Abstract
Purpose
The aim of this study was to evaluate serum levels of leptin, ghrelin, and adiponectin in obese and non-obese children with asthma and in healthy non-asthmatic children, and analyze their relationships with clinical outcomes.
Methods
This study enrolled 40 obese and 51 non-obese children with asthma and 20 healthy children. Body mass index and serum leptin, ghrelin, and adiponectin levels were determined in all children. Asthma symptom scores and lung function test results were recorded for subjects with asthma.
Results
Serum leptin levels (11.8±7.9, 5.3±6.8, and 2.1±2.4 ng/mL in the obese asthmatic, non-obese asthmatic, and control groups, respectively) and adiponectin levels (12,586.2±3,724.1; 18,089.3±6,452.3; and 20,297.5±3,680.7 ng/mL, respectively) differed significantly among the groups (P<0.001 for all). Mean ghrelin levels were 196.1±96.8 and 311.9±352.8 pg/mL in the obese and non-obese asthmatic groups, respectively, and 348.8±146.4 pg/mL in the control group (P=0.001). The asthma symptom score was significantly higher in the obese children with asthma than in the non-obese children with asthma (P<0.001). Leptin and adiponectin levels were correlated with the asthma symptom score in non-obese children with asthma (r=0.34 and r=-0.62, respectively).
Conclusions
Obesity leads to more severe asthma symptoms in children. Moreover, leptin, adiponectin, and ghrelin may play important roles in the inflammatory pathogenesis of asthma and obesity co-morbidity.
Keywords: Asthma, obesity, leptin, ghrelin, adiponectin, child
INTRODUCTION
Obesity increases the prevalence and severity of asthma in children and has a negative impact on the efficacy of conventional asthma therapy; however, the mechanism of the association between obesity and asthma is not clear.1-3 Changes in lung volume, including tidal volume due to obesity; the inflammatory effects of adipose tissue hormones and cytokines such as leptin, adiponectin, tumor necrosis factor (TNF) and interleukin (IL)-6; and conditions such as gastroesophageal reflux and sleep-disordered breathing associated with obesity have been implicated in the association between obesity and asthma.2,4 The disruption of immune tolerance mechanisms by obesity-associated adipokines and cytokines has been demonstrated to be involved in the association of obesity with asthma and autoimmune diseases.4
Leptin, adiponectin, and ghrelin are important mediators of energy metabolism and have been reported to have pro- or anti-inflammatory functions.5-8 Serum levels of leptin are increased in obesity, whereas those of adiponectin and ghrelin are decreased.5-8 Increased leptin levels lead to the downregulation of regulatory T cells, and the decrease in adiponectin causes the downregulation of IL-10 secretion.4 The reduction of serum Turkeyghrelin levels with obesity represses IL-1β, IL-6, and TNF-α levels, further disrupting anti-inflammatory mechanisms.9 The main outcome of these obesity-associated changes is a hyper-reactive immune system, which is susceptible to the development of allergic and autoimmune diseases.4
The aim of this study was to evaluate the levels of three hormones of energy metabolism (leptin, ghrelin, and adipokine) in obese and non-obese children with asthma and in healthy children without asthma, and to investigate the association between these levels and clinical outcome.
MATERIALS AND METHODS
Subjects
This study included 91 children (40 obese and 51 non-obese) with new-onset moderate asthma and 20 children without asthma. In accordance with the guidelines of the Global Initiative for Asthma, asthma was diagnosed based on a history of recurrent or chronic chest symptoms such as cough, wheezing, difficult breathing, and chest tightness that demonstrated clinical reversibility with short-acting bronchodilator treatment.10 The children with asthma had not received anti-inflammatory treatment.
Children with any other acute or chronic disease, including acute upper or lower respiratory tract infection, were excluded, as were children who had received treatment for asthma within the previous 3 months.
The children with asthma were grouped as obese and non-obese according to their body mass index (BMI) percentile, where obesity was defined as a BMI greater than the 95th percentile for age and gender.11,12 The BMI was calculated according to the formula: weight (kg)/height2 (m2).
Study design
Sociodemographic characteristics of the children, including age and gender, were recorded. Height and weight were measured using a stadiometer and an electronic scale, respectively, under the supervision of the researchers. Asthma symptom scores were assessed and recorded for the children with asthma. Serum leptin, ghrelin, and adiponectin levels were measured in fasting serum samples obtained in early morning. The study was approved by the university ethics committee; informed consent was obtained from the parents of the children.
Determination of symptom scores
Symptom scores in children with asthma were assessed according to a six-domain asthma symptom score.13 The items in the scale included dyspnea, tightness in the chest, wheezing during the day, wheezing during the night, and daily performance, scored from zero to three based on severity.
Blood sampling
For the measurement of leptin, ghrelin, and adiponectin levels, venous blood (2 mL) was obtained at 9:00 a.m. after an overnight fast. Blood samples were allowed to clot at room temperature for 60 minutes. The serum was separated by centrifugation at 1,200×g for 10 minutes and stored at -80℃. Sera were thawed at room temperature before measurements.
Measurement of serum leptin, ghrelin, and adiponectin
The serum leptin concentration was determined using a double antibody sandwich ELISA method with an antibody specific for human leptin (Biosource Leptin ELISA kit KAP2281; Biosource Europe S.A., Nivelles, Belgium). The assay sensitivity was 0.1 ng/mL, and the intra-assay %CV was 3.6.
The serum ghrelin concentration was measured using a double antibody sandwich ELISA method with an antibody specific for human ghrelin (Desacyl-Ghrelin ELISA kit; Linco Research Inc., St. Charles, MO, USA). The assay sensitivity was 12.5 mmol/mL, the inter-assay %CV was 8.6, and the intra-assay %CV was 3.6.
The serum adiponectin concentration measured by the double antibody sandwich ELISA method with an antibody specific for human adiponectin (Linco Research Inc.). The assay sensitivity was 0.78 ng/mL, the inter-assay %CV was 8.4, and the intra-assay %CV was 7.4.
Statistical analysis
Statistical analyses were performed using the SPSS software package, version 13.0 for Windows (SPSS, Chicago, IL, USA). Values of P<0.05 were taken to indicate significance. A Kruskall-Wallis test was used to compare leptin, ghrelin, and adiponectin levels and the mean age among the three groups. Frequencies were compared with a Chi-square test. The relationships between the adipokine levels and asthma severity parameters were examined using Pearson's correlation analysis.
RESULTS
Demographic and disease characteristics of the subjects
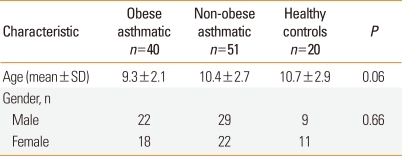
The mean ages of the obese children with asthma (n=40), non-obese children with asthma (n=51), and control children (n=20) were 9.3±2.1, 10.4±2.7, and 10.7±2.9 years, respectively (P=0.03). Males constituted 55, 56.9, and 45% of the respective groups (P=0.66) (Table 1).
Table 1.
Demographic characteristics of the children included in the study
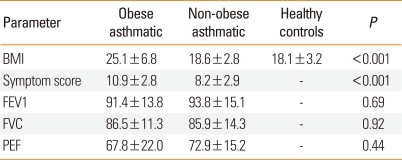
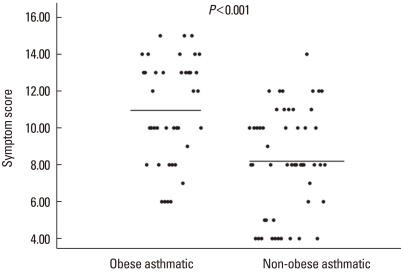
The BMI and abdominal circumference were significantly greater in the obese group than in the non-obese or control groups (P<0.001) (Table 2). The mean asthma symptom score was significantly higher in the obese children with asthma than in the non-obese children with asthma (10.9±2.8 vs. 8.2±2.9; P<0.001) (Table 2; Fig. 1).
Table 2.
BMI, lung function test results, and symptom scores in the study groups
BMI, body mass index.
Fig. 1.
Symptom scores of obese and non-obese children with asthma.
Lung function test results were not significantly different between the obese and non-obese children with asthma (P>0.05 for FEV1, FVC, and PEF) (Table 2).
Serum leptin, ghrelin, and adiponectin levels
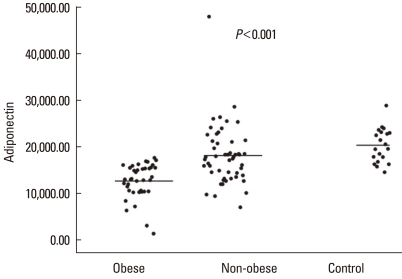
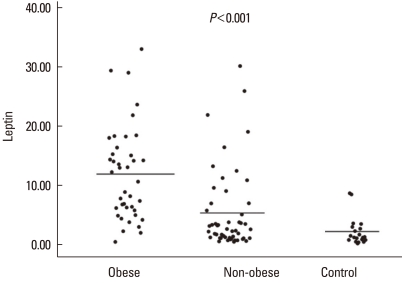
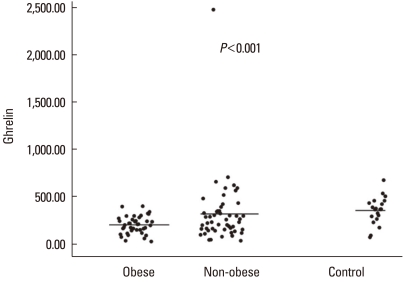
The serum leptin level differed significantly (P<0.001) among the obese asthmatic (11.8±7.9 ng/mL), non-obese asthmatic (5.3±6.8 ng/mL), and control groups (2.1±2.4 ng/mL) (Fig. 2). The serum adiponectin level also differed significantly (P<0.001) among the obese asthmatic (12,586.2±3,724.1 ng/mL), non-obese asthmatic (18,089.3±6,452.3 ng/mL), and control groups (20,297.5±3,680.7 ng/mL) (Fig. 3). The serum ghrelin levels in the obese and non-obese asthmatic groups were 196.1±96.8 and 311.9±352.8 pg/mL, respectively, and the level in the control group was 348.8±146.4 pg/mL (P=0.001) (Fig. 4).
Fig. 2.
Serum leptin levels (ng/mL) of the study groups.
Fig. 3.
Serum adiponectin levels (ng/mL) of the study groups.
Fig. 4.
Serum ghrelin levels (pg/mL) of the study groups.
Correlation between serum adipokine levels and clinical outcome
In the non-obese group, the leptin level was significantly correlated with age, BMI, and abdominal circumference (r=0.42, 0.68, and 0.67, respectively). The leptin level showed a significant negative correlation with the adiponectin level (r=-0.32), but not with the ghrelin level (r=-0.09). The leptin level also had a significant positive correlation with the symptom score (r=0.34), while the symptom score and adiponectin level were significantly negatively correlated (r=-0.62), and the symptom score and ghrelin level were not significantly correlated (r=-0.12).
In the obese group, the leptin level was not correlated with any of the sociodemographic characteristics, including age, BMI, and symptom score (P>0.05 for all). The leptin level was also not correlated with the adiponectin or ghrelin level (r=-0.09, and 0.11, respectively), and neither the adiponectin nor ghrelin level was correlated with any of the disease severity parameters (P>0.05).
In the control group, the leptin, adiponectin, and ghrelin levels were not significantly correlated with each other or with age or BMI (P>0.05).
DISCUSSION
There is a trend toward an increasing frequency of both obesity and asthma in the modern world, and elucidation of the pathogenic mechanism of the association between these two entities may aid in resolving therapeutic failure in obese children with asthma.1,2 Several mechanisms have been proposed, including a common etiological background such as fetal programming, worsening of asthma due to co-morbid conditions of obesity such as gastroesophageal reflux disease, and the influence of obesity on lung mechanics such as decreased tidal volume.2,3 Recently, hormones and cytokines released from adipose tissue, especially adipokines and ghrelin, have been the focus of research regarding this association.2,3,14 Our data demonstrate increased leptin levels in asthmatic children, and this increase was more prominent in obese children. Additionally, adiponectin and ghrelin levels were lower in obese than non-obese children with asthma. All of these data favor the hypothesis that adipokines play important roles in the inflammatory pathogenesis of asthma in obese individuals.
Leptin, a hormone of adipose tissue, is associated with inflammation,2 and its levels have been shown to be higher in children with asthma than in those without asthma.15,16 Leptin is a regulator of T cell proliferation and activation, monocyte recruitment and activation, and angiogenesis.17 Moreover, it plays a role in normal lung development.18 It has been shown to modulate the allergic airway response independent of obesity and to enhance the airway inflammatory response leading to higher antigen-induced T cell responses, increased mitogen-induced splenocyte interferon-γ production, and an increased number of tracheal mast cells.19,20 Our data indicate that leptin levels were significantly higher in obese and non-obese children with asthma than in healthy controls. This suggests a role of leptin as an inflammatory mediator in asthma, in concordance with previous reports of the inflammatory characteristics of this adipokine.19,20 Moreover, the higher leptin levels in obese children with asthma might have contributed to their higher symptom scores, compared with non-obese children with asthma.
Despite the higher symptom scores in obese compared with non-obese children with asthma, the leptin levels in obese children were not correlated with the asthma symptom scores. In contrast, leptin levels demonstrated a significant correlation with symptom scores in non-obese children with asthma. The absence of this correlation in obese children with asthma was attributed to the influence of obesity on leptin levels, such that the importance of obesity in determining leptin levels might have masked the presence of a correlation. However, it is more likely that a correlation was demonstrated in non-obese children with asthma because leptin plays a role in the inflammatory response in these children.
Adiponectin, similar to leptin, influences energy metabolism, but it has anti-inflammatory effects.5 Adiponectin is negatively associated with obesity because its concentration increases with weight loss.21 The decrease of adiponectin in obesity may be related to the association between obesity and asthma,5,22 as allergen challenge leads to less airway responsiveness and inflammation in animal models with higher adiponectin levels.5 Moreover, adiponectin inhibits the proliferation of cultured vascular smooth muscle cells.23 If adiponectin were to have a similar influence on airway smooth muscle, the decrease in adiponectin in obese individuals could contribute to increased smooth muscle mass in asthmatic individuals.24 The lower adiponectin levels in obese compared with non-obese children with asthma and the lower adiponectin levels in non-obese children with asthma compared with controls in the present study support an anti-inflammatory role for this adipokine.
Ghrelin is a gastric hormone that increases appetite and decreases fat utilization, playing a role in both short- and long-term regulation of energy balance.5,25 Ghrelin levels change in response to dieting to maintain body weight.26 There is little information available regarding ghrelin levels in obese asthmatics. A recent study showed that IgE levels are inversely correlated with ghrelin levels in obese children, leading the authors to postulate that ghrelin directly or indirectly inhibits IgE synthesis.27 Moreover, acting via its receptor, growth hormone secretagogue receptor (GHS-R),9 ghrelin has immuno-modulatory activities that contradict those of leptin.28 Ghrelin exerts anti-inflammatory actions primarily through dose- and time-dependent inhibition of IL-1β, IL-6, and TNF-α.9 It also decreases the leptin-induced pro-inflammatory response in mononuclear and T cells.9 However, no association could be found between the levels of interferon-γ-secreting T cells and ghrelin levels in obese children.29 Here, we report lower ghrelin levels in obese children with asthma than in non-obese children with asthma or controls, and lower ghrelin levels in non-obese children with asthma than in control subjects. These results support an anti-inflammatory role of ghrelin in the pathogenesis of asthma and obesity.
When compared with the non-obese asthmatic children, the obese children with asthma had more days of absenteeism from school, lower peak expiratory flow, more asthma drug prescriptions, and more emergency department visits.30 A linear relationship between asthma severity and BMI has been proposed.31,32 Although some research has demonstrated that asthma is more difficult to control in obese individuals, other studies were unable to demonstrate an association between obesity and asthma severity in children with mild asthma.33 Similar to these reports, the symptom scores of the obese children with asthma in our study were significantly higher than those of non-obese children with asthma.
The major limitation of this study was the absence of an evaluation of adipokine levels and asthma severity in the obese children with asthma after a weight loss intervention. This might have demonstrated a change in adipokine levels with weight loss, allowed an examination of the change in asthma control with altered adipokine levels, and enabled the determination of a causal relationship between adipokine levels and asthma severity. Moreover, measurement of an indicator of inflammation such as FeNO would have contributed to our understanding of the relationship between adipokine levels and inflammation in asthma. Another limitation is the small number of children enrolled, which decreased the power of the study.
In conclusion, the results of this study indicate that serum leptin is increased while serum adiponectin and ghrelin are decreased in children with asthma compared with non-asthmatic children. Moreover, the increase in leptin and decreases in adiponectin and ghrelin are more prominent in obese than in non-obese children with asthma. These data suggest that adipokines such as leptin, Adiponectin, and ghrelin are important in the inflammatory pathogenesis of the co-morbidity of asthma and obesity.
ACKNOWLEDGMENTS
We thank Ozlem Gunay for her help with data generation.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol. 2007;102:516–528. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 3.Shore SA. Obesity and asthma: implications for treatment. Curr Opin Pulm Med. 2007;13:56–62. doi: 10.1097/MCP.0b013e3280110196. [DOI] [PubMed] [Google Scholar]
- 4.Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007;62:1205–1213. doi: 10.1111/j.1398-9995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 5.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 6.Considine RV, Caro JF. Leptin and the regulation of body weight. Int J Biochem Cell Biol. 1997;29:1255–1272. doi: 10.1016/s1357-2725(97)00050-2. [DOI] [PubMed] [Google Scholar]
- 7.Bryson JM, Phuyal JL, Proctor DR, Blair SC, Caterson ID, Cooney GJ. Plasma insulin rise precedes rise in ob mRNA expression and plasma leptin in gold thioglucose-obese mice. Am J Physiol. 1999;276:E358–E364. doi: 10.1152/ajpendo.1999.276.2.E358. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 9.Dixit VD, Taub DD. Ghrelin and immunity: a young player in an old field. Exp Gerontol. 2005;40:900–910. doi: 10.1016/j.exger.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. 2006. [Google Scholar]
- 11.Barlow SE, Dietz WH. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998;102:E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002:1–190. [PubMed] [Google Scholar]
- 13.Yüksel H, Yilmaz O, Kirmaz C, Aydoğdu S, Kasirga E. Frequency of gastroesophageal reflux disease in nonatopic children with asthma-like airway disease. Respir Med. 2006;100:393–398. doi: 10.1016/j.rmed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Sood A. Obesity, adipokines, and lung disease. J Appl Physiol. 2010;108:744–753. doi: 10.1152/japplphysiol.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114:254–259. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 16.Gurkan F, Atamer Y, Ece A, Kocyigit Y, Tuzun H, Mete N. Serum leptin levels in asthmatic children treated with an inhaled corticosteroid. Ann Allergy Asthma Immunol. 2004;93:277–280. doi: 10.1016/S1081-1206(10)61501-3. [DOI] [PubMed] [Google Scholar]
- 17.Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 18.Torday JS, Sun H, Wang L, Torres E, Sunday ME, Rubin LP. Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation. Am J Physiol Lung Cell Mol Physiol. 2002;282:L405–L410. doi: 10.1152/ajplung.2002.282.3.L405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Mito N, Kitada C, Hosoda T, Sato K. Effect of diet-induced obesity on ovalbumin-specific immune response in a murine asthma model. Metabolism. 2002;51:1241–1246. doi: 10.1053/meta.2002.35196. [DOI] [PubMed] [Google Scholar]
- 21.Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 22.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, Szefler SJ, Sorkness CA, Morgan WJ, Teach SJ, Gan VN. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 24.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 26.Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschöp M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–673. [PubMed] [Google Scholar]
- 27.Matsuda K, Nishi Y, Okamatsu Y, Kojima M, Matsuishi T. Ghrelin and leptin: a link between obesity and allergy? J Allergy Clin Immunol. 2006;117:705–706. doi: 10.1016/j.jaci.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu R, Dong W, Cui X, Zhou M, Simms HH, Ravikumar TS, Wang P. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480–486. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luder E, Melnik TA, DiMaio M. Association of being overweight with greater asthma symptoms in inner city black and Hispanic children. J Pediatr. 1998;132:699–703. doi: 10.1016/s0022-3476(98)70363-4. [DOI] [PubMed] [Google Scholar]
- 31.Akerman MJ, Calacanis CM, Madsen MK. Relationship between asthma severity and obesity. J Asthma. 2004;41:521–526. doi: 10.1081/jas-120037651. [DOI] [PubMed] [Google Scholar]
- 32.Varraso R, Siroux V, Maccario J, Pin I, Kauffmann F. Asthma severity is associated with body mass index and early menarche in women. Am J Respir Crit Care Med. 2005;171:334–339. doi: 10.1164/rccm.200405-674OC. [DOI] [PubMed] [Google Scholar]
- 33.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]