Abstract
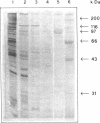
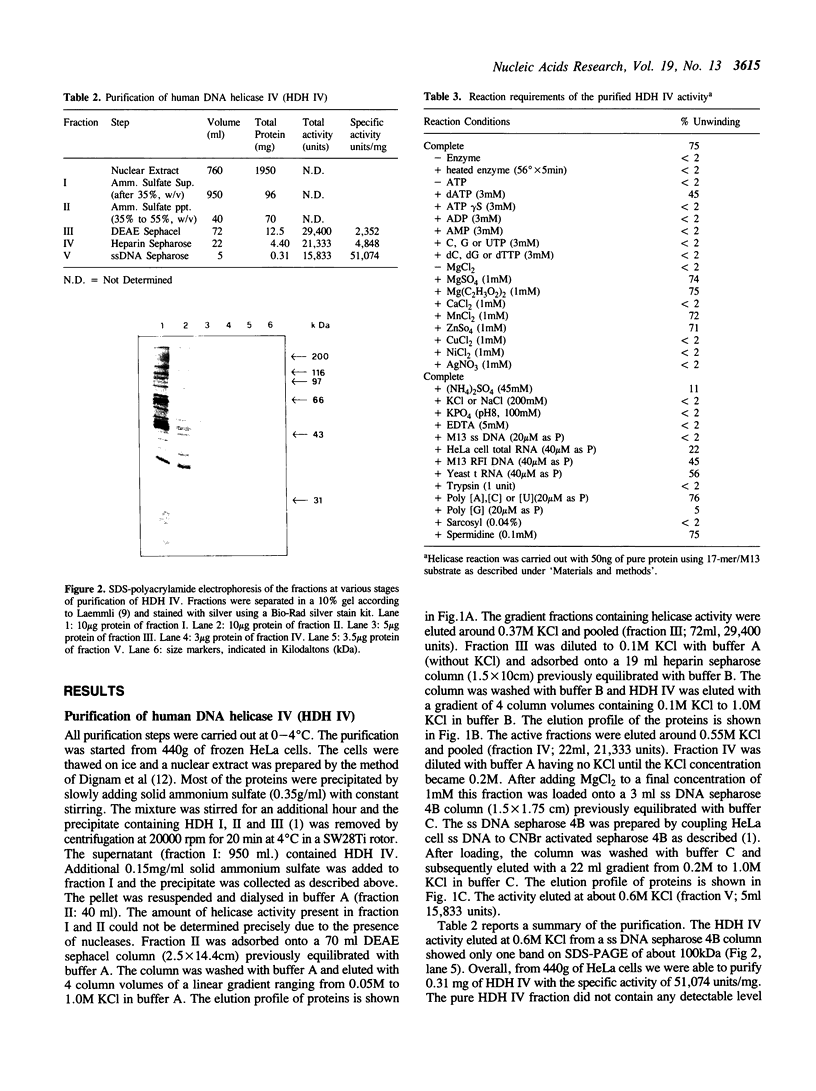
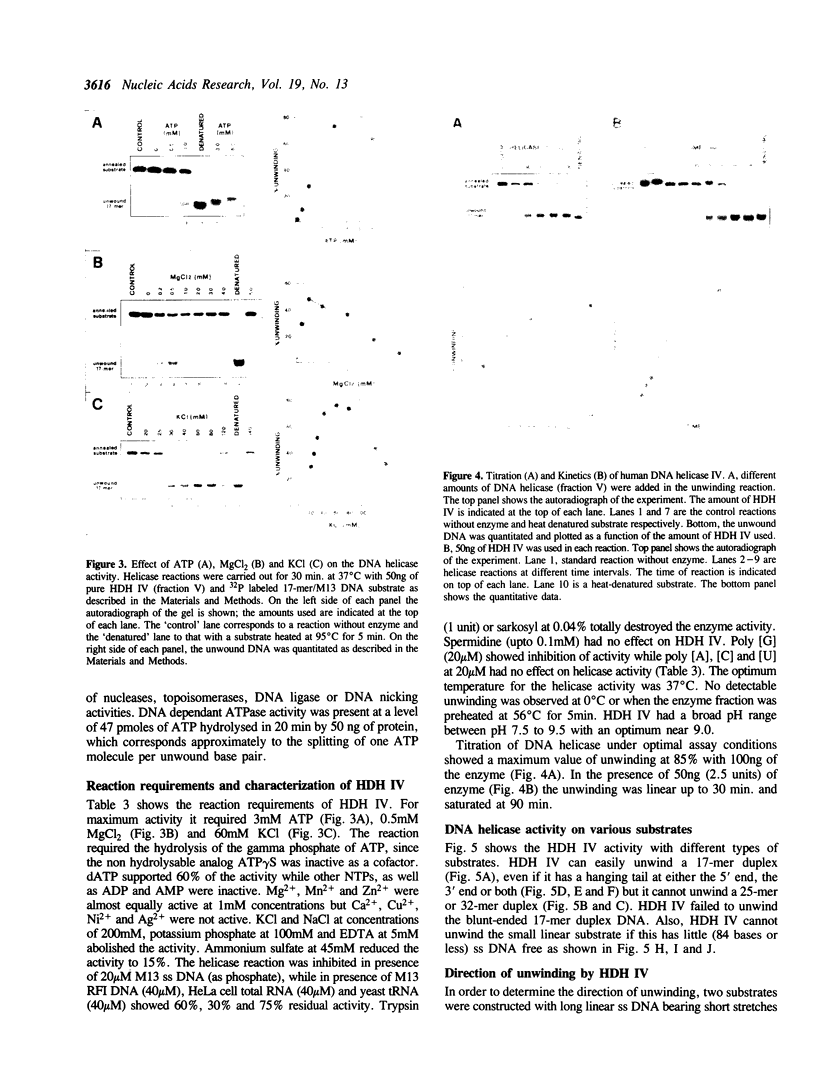
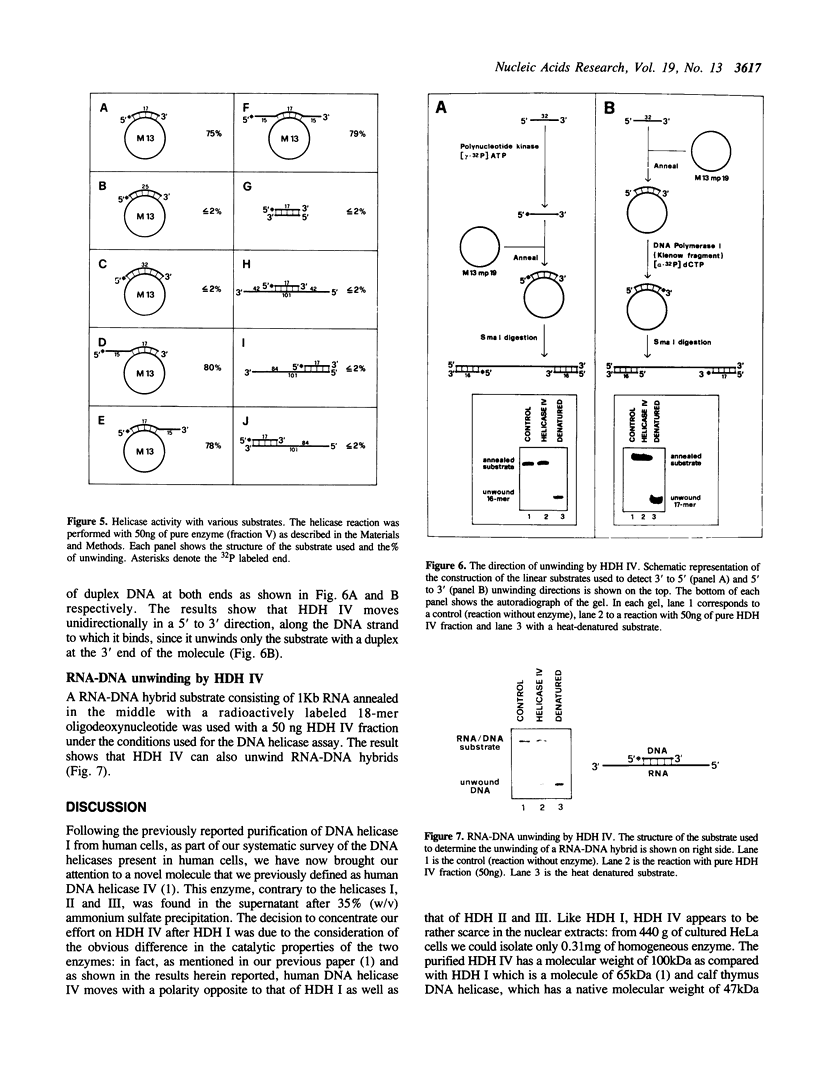
Human DNA helicase IV, a novel enzyme, was purified to homogeneity from HeLa cells and characterized. The activity was measured by assaying the unwinding of 32P labeled 17-mer annealed to M13 ss DNA. From 440g of HeLa cells we obtained 0.31 mg of pure protein. Helicase IV was free of DNA topoisomerases, DNA ligase and nuclease activities. The apparent molecular weight is 100 kDa. It requires a divalent cation for activity (Mg2+ = Mn2+ = Zn2+) and the hydrolysis of only ATP or dATP. The activity is destroyed by trypsin and is inhibited by 200 mM KCl or NaCl, 100 mM potassium phosphate, 45 mM ammonium sulfate, 5 mM EDTA, 20 microM ss M13 DNA or 20 microM poly [G] (as phosphate). The enzyme unwinds DNA by moving in the 5' to 3' direction along the bound strand, a polarity opposite to that of the previously described human DNA helicase I (Tuteja et al Nucleic Acids Res. 18, 6785-6792, 1990). It requires more than 84 bases of single-stranded DNA in order to exert its unwinding activity and does not require a replication fork-like structure. Like human DNA helicase I the enzyme can also unwind RNA-DNA hybrid.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biamonti G., Cobianchi F., Falaschi A., Riva S. Total purification of a DNA-dependent ATPase and of a DNA-binding protein from human cells. EMBO J. 1983;2(2):161–165. doi: 10.1002/j.1460-2075.1983.tb01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brennan C. A., Dombroski A. J., Platt T. Transcription termination factor rho is an RNA-DNA helicase. Cell. 1987 Mar 27;48(6):945–952. doi: 10.1016/0092-8674(87)90703-3. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., Biamonti G., Mastromei G., Falaschi A., Riva S. A DNA dependent ATPase from HeLa cells. Biochem Biophys Res Commun. 1982 Jan 29;104(2):402–409. doi: 10.1016/0006-291x(82)90651-9. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., Riva S., Mastromei G., Spadari S., Pedrali-Noy G., Falaschi A. Enhancement of the rate of DNA polymerase-alpha activity on duplex DNA by a DNA-binding protein and a DNA-dependent ATPase of mammalian cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):639–647. doi: 10.1101/sqb.1979.043.01.071. [DOI] [PubMed] [Google Scholar]
- Crute J. J., Mocarski E. S., Lehman I. R. A DNA helicase induced by herpes simplex virus type 1. Nucleic Acids Res. 1988 Jul 25;16(14A):6585–6596. doi: 10.1093/nar/16.14.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falaschi A., Biamonti G., Cobianchi F., Csordas-Toth E., Faulkner G., Giacca M., Pedacchia D., Perini G., Riva S., Tribioli C. Presence of transcription signals in two putative DNA replication origins of human cells. Biochim Biophys Acta. 1988 Dec 20;951(2-3):430–442. doi: 10.1016/0167-4781(88)90117-0. [DOI] [PubMed] [Google Scholar]
- Goetz G. S., Dean F. B., Hurwitz J., Matson S. W. The unwinding of duplex regions in DNA by the simian virus 40 large tumor antigen-associated DNA helicase activity. J Biol Chem. 1988 Jan 5;263(1):383–392. [PubMed] [Google Scholar]
- Hirling H., Scheffner M., Restle T., Stahl H. RNA helicase activity associated with the human p68 protein. Nature. 1989 Jun 15;339(6225):562–564. doi: 10.1038/339562a0. [DOI] [PubMed] [Google Scholar]
- Hughes M. J., Liang H. M., Jiricny J., Jost J. P. Purification and characterization of a protein from HeLa cells that binds with high affinity to the estrogen response element, GGTCAGCGTGACC. Biochemistry. 1989 Nov 14;28(23):9137–9142. doi: 10.1021/bi00449a027. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Stalder H. P. Mammalian DNA helicase. Nucleic Acids Res. 1985 Aug 12;13(15):5471–5483. doi: 10.1093/nar/13.15.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserman H. B., Ingebritsen T. S., Benbow R. M. Regulation of Xenopus laevis DNA topoisomerase I activity by phosphorylation in vitro. Biochemistry. 1988 May 3;27(9):3216–3222. doi: 10.1021/bi00409a014. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matson S. W. Escherichia coli DNA helicase II (uvrD gene product) catalyzes the unwinding of DNA.RNA hybrids in vitro. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4430–4434. doi: 10.1073/pnas.86.12.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson S. W., Kaiser-Rogers K. A. DNA helicases. Annu Rev Biochem. 1990;59:289–329. doi: 10.1146/annurev.bi.59.070190.001445. [DOI] [PubMed] [Google Scholar]
- Poll E. H., Benbow R. M. A DNA helicase from Xenopus laevis ovaries. Biochemistry. 1988 Nov 29;27(24):8701–8706. doi: 10.1021/bi00424a002. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Knippers R., Stahl H. RNA unwinding activity of SV40 large T antigen. Cell. 1989 Jun 16;57(6):955–963. doi: 10.1016/0092-8674(89)90334-6. [DOI] [PubMed] [Google Scholar]
- Seki M., Enomoto T., Eki T., Miyajima A., Murakami Y., Hanaoka F., Ui M. DNA helicase and nucleoside-5'-triphosphatase activities of polyoma virus large tumor antigen. Biochemistry. 1990 Jan 30;29(4):1003–1009. doi: 10.1021/bi00456a024. [DOI] [PubMed] [Google Scholar]
- Seki M., Enomoto T., Hanaoka F., Yamada M. DNA-dependent adenosinetriphosphatase B from mouse FM3A cells has DNA helicase activity. Biochemistry. 1987 May 19;26(10):2924–2928. doi: 10.1021/bi00384a038. [DOI] [PubMed] [Google Scholar]
- Stahl H., Knippers R. The simian virus 40 large tumor antigen. Biochim Biophys Acta. 1987 Oct 9;910(1):1–10. doi: 10.1016/0167-4781(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Thömmes P., Hübscher U. DNA helicase from calf thymus. Purification to apparent homogeneity and biochemical characterization of the enzyme. J Biol Chem. 1990 Aug 25;265(24):14347–14354. [PubMed] [Google Scholar]
- Tuteja N., Danciger M., Klisak I., Tuteja R., Inana G., Mohandas T., Sparkes R. S., Farber D. B. Isolation and characterization of cDNA encoding the gamma-subunit of cGMP phosphodiesterase in human retina. Gene. 1990 Apr 16;88(2):227–232. doi: 10.1016/0378-1119(90)90035-p. [DOI] [PubMed] [Google Scholar]
- Tuteja N., Tuteja R., Rahman K., Kang L. Y., Falaschi A. A DNA helicase from human cells. Nucleic Acids Res. 1990 Dec 11;18(23):6785–6792. doi: 10.1093/nar/18.23.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]