Abstract
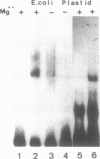
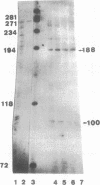
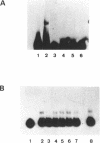
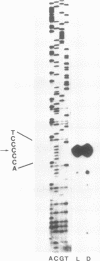
By means of mobility-shift assays and Exonuclease III mapping we have determined a 14 bp sequence (named CDF2 binding site) located in front of the 16S rRNA initiation start site which is protected by a spinach chloroplast extract. This region does not include neither one of the two '-35' nor of the two '-10' E. coli-like promoter elements which are recognised by E. coli RNA polymerase in vitro. The CDF2 binding site is specifically recognized by two small polypeptides which migrate corresponding to 35 and 33 kDa respectively as shown by UV cross-linking experiments. In vivo transcription initiation of the 16S rRNA gene occurs 13 nucleotides downstream of the 14 bp sequence and is different from the transcription start site which is used by E.coli polymerase in vitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audren H., Bisanz-Seyer C., Briat J. F., Mache R. Structure and transcription of the 5S rRNA gene from spinach chloroplasts. Curr Genet. 1987;12(4):263–269. doi: 10.1007/BF00435288. [DOI] [PubMed] [Google Scholar]
- Briat J. F., Dron M., Loiseaux S., Mache R. Structure and transcription of the spinach chloroplast rDNA leader region. Nucleic Acids Res. 1982 Nov 11;10(21):6865–6878. doi: 10.1093/nar/10.21.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B. M., Narita J. O., DeLuca-Flaherty C., Gruissem W., Rushlow K. A., Hallick R. B. Evidence for two RNA polymerase activities in Euglena gracilis chloroplasts. J Biol Chem. 1984 Dec 10;259(23):14880–14887. [PubMed] [Google Scholar]
- Gruissem W., Zurawski G. Analysis of promoter regions for the spinach chloroplast rbcL, atpB and psbA genes. EMBO J. 1985 Dec 16;4(13A):3375–3383. doi: 10.1002/j.1460-2075.1985.tb04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruissem W., Zurawski G. Identification and mutational analysis of the promoter for a spinach chloroplast transfer RNA gene. EMBO J. 1985 Jul;4(7):1637–1644. doi: 10.1002/j.1460-2075.1985.tb03831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Keus R. J., Dekker A. F., van Roon M. A., Groot G. S. The nucleotide sequences of the regions flanking the genes coding for 23S, 16S and 4.5S ribosomal RNA on chloroplast DNA from Spirodela oligorhiza. Nucleic Acids Res. 1983 Sep 24;11(18):6465–6474. doi: 10.1093/nar/11.18.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Light-induced transcription of chloroplast genes. psbA transcription is differentially enhanced in illuminated barley. J Biol Chem. 1990 Feb 5;265(4):1895–1902. [PubMed] [Google Scholar]
- Lam E., Hanley-Bowdoin L., Chua N. H. Characterization of a chloroplast sequence-specific DNA binding factor. J Biol Chem. 1988 Jun 15;263(17):8288–8293. [PubMed] [Google Scholar]
- Lescure A. M., Bisanz-Seyer C., Pesey H., Mache R. In vitro transcription initiation of the spinach chloroplast 16S rRNA gene at two tandem promoters. Nucleic Acids Res. 1985 Dec 20;13(24):8787–8796. doi: 10.1093/nar/13.24.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G. DNA sequence requirements for the accurate transcription of a protein-coding plastid gene in a plastid in vitro system from mustard (Sinapis alba L.). EMBO J. 1984 Aug;3(8):1697–1704. doi: 10.1002/j.1460-2075.1984.tb02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler J. J., Marsden M. P., Price C. A. Factors affecting the separation of photosynthetically competent chloroplasts in gradients of silica sols. Arch Biochem Biophys. 1975 May;168(1):289–301. doi: 10.1016/0003-9861(75)90253-2. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Klein R. R. Transcription and RNA stability are important determinants of higher plant chloroplast RNA levels. EMBO J. 1987 Jun;6(6):1571–1579. doi: 10.1002/j.1460-2075.1987.tb02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita J. O., Rushlow K. E., Hallick R. B. Characterization of a Euglena gracilis chloroplast RNA polymerase specific for ribosomal RNA genes. J Biol Chem. 1985 Sep 15;260(20):11194–11199. [PubMed] [Google Scholar]
- Straney D. C., Crothers D. M. Intermediates in transcription initiation from the E. coli lac UV5 promoter. Cell. 1985 Dec;43(2 Pt 1):449–459. doi: 10.1016/0092-8674(85)90175-8. [DOI] [PubMed] [Google Scholar]
- Strittmatter G., Gozdzicka-Jozefiak A., Kössel H. Identification of an rRNA operon promoter from Zea mays chloroplasts which excludes the proximal tRNAValGAC from the primary transcript. EMBO J. 1985 Mar;4(3):599–604. doi: 10.1002/j.1460-2075.1985.tb03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun E., Wu B. W., Tewari K. K. In vitro analysis of the pea chloroplast 16S rRNA gene promoter. Mol Cell Biol. 1989 Dec;9(12):5650–5659. doi: 10.1128/mcb.9.12.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohdoh N., Shinozaki K., Sugiura M. Sequence of a putative promoter region for the rRNA genes of tobacco chloroplast DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5399–5406. doi: 10.1093/nar/9.20.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Allmen J. M., Stutz E. The soybean chloroplast genome: nucleotide sequence of a region containing tRNA-Val (GAC) and 16S rRNA gene. Nucleic Acids Res. 1988 Feb 11;16(3):1200–1200. doi: 10.1093/nar/16.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]