Abstract
The neuropilins (NRPs) contribute to the function of cancer cells in their capacity as VEGF receptors. Given that NRP2 is induced in breast cancer and correlates with aggressive disease, we examined the role of NRP2 in regulating the interaction of breast cancer cells with the ECM. Using epithelial cells from breast tumors, we defined NRP2high and NRP2low populations that differed in integrin expression and adhesion to laminin. Specifically, the NRP2high population adhered more avidly to laminin and expressed high levels of the α6β1 integrin than the NRP2low population. The NRP2high population formed numerous focal adhesions on laminin that were not seen in the NRP2low population. These results were substantiated using breast carcinoma cell lines that express NRP2 and α6β1 integrin. Depletion experiments revealed that adhesive strength on laminin but not collagen is dependent on NRP2, and that VEGF is needed for adhesion on laminin. A specific interaction between NRP2 and α6β1 integrin was detected by co-immunoprecipitation. NRP2 is necessary for focal adhesion formation on laminin and for the association of α6β1 integrin with the cytoskeleton. NRP2 also facilitates α6β1-integrin-mediated activation of FAK and Src. Unexpectedly, we discovered that NRP2 is located in focal adhesions on laminin. The mechanism by which NRP2 regulates the interaction of α6β1 integrin with laminin to form focal adhesions involves PKC activation. Together, our data reveal a new VEGF–NRP2 signaling pathway that activates the α6β1 integrin and enables it to form focal adhesions and signal. This pathway is important in the pathogenesis of breast cancer.
Key words: Cancer, Neuropilin, Integrin, Laminin, FAK
Introduction
An emerging area of importance in cancer biology is the function of receptors for VEGF on tumor cells. Although most studies on VEGF receptors have focused on endothelial cells and their role in angiogenesis, it has become apparent that tumor cells also express specific VEGF receptors and that these receptors contribute to tumor initiation, migration, invasion and survival (Bachelder et al., 2001; Gray et al., 2008; Hu et al., 2007; Lichtenberger et al., 2010; Matsushita et al., 2007; Miao et al., 2000; Muders et al., 2009; Sulpice et al., 2008; Wang et al., 2007). The neuropilins (NRPs) are one class of VEGF receptors that are particularly interesting with respect to cancer biology. NRP1 and NRP2 were identified initially as neuronal receptors for semaphorins, which are axon guidance factors that function primarily in the developing nervous system (Uniewicz and Fernig, 2008). The seminal finding by Klagsbrun that neuropilins can also function as VEGF receptors and that they are expressed on endothelial and tumor cells launched studies aimed at understanding their function in angiogenesis and tumor biology (Soker et al., 1998). NRPs have the ability to interact with and modulate the function of tyrosine kinase VEGF receptors (VEGFR1 and VEGFR2), as well as other growth factor receptors (Neufeld et al., 2002; Sulpice et al., 2008). There is also evidence that NRPs can function independently of other receptors (Gray et al., 2005) and that they are valid targets for therapeutic inhibition of angiogenesis and cancer (Caunt et al., 2008; Gray et al., 2008; Pan et al., 2007). The observation that the expression of NRP2 is induced is some cancers such as breast cancer (Yasuoka et al., 2009) and that its expression correlates with aggressive disease and poor survival suggests that this NRP has a substantial influence on the behavior of breast carcinoma cells.
The possibility that NRP2 influences the activation and function of specific integrins that contribute to tumor behavior merits consideration. Previous studies demonstrated that VEGF signaling activates specific integrins in endothelial cells (Byzova et al., 2000) and that integrins can be regulated by NRP1 (Valdembri et al., 2009). In this study we observed that loss of NRP2 expression in breast carcinoma cells impedes their ability to interact with laminin matrices. This later observation suggested that NRP2 regulates the α6 integrins, which function as laminin receptors. This hypothesis is compelling because the α6 integrins have been implicated in breast tumor formation and progression (Chung and Mercurio, 2004; Guo et al., 2006; Lipscomb et al., 2005), so we pursued this hypothesis in this study. Unexpectedly, we discovered that NRP2 is located in focal adhesions, that it associates with the α6β1 integrin specifically and that it regulates the ability of this integrin to form focal adhesions and signal. These studies add a new dimension to the function of the NRPs and their contribution to cell biology. Moreover, the fact that both NRP2 (Yasuoka et al., 2009) and the α6β1 integrin (Friedrichs et al., 1995; Wewer et al., 1997) have been implicated in aggressive breast cancer underscores the importance of NRP2-mediated regulation of α6β1 integrin function.
Results
Characterization of NRP2high and NRP2low populations of epithelial cells isolated from human breast tumors
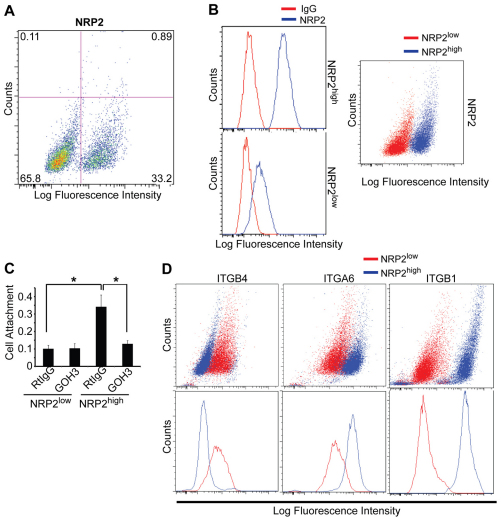
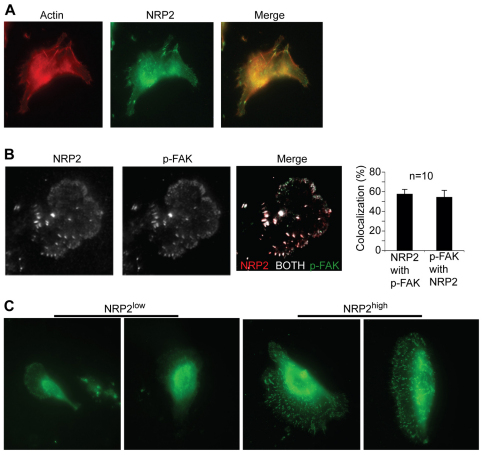
We isolated epithelial cells (EpCAM+) from human breast tumors to characterize the properties of NRP2-expressing cells. This approach is based on the report that NRP2 is expressed at low levels in normal breast epithelium and that its expression increases in breast cancer and correlates with aggressive disease (Yasuoka et al., 2009). Epithelial cells were isolated from four separate, invasive breast tumors and analyzed for NRP2 expression. We observed that a small but significant proportion of these cells expressed high levels of NRP2, which ranged from 12–33% of the total population (Fig. 1A). These cells, as well as the population of cells expressing low levels of NRP2, were pooled to generate NRP2high and NRP2low populations, respectively (Fig. 1B). Given our interest in laminin interactions, we observed that the NRP2high population adhered much more robustly to laminin than did the NRP2low population, and that this adhesion was inhibited by an α6 integrin function-blocking antibody (Fig. 1C). We therefore compared the expression of the α6-, β1- and β4-integrin subunits in the NRP2high and NRP2low populations. Indeed, the NRP2high population expressed considerably more α6 and αβ1 integrin than did the NRP2low population (Fig. 1D). Interestingly, β4 integrin expression was very low in the NRP2high population (Fig. 1D) indicating that α6β1 integrin is the predominant laminin receptor in these cells.
Fig. 1.
Characterization of NRP2high and NRP2low populations of epithelial cells isolated from human breast tumors. (A) Epithelial cells (EpCAM+) were isolated from breast tumors and analyzed for NRP2 expression by flow cytometry. Approximately 33% of cells express high levels of NRP2. (B) Epithelial cells isolated from four different breast tumors were sorted into NRP2high and NRP2low populations. These populations were stained with either an NRP2 antibody or goat IgG to confirm the relative expression of NRP2. Histograms in the left panels show NRP2 expression relative to control IgG in each population; pseudocolored dot plot in the right panel show NRP2 expression in the NRP2high and NRP2low cell populations. (C) NRP2high and NRP2low populations were incubated with either an α6 antibody (GoH3) or rat IgG for 1 hour, and assayed for adhesion to laminin. NRP2high cells adhere much more avidly to laminin and this adhesion is blocked by GoH3. (D) The relative expression of α6, β1 and β4 integrins in the NRP2high and NRP2low populations was quantified by flow cytometry. The NRP2high population expressed relatively high levels of α6 and β1 integrins, but low levels of the β4 integrin subunit.
NRP2 regulates the interaction of breast carcinoma cells with laminin matrices and the function of the α6β1 integrin
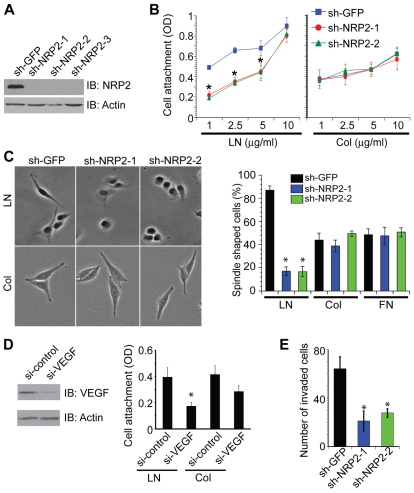
To explore the relationship between NRP2 and α6β1 integrin more rigorously, we made use of MDA-MBA-435 cells, a breast carcinoma cell line (Chambers, 2009; Montel et al., 2009) that expresses NRP2 and α6β1 integrin but not α6β4 integrin. Depletion of NRP2 expression in these cells using short hairpin RNA (shRNAs; Fig. 2A) diminished their adhesive strength on laminin but not on collagen (Fig. 2B). Specifically, NRP2-depleted cells adhered less avidly to low concentrations of laminin than did control cells (Fig. 2B). NRP2-depleted cells also exhibited reduced spreading on laminin compared with control cells (Fig. 2C). Loss of NRP2, however, did not affect cell proliferation or morphology on tissue culture plastic (data not shown) or on collagen (Fig. 2C). Adhesion to laminin is also dependent on VEGF because depletion of VEGF using siRNA impeded adhesion to laminin but not to collagen (Fig. 2D). NRP2-depleted cells also invaded Matrigel poorly compared with control cells (Fig. 2E).
Fig. 2.
Neuropilin-2 regulates the interaction of breast carcinoma cells with laminin. (A,B) MDA-MB-435 transfectants (shGFP, shNRP2-1 or shNRP2-2) were serum-deprived overnight, detached and plated on either laminin (1, 2.5, 5 or 10 μg/ml) or collagen (1, 2.5, 5 or 10 μg/ml). Cells were incubated for 30 minutes, fixed, and relative adhesion was quantified using a colorimetric assay (B). Immunoblotting verified shRNA-mediated NRP2 depletion in these cells (A). (C) MDA-MB-435 transfectants (shGFP, shNRP2-1 or shNRP2-2) were plated on either laminin, collagen or fibronectin (5 μg/ml) for 2 hours and cells were imaged using phase contrast microscopy (20× objective). The number of spread, spindle-shaped cells was counted in 20 random fields and plotted as a percentage of total cells. (D) MDA-MB-435 cells were transiently transfected with either scrambled (control) siRNA or VEGF siRNA. Cells were plated on either collagen- or laminin-coated plates (2.5 μg/ml) 48 hours after transfection. Cells were incubated for 30 minutes, fixed, and relative adhesion was quantified using a colorimetric assay. Immunoblotting verified siRNA-mediated VEGF depletion in cells. (E) MDA-MB-435 transfectants (shGFP, shNRP2-1 or shNRP2-2) were plated in the upper chamber of Matrigel-coated Transwell plates. After 12 hours, cells that had migrated through the membranes were stained with DAPI and counted in 20 random fields.
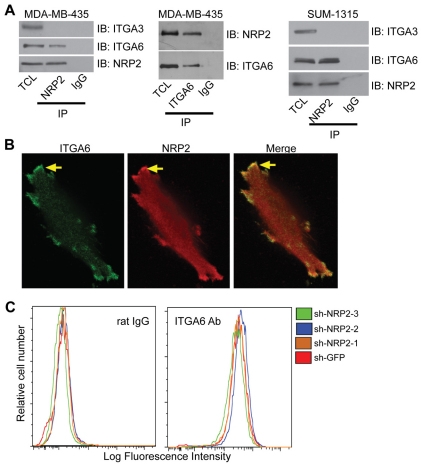
The effect of NRP2 on laminin interactions suggested that this receptor influences the function of α6β1 integrin. Given that the NRPs are known to interact with other receptors including integrins (Fukasawa et al., 2007; Robinson et al., 2009; Valdembri et al., 2009), we initially assessed the possibility of a specific interaction between NRP2 and α6β1 integrin using co-immunoprecipitation experiments. These data revealed that NRP2 interacts specifically with α6β1 integrin and not with α3β1 integrin, which can also function as a laminin receptor (Delwel et al., 1994) (Fig. 3A). This interaction was detected by NRP2 immunoprecipitation (IP) and α6 integrin immunoblotting assays, and by α6 integrin IP and NRP2 immunoblotting. This interaction was also detected in SUM-1315 cells, another breast carcinoma cell line that expresses NRP2 and α6β1 integrin but not α6β4 integrin (supplementary material Fig. S1A,B). These biochemical interactions were substantiated using immunofluorescence microscopy, which revealed substantial colocalization of NRP2 and α6 integrin (Fig. 3B). No colocalization of NRP2 and α3β1 integrin was observed (supplementary material Fig. S3A). Moreover, NRP2 does not appear to regulate α6 integrin or β4 integrin surface expression as evidenced by flow cytometry (Fig. 3C; supplementary material Fig. S1A), in contrast to the reported regulation of α5β1 integrin surface trafficking by NRP1 (Valdembri et al., 2009). The NRP2 antibody used is highly specific (supplementary material Fig. S1C) and it does not cross-react with NRP1 (Bae et al., 2008).
Fig. 3.
Neuropilin-2 associates with the α6β1 integrin. (A) Left panel: MDA-MB-435 cells were extracted in a Triton X-100 buffer and immunoprecipitated using either a NRP2 antibody (C9) or mouse IgG. Immunoprecipitated proteins were separated by SDS-PAGE and immunoblotted using antibodies to either α6 integrin, α3 integrin or NRP2. Middle panel: extracts of MDA-MB-435 cells were immunoprecipitated using either an α6 antibody (J8H) or mouse IgG. Immunoprecipitated proteins were separated on SDS-PAGE and immunoblotted using antibodies to either α6 integrin or NRP2. Right panel: SUM-1315 cells were extracted in a Triton X-100 buffer and immunoprecipitated using either a NRP2 antibody (C9) or mouse IgG. Immunoprecipitated proteins were separated by SDS-PAGE and immunoblotted using antibodies to either α6 integrin, α3 integrin or NRP2. TCL, total cell lysate. (B) MDA-MB-435 cells were plated on laminin (5 μg/ml) and immunofluorescence was examined using antibodies to NRP2 or α6 integrin. Samples were imaged by confocal microscopy (60× objective). Arrows indicate an area of colocalization. (C) MDA-MB-435 transfectants (shGFP, shNRP2-1, shNRP2-2 and shNRP-3) were analyzed by flow cytometry using an α6 antibody (Ab; GoH3) or rat IgG.
Focal adhesion formation mediated by the α6β1 integrin is dependent on NRP2
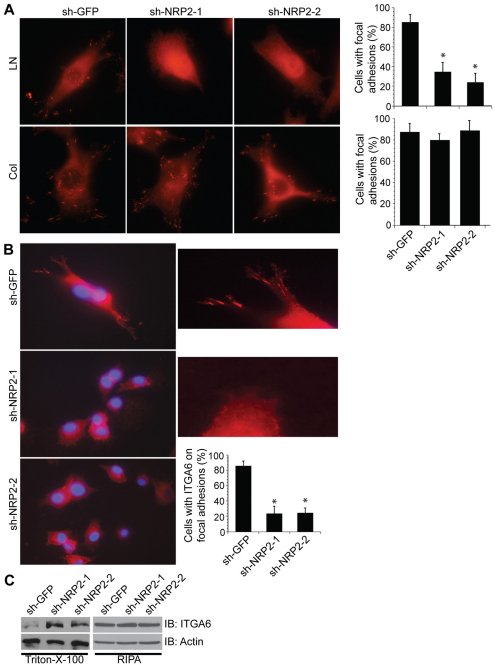
The association of NRP2 with α6β1 integrin and its effect on cell morphology caused us to examine the location of NRP2 in cells adherent to laminin. MDA-MB-435 cells form well-defined focal adhesions when plated on either laminin or collagen as evidenced by phosphorylated FAK (FAK-P; at Y397; Fig. 4A) or vinculin (supplementary material Fig. S2) immunofluorescence. Depletion of NRP2 expression in these cells diminished focal adhesions formation on laminin significantly (P=0.01) but it had no effect on collagen (Fig. 4A). The possibility that the reduction in focal adhesions was caused by the inability of NRP2-depleted cells to spread was discounted by the observation that those NRP2-depleted cells that did spread on laminin lacked focal adhesions (Fig. 4A). The localization of α6β1 integrin to focal adhesions is also dependent on NRP2 expression. In cells plated on laminin, α6β1 integrin was located in focal adhesions at the leading edge (Fig. 4B). The distribution of α6 was more diffuse in NRP2-depleted cells, which were more ‘rounded’. These observations are consistent with our finding that loss of NRP2 increased the solubility of α6 in Triton X-100 considerably (Fig. 4C), suggesting that NRP2 facilitates α6 integrin association with the cytoskeleton. No change in α3β1 integrin localization was observed upon NRP2 downregulation (supplementary material Fig. S3B).
Fig. 4.
Neuropilin-2 regulates the localization of α6β1 integrin in focal adhesions and its interaction with the cytoskeleton. (A) MDA-MB-435 transfectants (shGFP, shNRP2-1 and shNRP2-2) were plated on laminin or collagen and stained with a FAK-P (Y397) antibody. The percentages of cells with focal adhesions were quantified as shown in the bar graphs. Original magnification: 60×. (B) MDA-MB-435 transfectants (shGFP, shNRP2-1 or shNRP2-2) were used to identify the location of α6β1 integrin by immunofluorescence microscopy. Original magnification: 60×. The photomicrographs on the right are higher magnification images of the left panel to provide better resolution of focal adhesions at the leading edge. The number of cells with discrete localization of α6β1 integrin in focal adhesions was counted and plotted as a percentage of total cells. This experiment was repeated three times with consistent results. *P<0.05. (C) MDA-MB-435 transfectants (shGFP, shNRP2-1 and shNRP2-2) were extracted in either Triton X-100 or RIPA lysis buffer and immunoblotted using antibodies to α6 (AA6A) and actin.
Unexpectedly, we observed that NRP2 itself was located in focal adhesions on laminin and that it colocalized with F-actin (Fig. 5A). NRP2 and α6β1 integrin also colocalized in focal adhesions (Fig. 3B). To substantiate the NRP2 localization to focal adhesions, we obtained more definitive data using TIRF microscopy. Using this technique, we detected NRP2 in focal adhesions where it colocalized with active FAK (FAK-P; Fig. 5B). An ~60% colocalization of NRP2 and FAK-P in these structures was revealed by quantification of these TIRF images. Together, these data suggest that NRP2 is located in focal adhesions on laminin and that it is necessary for such focal adhesions to form. To validate this hypothesis, we compared the ability of the NRP2high and NRP2low populations of freshly isolated tumor cells described in Fig. 1 to form focal adhesions on laminin. Consistent with our hypothesis, the NRP2high cells formed numerous, well-defined focal adhesions on laminin as assessed by FAK-P staining, in marked contrast to the NRP2low population (Fig. 5C).
Fig. 5.
Neuropilin-2 localizes to focal adhesions and contributes to focal adhesion formation on laminin. (A) MDA-MB-435 cells were plated on laminin and immunofluorescence staining was performed using a NRP2 antibody and phalloidin. Original magnification: 60×. (B) MDA-MB-435 cells were plated on laminin and immunofluorescence staining was performed using antibodies to FAK-P (Y397) and NRP2. Samples were imaged using TIRF microscopy. Analysis of the TIRF images was performed as described in Materials and Methods. NRP2 and FAK colocalization was calculated as number of pixels where NRP2>threshold and FAK>threshold divided by the number of pixel where NRP2>threshold; and FAK colocalization with NRP-2 was calculated as the number of pixels where NRP2>threshold and FAK>threshold divided by the number of pixel where FAK>threshold. Averaging all experiments, the colocalization of NRP2 with FAK was 57.8±4.6% and FAK with NRP-2 was 54.6±6.8% (means ± s.e.m., n=10). (C) NRP2high and NRP2low populations (Fig. 1) were plated on laminin and stained with a FAK-P (Y397) antibody. Original magnification: 60×.
PKC mediates NRP2-dependent α6β1 activation and focal adhesion formation
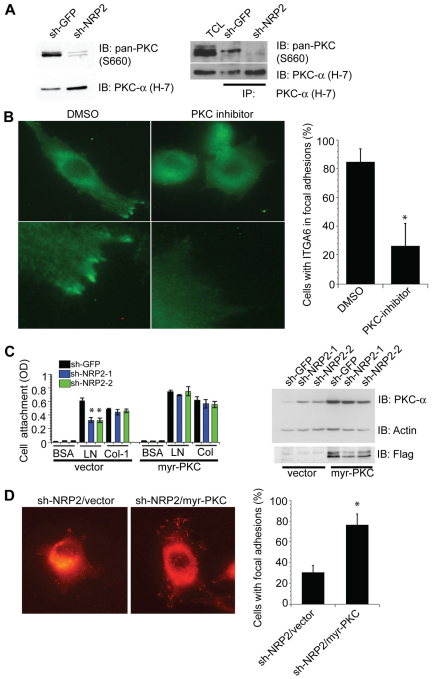
To investigate the mechanism by which NRP2 promotes α6β1 integrin activation and focal adhesion formation, we focused on a previous finding from our lab that PKC stimulates α6β1 integrin activation and its association with the cytoskeleton (Shaw et al., 1990). On the basis of this observation, we hypothesized that NRP2 contributes to PKC activation and that PKC enhances α6β1 integrin interactions with laminin and focal adhesion formation. Indeed, loss of NRP2 expression reduced PKC activation substantially as assayed using a phosphorylated-pan-PKC (Ser660) antibody (Fig. 6A). This effect of NRP2 on PKC activation is not dependent on laminin adhesion (data not shown), excluding the possibility that α6 signaling mediates this activation. We assessed whether NRP2 contributes to PKCα activation by immunoprecipitating extracts with a PKCα-specific antibody and blotting with the phosphorylated-pan-PKC antibody. As shown in Fig. 6A, loss of NRP2 expression reduced the amount of phosphorylated-pan-PKC captured by the PKCα-specific antibody specifically. This result provides evidence that NRP2 contributes to PKCα activation. Additional evidence to support a role for PKC in regulating α6β1 integrin function was obtained using a PKC inhibitor (G06983). This inhibitor prevented the localization of α6β1 integrin to focal adhesions at the leading edge, although it did not have much effect on cell adhesion (Fig. 6B). We also assessed whether the effects of NRP2 depletion on laminin adhesion and focal adhesion formation could be ‘rescued’ by expressing a constitutively active form of PKC (myr-PKC). Expression of this construct in NRP2-depleted cells increased adhesion to laminin specifically (Fig. 6C) and it increased focal adhesion formation significantly (P=0.01; Fig. 6D).
Fig. 6.
PKC mediates NRP2-dependent α6β1 integrin activation and focal adhesion formation. (A) Extracts from MDA-MB-435 transfectants (shGFP and shNRP2) were immunoblotted using antibodies to pan-phosphorylated-PKC (Ser660) or PKCα. These extracts were also immunoprecipitated using the PKCα and immunoblotted using antibodies to pan-phosphorylated PKC (Ser660) and PKCα. (B) MDA-MB-435 cells were treated with either DMSO or a PKC inhibitor (G06983; 10 nM) for 30 minutes and α6β1 integrin location was analyzed by immunofluorescence microscopy. Original magnification: 60×. (C,D) MDA-MB-435 transfectants (shGFP and shNRP2) were transfected with either vector alone or a FLAG-tagged, myristylated PKC construct (myr-PKC). Expression of this construct was verified by immunoblotting for PKCα and FLAG (C, right panel). Cells were detached after 48 hours and cell adhesion assays were performed using BSA, laminin (2.5 μg/ml) or collagen (2.5 μg/ml; C, left panel). These cells were also plated on laminin and FAK-P (Y397) and the location was analyzed by immunofluorescence microscopy (D). Original magnification: 60×. The percentage of cells with focal adhesions was determined from three independent experiments. *P<0.05.
Focal adhesion signaling on laminin is dependent on NRP2
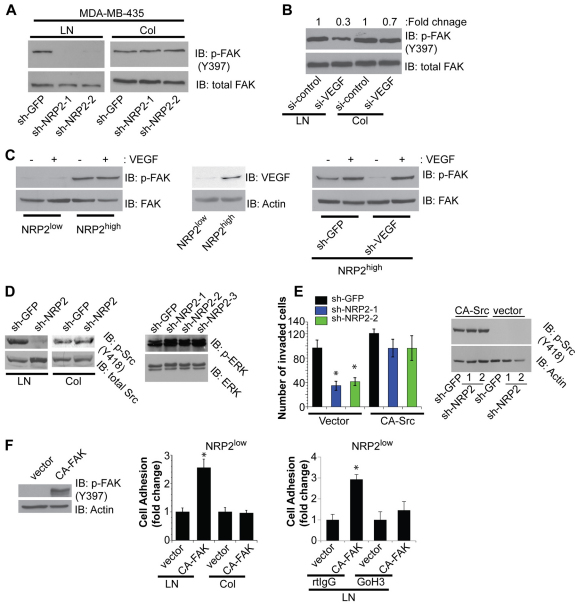
Our data on the importance of NRP2 in regulating the localization and function of α6β1 integrin suggest that it contributes to α6β1-integrin-mediated signaling. To test this possibility, we assessed FAK activation in control and NRP2-depleted cells by immunoblotting with a FAK-P antibody. As shown in Fig. 7A, NRP2-depleted MDA-MB-435 cells were unable to activate FAK upon adhesion to laminin, unlike the control cells, but they were able to activate FAK on collagen. To investigate the role of VEGF in FAK activation, we measured FAK activation upon VEGF depletion and detected a substantial reduction in FAK activation on laminin compared with collagen (Fig. 7B). We extended our analysis of FAK activation to the NRP2high and NRP2low populations of cells isolated from tumors (Fig. 1). Indeed, the NRP2high population expressed much higher levels of FAK-P than did the NRP2low population, as assessed by immunofluorescence (Fig. 5C). This result was substantiated by immunoblotting using the FAK-P antibody (Fig. 7C). Interestingly, VEGF expression was much higher in the NRP2high cells than in the NRP2low cells (Fig. 7C). Therefore, we depleted endogenous VEGF and observed a substantial reduction in FAK activation. Importantly, stimulation of these VEGF-depleted cells with exogenous VEGF restored FAK activation (Fig. 7C).
Fig. 7.
Focal adhesion signaling on laminin is dependent on NRP2. (A) MDA-MB-435 and transfectants (shGFP, shNRP2-1 and shNRP2-2) were serum-deprived and plated on laminin or collagen. Cell extracts were immunoblotted using antibodies to FAK-P (Y397) or total FAK. (B) MDA-MB-435 cells were transfected with either control siRNA or VEGF siRNA. Cells were either plated on laminin or collagen and cell extracts were analyzed by immunoblotting to assess the expression of FAK-P and total FAK. Densitometric analysis was performed to quantify the immunoblotting results. (C) Left panel: NRP2low and NRP2high populations (Fig. 1) plated on laminin were treated with VEGF (100 ng/ml for 30 minutes) and cell extracts were analyzed by immunoblotting to assess expression of FAK-P (Y397) and total FAK. Middle panel: NRP2low and NRP2high populations were plated on laminin and cell extracts were analyzed by immunoblotting to assess expression of VEGF and actin. Right panel: NRP2high population were infected with either shGFP- or shVEGF-expressing lentivirus and plated on laminin. Cells were treated with VEGF and cell extracts were analyzed by immunoblotting to assess expression of FAK-P (Y397) and total FAK. (D) Left panel: MDA-MB-435 transfectants (shGFP or shNRP2) were plated on laminin or collagen for 30 minutes, and Src-P (Y418) and total Src levels were assessed by immunoblotting. Right panel: MDA-MB-435 transfectants (shGFP, shNRP2-1, shNRP2-2 and shNRP-3) were plated on tissue culture plates, and ERK-P and total ERK levels were assessed by immunoblotting. (E) MDA-MB-435 transfectants (shGFP, shNRP2-1 and shNRP2-2) were transfected with either vector alone or a constitutively active Src construct (CA-Src). Expression of this construct was verified by immunoblotting with a Src-P (Y418). Invasion assays were performed as described in the legend to Fig. 2. (F) Left and middle panels: the NRP2low population of tumor cells (Fig. 1) was transfected with either vector or CA-FAK K38A. Cells were plated on laminin or collagen for 30 minutes and number of attached cells was counted in 20 fields; the results are shown as fold change relative to vector (middle). *P<0.01. Immunoblotting verified expression of CA-FAK in cells (left). Right panel: the NRP2low population was transfected with either vector or CA-FAK K38A. Cells were incubated with either IgG or α6 antibody (GoH3) for 1 hour at 4°C. Cells were plated on laminin for 30 minutes and the number of attached cells was counted in 20 fields and is shown as fold change relative to vector-transfected cells.
We also evaluated Src activation as a function of NRP2 expression because Src is the kinase involved in FAK tyrosine phosphorylation (Zhao and Guan, 2009). Similar to FAK activation, NRP2 is necessary for Src activation induced by laminin but not collagen attachment (Fig. 7D). However, NRP2 downregulation did not cause any change in ERK activation (Fig. 7D, right panel). We also assessed whether the effects of NRP2 depletion on cell invasion could be ‘rescued’ by expressing a constitutively active form of Src (CA-Src). Expression of this construct in NRP2-depleted cells increased cell invasion on Matrigel (Fig. 7E).
Given that the NRP2low population adheres much less avidly to laminin than does the NRP2high population (Fig. 1), we hypothesized that FAK activation is crucial for strong adhesion on laminin. To test this hypothesis we expressed constitutively active FAK K38A in NRP2low cells and observed a significant (P=0.001) increase in adhesion to laminin but not to collagen and this adhesion was dependent on α6β1 integrin (Fig. 7F). These data suggest a positive feedback mechanism in which CA-FAK enhances the activity of α6β1 integrin.
Discussion
The data reported here reveal a previously unknown mechanism for activation of the α6β1 integrin by NRP2. Specifically, we conclude that VEGF–NRP2 signaling activates PKC and that PKC contributes to the functional activation of α6β1 integrin enabling it to interact more avidly with laminin, form focal adhesions and signal FAK activation. Unexpectedly, we discovered that NRP2 itself is located in focal adhesions on laminin and that it is essential for the formation of these structures. The relevance of these findings is validated by our observation that the NRP2high population of tumor cells harvested from freshly resected, invasive breast carcinomas expressed high levels of α6β1 integrin and active FAK, adhered avidly to laminin and formed focal adhesions in contrast to the NRP2low population.
Our study supports the hypothesis that the activation state of the α6β1 integrin is tightly regulated by autocrine and paracrine factors present in the tumor microenvironment, highlighting the reported functional importance of this integrin in cancer (e.g. Lathia et al., 2010; Sroka et al., 2010; Wewer et al., 1997). Moreover, the fact that both NRP2 (Yasuoka et al., 2009) and α6β1 integrin (Friedrichs et al., 1995; Wewer et al., 1997) have been associated with aggressive breast cancer supports this pathophysiological mechanism. This mode of α6β1 integrin regulation was foreshadowed in earlier work from our lab demonstrating that activation of α6β1 integrin in macrophages is regulated by inflammatory stimuli such as IFN-γ and LPS (Shaw and Mercurio, 1989). A common theme in these studies is that PKC contributes to the functional activation of α6β1 integrin and its association with the cytoskeleton (Shaw et al., 1990). The possibility that PKC activation is dependent on laminin adhesion and not NRP2 signaling in our studies is discounted by our finding that NRP2 contributes to PKC activation on all substrata tested. The mechanism by which VEGF–NRP2 signaling activates PKC is worth considering in light of recent findings. Specifically, we note that VEGF–NRP2 can activate TORC2 and that TORC2 can phosphorylate and stabilize conventional PKCs (Muders et al., 2009; Sarbassov et al., 2004). This potential mechanism is relevant because TORC2 has been implicated in regulating the actin cytoskeleton by modulating PKC phosphorylation (Sarbassov et al., 2004).
We are particularly intrigued by our observation that VEGF–NRP2 is needed for α6β1 integrin to activate FAK, because FAK is emerging as a central player in the biology of mammary gland development and breast cancer (Nagy et al., 2007; Provenzano et al., 2008; van Miltenburg et al., 2009). In fact, we reported recently that VEGF–NRP2-mediated activation of FAK contributes to branching morphogenesis in the developing mammary gland (Goel et al., 2011). Interestingly, FAK activation is dependent on α6β1-integrin-mediated adhesion to laminin, which excludes the possibility that VEGF–NRP2 activates FAK directly. The most compelling data we obtained that highlight the importance of FAK is that the NRP2high population of tumors isolated from breast carcinomas expresses considerably higher levels of FAK-P than the NRP2low population and that the ability of NRP2low cells to adhere to laminin and form focal adhesions could be rescued by expression of constitutively active FAK. This rescue experiment suggests that CA-FAK can enhance the activity of α6β1 integrin, perhaps by a mechanism that involves FAK stimulation of VEGF expression.
The potential relationship of our FAK data to the biology of breast tumor stem cells merits consideration. FAK has been implicated in the function of such cells (Luo et al., 2009), which are characterized by high expression of α6 integrin (CD49f) (Lathia et al., 2010). Given our findings in this study including the observation that the NRP2high population isolated from tumors expresses high levels of α6 integrin, it is reasonable to postulate that VEGF–NRP2 signaling regulates α6β1-integrin-mediated activation of FAK in breast tumor stem cells. This hypothesis is consistent with other reports that have implicated both VEGF signaling (Bao et al., 2006; Lichtenberger et al., 2010) and the α6 integrin (Lathia et al., 2010) in the function of tumor stem cells.
Our findings on the regulation of α6β1 integrin by NRP2 should be considered in the context of previous studies on the regulation of integrin function by the NRPs. These studies have focused entirely on NRP1 and no published data exist on integrin regulation by NRP2. Interestingly, we found that the α6 integrins do not associate with NRP1 (data not shown). NRP1 has been reported to interact with the α5β1 integrin in endothelial cells and promote FN adhesion independently of the known NRP1 ligands, VEGF and semaphorins (Valdembri et al., 2009). The mechanism involves NRP1-mediated trafficking and internalization of α6β1 integrin. Although this study is significant, we found no evidence that NRP2 regulates the surface expression of α6β1 integrin. The importance of integrin–NRP interactions in cancer was indicated initially by the report that NRP1 interacts with β1 integrins in pancreatic carcinoma cells and it modulates their growth, survival and invasion (Fukasawa et al., 2007). Integrins might also regulate NRP function. For example, the αvβ3 integrin was shown to inhibit the contribution of NRP1 to angiogenesis by sequestering it away from VEGFR2 (Robinson et al., 2009). Together, the previously published data have established the importance of NRP1-integrin interactions.
The distinguishing aspect of our results is not only that NRP2 interacts with a specific integrin and regulates its function but also that NRP2 is located in the focal adhesion, which is the nexus of integrin signaling (Dubash et al., 2009), and it contributes to the formation of this structure through its ability to activate α6β1 integrin and promote its association with the cytoskeleton. Of note, a recent study concluded that NRP1 does not localize to focal adhesions, establishing a key difference between these two receptors (Evans et al., 2011). Importantly, we also implicate FAK activation as the prime consequence of VEGF–NRP2 regulation of α6β1 integrin and demonstrate the significance of this mechanism in tumor cells isolated from invasive breast carcinomas. The implication of these findings for the pathogenesis of breast cancer, especially the function of tumor stem cells, is significant.
Materials and Methods
Reagents and antibodies
Matrigel and collagen I were purchased from BD Biosciences (San Jose, CA); laminin-1 (LN-1) from Invitrogen (Carlsbad, CA), fibronectin from Sigma (St Louis, MO); VEGF-165 from Peprotech (Rocky Hill, NJ) and G06983 from Calbiochem (Darmstadt, Germany). Antibodies against the following proteins were used: α3 integrin (Millipore, Billerica, MA) used for immunoblotting, or P1B5 (Gibco, Invitrogen, used for immunofluorescence); α6 integrin (AA6A, provided by Anne Cress, University of Arizona Cancer Center, Tucson, AZ, USA; J8H, provided by Arnoud Sonnenberg, The Netherlands Cancer Center, Amsterdam, The Netherlands; and GoH3, purchased from Millipore); β1 integrin (AIIB2, Developmental Studies Hybridoma Bank, Iowa); β4 integrin (439-9b, provided by Rita Falcioni (Regina Elena Cancer Institute, Rome, Italy); NRP2 (goat IgG, R&D, Minneapolis, MN; C9 and H300, Santa Cruz Biotechnology, Santa Cruz, CA); vinculin (Sigma); actin (Sigma); FAK-P (Y397) [mouse IgG (BD Bioscience) used for immunoblotting; rabbit IgG (AbCaM, Cambridge, MA, USA) used for immunofluorescence]; FLAG (Sigma); anti-rabbit-FITC; anti-goat FITC; anti-goat TRITC; rat IgG; mouse IgG (Jackson, West Grove, PA, USA); pan-phosphorylated-PKC S660; Src, phosphorylated-Src Y418; ERK, phosphorylated ERK (Cell Signaling, Beverly, MA, USA); PKCα (H7) and FAK (Santa Cruz Biotechnology); EpCAM (AbCaM) and VEGF (Calbiochem). Rhodamine-conjugated phalloidin was purchased from Sigma.
Isolation of epithelial cells from breast tumors
All human breast tissue was obtained in compliance with the laws and institutional guidelines, as approved by the Institutional Review Board committee of the University of Massachusetts Medical School. Epithelial cells were isolated from discarded but freshly resected, invasive breast tumors as described previously (Fillmore et al., 2010). Briefly, the tissue was minced and digested overnight with a mixture of collagenase (Roche, Indianapolis, IN, USA) and hyaluronidase (MP Biomedicals, Solon, OH, USA). The digested cells were plated briefly in serum (1–2 hours) to deplete mammary fibroblasts. The organoids were dissociated into a single cell suspension by trypsinization and filtered (40-μm filter; BD Biosciences) to remove residual clustered cells. Immediately after dissociation, cells were sorted on the basis of EpCAM and NRP2 expression and subsequently analyzed by flow cytometry using GoH3 (α6), NRP2 (R&D), β4 (439-9B) or control IgG to quantify expression of these receptors. In some experiments, cells were infected with lentiviruses expressing VEGF shRNA (Open Biosystems, Huntsville, AL, USA; clone ID TRCN0000003343 or TRCN0000003344).
Cell lines and transfectants
SUM-1315 cells were provided by Steve Ethier (Wayne State University School of Medicine). MDA-MB-435 cells were obtained from the Lombardi Cancer Center Breast Cancer Repository. Lentiviruses containing NRP2 shRNAs (Open Biosystems; clone ID TRCN0000063308, TRCN0000063309 or TRCN0000063312) or a GFP control (Open Biosystems; RHS4459) were generated, titrated according to the manufacturer's instructions and used to infect cells following standard protocols. Stable cell transfectants were generated by puromycin selection (2 μg/ml). In some experiments, cells were transfected with VEGF siRNA (Smartpool, Dharmacon, Lafayette, CO, USA) or scrambled control siRNA.
Cell-based assays
To assay cell adhesion, 96-well plates were coated with varying concentrations of either laminin or collagen overnight at 4°C, blocked with BSA and washed with PBS. Cells were detached, washed with PBS and plated (105 cells per well). Cells were incubated at 37°C for 30 minutes in serum-free medium, washed, and adherent cells were stained with Crystal Violet to quantify adhesion using a colorimetric assay. In some experiments, cells were incubated with function blocking antibodies on ice for 1 hour. Invasion assays were performed as described previously (Shaw et al., 1997).
Biochemical experiments
Cells were extracted in either a Triton X-100 buffer (1% Triton X-100, 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1 mM PMSF and protease inhibitors) or RIPA (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1 mM PMSF and protease inhibitors). The extracts were pre-cleared by centrifugation and proteins were immunoprecipitated by incubating with primary antibody and protein-A–Sepharose. Immunocomplexes were dissociated and proteins were separated by SDS-PAGE and immunoblotted using antibodies as specified in the figure legends. In some experiments (Fig. 4C), cell extracts were prepared using a modified Triton X-100 buffer that removes most of the soluble protein and phospholipid but leaves the cytoskeleton intact, to assess the interaction of α6β1 integrin with the cytoskeleton (Rabinovitz and Mercurio, 1997).
Immunofluorescence and TIRF microscopy
Cells were seeded onto ECM-coated coverslips and processed for immunofluorescence microscopy. Cells were fixed, permeabilized and blocked using BSA. Cells were incubated with primary antibody overnight at 4°C, washed and incubated with fluorochrome-conjugated secondary antibodies. Images were captured using fluorescence, confocal or TIRF microscopy. For TIRF, there were 10 data sets (i.e. n=10). For each color image (either NRP-2 or FAK) of each data set, we first estimated the background using morphological filter, a gray level ‘opening’ operation, and subtracted it from the image. Subsequently, we selected a global intensity threshold (same for all pixels in that given image) for that background-subtracted image and eliminated all pixels having intensity less than this threshold. Colocalization was calculated on a pixel basis as follows. NRP-2 with FAK: number of pixels where NRP2>threshold and FAK>threshold divided by the number of pixel where NRP2>threshold; FAK with NRP2: number of pixels where NRP2>threshold and FAK>threshold divided by the number of pixel where FAK>threshold.
Supplementary Material
Acknowledgements
We thank Isaac Rabinovitz and Chris Turner for helpful discussions. We also thank Arnoud Sonnenberg and Anne Cress for providing α6antibodies, Jun-Lin Guan for providing K38A FAK, Alex Toker for providing myr-PKC and Leslie Shaw for CA-Src.
Footnotes
Funding
This work was supported by the National Institutes of Health [grant number R01CA80789 to A.M.M.] Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.094433/-/DC1
References
- Bachelder R. E., Crago A., Chung J., Wendt M. A., Shaw L. M., Robinson G., Mercurio A. M. (2001). Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 61, 5736-5740 [PubMed] [Google Scholar]
- Bae D., Lu S., Taglienti C. A., Mercurio A. M. (2008). Metabolic stress induces the lysosomal degradation of neuropilin-1 but not neuropilin-2. J. Biol. Chem. 283, 28074-28080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S., Wu Q., Sathornsumetee S., Hao Y., Li Z., Hjelmeland A. B., Shi Q., McLendon R. E., Bigner D. D., Rich J. N. (2006). Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 66, 7843-7848 [DOI] [PubMed] [Google Scholar]
- Byzova T. V., Goldman C. K., Pampori N., Thomas K. A., Bett A., Shattil S. J., Plow E. F. (2000). A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6, 851-860 [PubMed] [Google Scholar]
- Caunt M., Mak J., Liang W. C., Stawicki S., Pan Q., Tong R. K., Kowalski J., Ho C., Reslan H. B., Ross J., et al. (2008). Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 13, 331-342 [DOI] [PubMed] [Google Scholar]
- Chambers A. F. (2009). MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 69, 5292-5293 [DOI] [PubMed] [Google Scholar]
- Chung J., Mercurio A. M. (2004). Contributions of the alpha6 integrins to breast carcinoma survival and progression. Mol. Cells 17, 203-209 [PubMed] [Google Scholar]
- Delwel G. O., de Melker A. A., Hogervorst F., Jaspars L. H., Fles D. L., Kuikman I., Lindblom A., Paulsson M., Timpl R., Sonnenberg A. (1994). Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol. Biol. Cell 5, 203-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubash A. D., Menold M. M., Samson T., Boulter E., Garcia-Mata R., Doughman R., Burridge K. (2009). Chapter 1. Focal adhesions: new angles on an old structure. Int. Rev. Cell Mol. Biol. 277, 1-65 [DOI] [PubMed] [Google Scholar]
- Evans I. M., Yamaji M., Britton G., Pellet-Many C., Lockie C., Zachary I. C., Frankel P. (2011). Neuropilin-1 signaling through p130Cas tyrosine phosphorylation is essential for growth factor-dependent migration of glioma and endothelial cells. Mol. Cell Biol. 31, 1174-1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore C. M., Gupta P. B., Rudnick J. A., Caballero S., Keller P. J., Lander E. S., Kuperwasser C. (2010). Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc. Natl. Acad. Sci. USA 107, 21737-21742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs K., Ruiz P., Franke F., Gille I., Terpe H. J., Imhof B. A. (1995). High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 55, 901-906 [PubMed] [Google Scholar]
- Fukasawa M., Matsushita A., Korc M. (2007). Neuropilin-1 interacts with integrin beta1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol. Ther. 6, 1173-1180 [DOI] [PubMed] [Google Scholar]
- Goel H. L., Bae D., Pursell B., Gouvin L. M., Lu S., Mercurio A. M. (2011). Neuropilin-2 promotes branching morphogenesis in the mouse mammary gland. Development 138, 2969-2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. J., Wey J. S., Belcheva A., McCarty M. F., Trevino J. G., Evans D. B., Ellis L. M., Gallick G. E. (2005). Neuropilin-1 suppresses tumorigenic properties in a human pancreatic adenocarcinoma cell line lacking neuropilin-1 coreceptors. Cancer Res. 65, 3664-3670 [DOI] [PubMed] [Google Scholar]
- Gray M. J., Van Buren G., Dallas N. A., Xia L., Wang X., Yang A. D., Somcio R. J., Lin Y. G., Lim S., Fan F., et al. (2008). Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J. Natl. Cancer Inst. 100, 109-120 [DOI] [PubMed] [Google Scholar]
- Guo W., Pylayeva Y., Pepe A., Yoshioka T., Muller W. J., Inghirami G., Giancotti F. G. (2006). Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126, 489-502 [DOI] [PubMed] [Google Scholar]
- Hu B., Guo P., Bar-Joseph I., Imanishi Y., Jarzynka M. J., Bogler O., Mikkelsen T., Hirose T., Nishikawa R., Cheng S. Y. (2007). Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene 26, 5577-5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia J. D., Gallagher J., Heddleston J. M., Wang J., Eyler C. E., Macswords J., Wu Q., Vasanji A., McLendon R. E., Hjelmeland A. B., et al. (2010). Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 6, 421-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberger B. M., Tan P. K., Niederleithner H., Ferrara N., Petzelbauer P., Sibilia M. (2010). Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 140, 268-279 [DOI] [PubMed] [Google Scholar]
- Lipscomb E. A., Simpson K. J., Ring J. E., Dugan A. S., Mercurio A. M. (2005). The α6β4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res. 65, 10970-10976 [DOI] [PubMed] [Google Scholar]
- Luo M., Fan H., Nagy T., Wei H., Wang C., Liu S., Wicha M. S., Guan J. L. (2009). Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 69, 466-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita A., Gotze T., Korc M. (2007). Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 67, 10309-10316 [DOI] [PubMed] [Google Scholar]
- Miao H. Q., Lee P., Lin H., Soker S., Klagsbrun M. (2000). Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 14, 2532-2539 [DOI] [PubMed] [Google Scholar]
- Montel V., Suzuki M., Galloy C., Mose E. S., Tarin D. (2009). Expression of melanocyte-related genes in human breast cancer and its implications. Differentiation 78, 283-291 [DOI] [PubMed] [Google Scholar]
- Muders M. H., Zhang H., Wang E., Tindall D. J., Datta K. (2009). Vascular endothelial growth factor-C protects prostate cancer cells from oxidative stress by the activation of mammalian target of rapamycin complex-2 and AKT-1. Cancer Res. 69, 6042-6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy T., Wei H., Shen T. L., Peng X., Liang C. C., Gan B., Guan J. L. (2007). Mammary epithelial-specific deletion of the focal adhesion kinase gene leads to severe lobulo-alveolar hypoplasia and secretory immaturity of the murine mammary gland. J. Biol. Chem. 282, 31766-31776 [DOI] [PubMed] [Google Scholar]
- Neufeld G., Kessler O., Herzog Y. (2002). The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv. Exp. Med. Biol. 515, 81-90 [DOI] [PubMed] [Google Scholar]
- Pan Q., Chanthery Y., Liang W. C., Stawicki S., Mak J., Rathore N., Tong R. K., Kowalski J., Yee S. F., Pacheco G., et al. (2007). Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11, 53-67 [DOI] [PubMed] [Google Scholar]
- Provenzano P. P., Inman D. R., Eliceiri K. W., Beggs H. E., Keely P. J. (2008). Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am. J. Pathol. 173, 1551-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I., Mercurio A. M. (1997). The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J. Cell Biol. 139, 1873-1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. D., Reynolds L. E., Kostourou V., Reynolds A. R., da Silva R. G., Tavora B., Baker M., Marshall J. F., Hodivala-Dilke K. M. (2009). Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. J. Biol. Chem. 284, 33966-33981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296-1302 [DOI] [PubMed] [Google Scholar]
- Shaw L. M., Mercurio A. M. (1989). Interferon gamma and lipopolysaccharide promote macrophage adherence to basement membrane glycoproteins. J. Exp. Med. 169, 303-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L. M., Messier J. M., Mercurio A. M. (1990). The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the alpha 6 beta 1 integrin. J. Cell Biol. 110, 2167-2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L. M., Rabinovitz I., Wang H. H., Toker A., Mercurio A. M. (1997). Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell 91, 949-960 [DOI] [PubMed] [Google Scholar]
- Soker S., Takashima S., Miao H. Q., Neufeld G., Klagsbrun M. (1998). Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735-745 [DOI] [PubMed] [Google Scholar]
- Sroka I. C., Anderson T. A., McDaniel K. M., Nagle R. B., Gretzer M. B., Cress A. E. (2010). The laminin binding integrin alpha6beta1 in prostate cancer perineural invasion. J. Cell Physiol. 224, 283-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice E., Plouet J., Berge M., Allanic D., Tobelem G., Merkulova-Rainon T. (2008). Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 111, 2036-2045 [DOI] [PubMed] [Google Scholar]
- Uniewicz K. A., Fernig D. G. (2008). Neuropilins: a versatile partner of extracellular molecules that regulate development and disease. Front. Biosci. 13, 4339-4360 [DOI] [PubMed] [Google Scholar]
- Valdembri D., Caswell P. T., Anderson K. I., Schwarz J. P., Konig I., Astanina E., Caccavari F., Norman J. C., Humphries M. J., Bussolino F., et al. (2009). Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 7, e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Miltenburg M. H., Lalai R., de Bont H., van Waaij E., Beggs H., Danen E. H., van de Water B. (2009). Complete focal adhesion kinase deficiency in the mammary gland causes ductal dilation and aberrant branching morphogenesis through defects in Rho kinase-dependent cell contractility. FASEB J. 23, 3482-3493 [DOI] [PubMed] [Google Scholar]
- Wang L., Dutta S. K., Kojima T., Xu X., Khosravi-Far R., Ekker S. C., Mukhopadhyay D. (2007). Neuropilin-1 modulates p53/caspases axis to promote endothelial cell survival. PLoS ONE 2, e1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer U. M., Shaw L. M., Albrechtsen R., Mercurio A. M. (1997). The integrin alpha 6 beta 1 promotes the survival of metastatic human breast carcinoma cells in mice. Am. J. Pathol. 151, 1191-1198 [PMC free article] [PubMed] [Google Scholar]
- Yasuoka H., Kodama R., Tsujimoto M., Yoshidome K., Akamatsu H., Nakahara M., Inagaki M., Sanke T., Nakamura Y. (2009). Neuropilin-2 expression in breast cancer: correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer 9, 220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Guan J. L. (2009). Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 28, 35-49 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.