significantly higher in group I (120.2±46.7 pg/mL) than in groups III and IV (92.5±36.2 and 91.0±34.9 pg/mL, respectively; P<0.01) at admission phase. Serum IL-5 levels were increased at recovery phase compared with those at admission phase in all four groups, but the increase was significant only in group I (150.5±63.9 vs. 120.2±46.7 pg/mL, recovery phase vs. admission phase; P<0.05). At the time of symptomatic recovery phase, serum IL-5 was significantly higher in group I (150.5±63.9 pg/mL) than in groups III and IV (98.6±33.1 and 96.3±49.9 pg/mL, respectively; P<0.05) (Fig. 2; Table 2).
Fig. 2.
Comparison of serum IL-5 levels at admission phase (A) and at clinical recovery phase (R) in the four subject groups.The mean serum IL-5 level was higher at clinical recovery phase than at admission phase in group I, whereas the mean serum IL-5 levels did not differ significantly between recovery phase and admission phase in the other groups.
Serum levels of IL-13 and TNF-α were not significantly different among the four groups, at admission phase or at recovery phase. Serum IL-13 and TNF-α levels did not differ significantly between admission phase and symptomatic recovery phase in any group (Table 2).
DISCUSSION
In this study, serum VEGF levels were significantly higher in group I than in groups II and III, and IV at admission phase. Our findings, which included atopic children with and without wheezing, are consistent with those of previous studies that included children with wheezing.9,12 It has been suggested that compared with allergy, M. pneumoniae can more frequently aggravate or initiate asthma symptoms, and that M. pneumoniae pneumonia and asthma are significantly correlated in terms of clinical symptoms.17,18 It has also been shown that allergic reactions are induced or aggravated by IgE-related hypersensitivity and Th2 immune reactions.19-21 M. pneumoniae induces upper airway infections such as acute pharyngitis and laryngitis more frequently than lower airway infections.22 Clyde23 reported that 20% of patients with M. pneumoniae infection were asymptomatic, and that less than 5% of all patients with M. pneumoniae infection had lower airway infections such as pneumonia. Based on these results, it is conceivable that M. pneumoniae infection may stimulate or aggravate allergic reactions.
In the present study, VEGF levels were significantly higher in atopic children with M. pneumoniae pneumonia than in those with viral pneumonia. VEGF, an endothelial cell-specific mitogenic peptide, serves as a vascular permeability factor and plays a crucial role in vasculogenesis and angiogenesis. It also induces extravasation of protein-rich plasma from the airway, increases vascular permeability, and causes airway inflammation through edema formation.24,25 Increased VEGF levels with M. pneumoniae infection suggest that M. pneumoniae stimulates allergic inflammation, as well as airway vascular angiogenesis and edema formation, consequently aggravating airway inflammation. In previous studies, the levels of IL-13, IL-4, and TNF-α were increased with M. pneumoniae infection, suggesting that this infection can aggravate asthma symptoms.26,27 However, in our study, there were no significant differences in serum IL-13 and TNF-α levels between admission phase and symptomatic recovery phase in any of the four study groups. This apparent discrepancy may be attributable to differences between the two study cohorts.
In the present study, serum IL-5 levels at admission were higher in groups I and II (M. pneumonia groups) than in groups III and IV (viral pneumonia groups), although these differences did not reach statistical significance. This result agrees with previous results reported by Esposito et al.28
Serum levels of VEGF and IL-5 were significantly higher at the time of symptomatic recovery phase than at admission phase. This implies that M. pneumonia infection stimulates bacterial inflammation as well as allergic inflammation, and that inflammation reactions related to VEGF and IL-5 persist even after recovery from M. pneumoniae pneumonia, prolonging allergic inflammation. The increased VEGF levels at the time of symptomatic recovery phase in our study suggest the presence of VEGF-induced increases in vascular permeability, airway vascular angiogenesis, and edema formation, all of which aggravate airway inflammation. Serum IL-5 levels at recovery phase were significantly higher in group I than in the other groups, indicating that IL-5-dependent allergic inflammatory reactions are persistently induced by M. pneumoniae infection in atopic children, but not in non-atopic children.
In the present study, serum levels of IL-13 and TNF-α tended to be higher in atopic children with viral pneumonia (group III) compared with the other groups; however, the differences were not significant. We also did not detect differences in serum cytokine levels between children with viral pneumonia caused by respiratory syncytial virus or rhinovirus and children infected by other viruses (data not shown).
The serum levels of VEGF and IL-5 were more increased in atopic children with M. pneumonia pneumonia than in the other groups. In this group, the serum levels of VEGF and IL-5 were more increased at recovery phase than at admission phase.
In conclusion, our study results suggest that M. pneumoniae pneumonia may induce allergic inflammatory reactions as well as bacterial inflammatory reactions and can persistently aggravate existing allergic inflammation. Further studies with larger sample sizes are needed to confirm our results. Mechanisms of allergic reactions through other cytokine mediators also merit additional investigation.
ACKNOWLEDGMENTS
This study was supported by the 2010 Hanyang University, College of Medicine, Research Fund (HY-2010-MC) and a Research Grant of Alumni of Pediatrics, College of Medicine, Hanyang University.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Kim KW, Kim KE. Mycoplasma and chlamydia infection in Korea. Korean J Pediatr. 2009;52:277–282. [Google Scholar]
- 2.Kim JW, Seo HK, Yoo EG, Park SJ, Yoon SH, Jung HY, Han MY. Mycoplasma pneumoniae pneumonia in Korean children, from 1979 to 2006-a meta-analysis. Korean J Pediatr. 2009;52:315–323. [Google Scholar]
- 3.Hansbro PM, Beagley KW, Horvat JC, Gibson PG. Role of atypical bacterial infection of the lung in predisposition/protection of asthma. Pharmacol Ther. 2004;101:193–210. doi: 10.1016/j.pharmthera.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Esposito S, Blasi F, Arosio C, Fioravanti L, Fagetti L, Droghetti R, Tarsia P, Allegra L, Principi N. Importance of acute Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with wheezing. Eur Respir J. 2000;16:1142–1146. doi: 10.1034/j.1399-3003.2000.16f21.x. [DOI] [PubMed] [Google Scholar]
- 5.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, Gaydos CA, Martin RJ. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 6.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 7.Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, Heilbronner C, Iniguez JL, Chaussain M, Nicand E, Raymond J, Gendrel D. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–1346. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 8.Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS. 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- 9.Seo Y, Yu BK, Oh YJ, Lee Y, Yoo Y, Choung JT, Koh YY. Increased vascular endothelial growth factor in children with acute Mycoplasma pneumoniae pneumonia and wheezing. Korean J Pediatr. 2008;51:487–491. [Google Scholar]
- 10.Mould AW, Ramsay AJ, Matthaei KI, Young IG, Rothenberg ME, Foster PS. The effect of IL-5 and eotaxin expression in the lung on eosinophil trafficking and degranulation and the induction of bronchial hyperreactivity. J Immunol. 2000;164:2142–2150. doi: 10.4049/jimmunol.164.4.2142. [DOI] [PubMed] [Google Scholar]
- 11.Rosenwasser LJ, Rothenberg ME. IL-5 pathway inhibition in the treatment of asthma and Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:1245–1246. doi: 10.1016/j.jaci.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Choi IS, Byeon JH, Yoo Y, Lee KC, Choung JT. Increased serum interleukin-5 and vascular endothelial growth factor in children with acute mycoplasma pneumonia and wheeze. Pediatr Pulmonol. 2009;44:423–428. doi: 10.1002/ppul.20961. [DOI] [PubMed] [Google Scholar]
- 13.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thavagnanam S, Parker JC, McBrien ME, Skibinski G, Heaney LG, Shields MD. Effects of IL-13 on mucociliary differentiation of pediatric asthmatic bronchial epithelial cells. Pediatr Res. 2011;69:95–100. doi: 10.1203/PDR.0b013e318204edb5. [DOI] [PubMed] [Google Scholar]
- 15.Stirling RG, Chung KF. New immunological approaches and cytokine targets in asthma and allergy. Eur Respir J. 2000;16:1158–1174. doi: 10.1034/j.1399-3003.2000.16f24.x. [DOI] [PubMed] [Google Scholar]
- 16.Lack G, Bradley KL, Hamelmann E, Renz H, Loader J, Leung DY, Larsen G, Gelfand EW. Nebulized IFN-gamma inhibits the development of secondary allergic responses in mice. J Immunol. 1996;157:1432–1439. [PubMed] [Google Scholar]
- 17.MacDowell AL, Bacharier LB. Infectious triggers of asthma. Immunol Allergy Clin North Am. 2005;25:45–66. doi: 10.1016/j.iac.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nisar N, Guleria R, Kumar S, Chand Chawla T, Ranjan Biswas N. Mycoplasma pneumoniae and its role in asthma. Postgrad Med J. 2007;83:100–104. doi: 10.1136/pgmj.2006.049023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tipirneni P, Moore BS, Hyde JS, Schauf V. IgE antibodies to Mycoplasma pneumoniae in asthma and other atopic diseases. Ann Allergy. 1980;45:1–7. [PubMed] [Google Scholar]
- 20.Yano T, Ichikawa Y, Komatu S, Arai S, Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1994;149:1348–1353. doi: 10.1164/ajrccm.149.5.8173777. [DOI] [PubMed] [Google Scholar]
- 21.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32:956–973. doi: 10.1111/j.1574-6976.2008.00129.x. [DOI] [PubMed] [Google Scholar]
- 23.Clyde WA., Jr Mycoplasma pneumoniae respiratory disease symposium: summation and significance. Yale J Biol Med. 1983;56:523–527. [PMC free article] [PubMed] [Google Scholar]
- 24.Hirakawa S, Okazaki H, Sayama K, Tohyama M, Hashimoto K. Possible association of vascular endothelial growth factor with the development of edema in drug-induced hypersensitivity syndrome. J Dermatol. 2011;38:292–294. doi: 10.1111/j.1346-8138.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 25.Corrigan CJ, Wang W, Meng Q, Fang C, Wu H, Reay V, Lv Z, Fan Y, An Y, Wang YH, Liu YJ, Lee TH, Ying S. T-helper cell type 2 (Th2) memory T cell-potentiating cytokine IL-25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci U S A. 2011;108:1579–1584. doi: 10.1073/pnas.1014241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraft M, Cassell GH, Duffy LB, Metze T, Pak J, Gaydos CA, Martin RJ. Mycoplasma and Chlamydia are present in the airways of chronic, stable asthmatics. Am J Respir Crit Care Med. 1999;159:A517. [Google Scholar]
- 27.Hassan J, Irwin F, Dooley S, Connell J. Mycoplasma pneumoniae infection in a pediatric population: analysis of soluble immune markers as risk factors for asthma. Hum Immunol. 2008;69:851–855. doi: 10.1016/j.humimm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Esposito S, Droghetti R, Bosis S, Claut L, Marchisio P, Principi N. Cytokine secretion in children with acute Mycoplasma pneumoniae infection and wheeze. Pediatr Pulmonol. 2002;34:122–127. doi: 10.1002/ppul.10139. [DOI] [PubMed] [Google Scholar]