Abstract
The epithelial-to-mesenchymal transition (EMT) genetic program is a molecular convergence point in the life-threatening progression of organ fibrosis and cancer toward organ failure and metastasis, respectively. Here, we employed the EMT process as a functional screen for testing crude natural extracts for accelerated drug development in fibrosis and cancer. Because extra virgin olive oil (EVOO) (i.e., the juice derived from the first cold pressing of the olives without any further refining process) naturally contains high levels of phenolic compounds associated with the health benefits derived from consuming an EVOO-rich Mediterranean diet, we have tested the ability of an EVOO-derived crude phenolic extract to regulate fibrogenic and oncogenic EMT in vitro. High-performance liquid chromatography (HPLC) coupled to time-of-flight (TOF) mass spectrometry assays revealed that the EVOO phenolic extract was mainly composed (∼70%) of two members of the secoiridoid family of complex polyphenols, namely oleuropein aglycone—the bitter principle of olives—and its derivative decarboxymethyl oleuropein aglycone. EVOO secoiridoids efficiently prevented loss of proteins associated with polarized epithelial phenotype (i.e., E-cadherin) as well as de novo synthesis of proteins associated with mesenchymal migratory morphology of transitioning cells (i.e., vimentin). The ability of EVOO to impede transforming growth factor-β (TGF-β)–induced disintegration of E-cadherin-mediated cell–cell contacts apparently occurred as a consequence of the ability of EVOO phenolics to prevent the upregulation of SMAD4—a critical mediator of TGF-β signaling—and of the SMAD transcriptional cofactor SNAIL2 (Slug)—a well-recognized epithelial repressor. Indeed, EVOO phenolics efficiently prevented crucial TGF-β–induced EMT transcriptional events, including upregulation of SNAI2, TCF4, VIM (Vimentin), FN (fibronectin), and SERPINE1 genes. While awaiting a better mechanistic understanding of how EVOO phenolics molecularly shut down the EMT differentiation process, it seems reasonable to suggest that nontoxic Oleaceae secoiridoids certainly merit to be considered for aging studies and, perhaps, for ulterior design of more pharmacologically active second-generation anti-EMT molecules.
Introduction
Aderegulated epithelial-to-mesenchymal (EMT) trans-differentiation process (i.e., the generation of motile mesenchymal cells from epithelial sheets)1–3 is an ideal molecular scenario to test the notion that “an anti-aging drug must delay age-related diseases in order to extend lifespan (i.e., unless a drug delays age-related diseases, it will not extend lifespan and vice versa, if a drug prevents age-related diseases, it must extend lifespan).”4 On the one hand, EMT overactivation is a critical phenomenon in age-related human diseases exhibiting end-state organ fibrosis (e.g., kidney fibrosis in chronic renal disease, liver fibrosis in nonalcoholic steatohepatitis, or myocardial fibrosis in heart failure).5–8 The pleiotropic cytokine transforming growth factor-β (TGF-β) is a major regulator of these types of pathophysiological EMT.
Indeed, chronic diseases characterized by excessive fibrosis can be explained in terms of repeated and sustained infliction of TGF-β-induced EMT, which significantly increases synthesis and accumulation of collagen and extracellular matrix (ECM) in the affected organ.7,9–11 In this scenario, highly differentiated epithelial cells in the specific organs (e.g., tubular epithelial cells in the kidney) will respond to noxious stimuli by undergoing TGF-β-driven EMT. Then, the transdifferentiated epithelial cells migrate into the interstitial space and some of them will become activated (i.e., myofibroblasts de novo expressing mesenchymal markers such as α-smooth muscle cell actin [SMA]) to become the main source of excessive ECM. TGF-β–induced EMT not only constitutes a pivotal mechanism contributing to the supply of activated myofibroblasts in renal fibrosis but it also similarly turns adult hepatocytes into activated fibroblasts, contributing to liver fibrosis in nonalcoholic steatohepatitis.8 Spontaneous activation of TGF-β–driven EMT has also been shown to profibrotic responses during myocardial fibrosis in aged mice hearts.12
While end-stage renal disease will need lifelong renal replacement therapy with maintenance dialysis or kidney transplantation, it is also noteworthy that renal transplant recipients frequently start or restart dialysis because of the unusual propensity of these allografts to develop interstitial fibrosis and tubular atrophy as tubular epithelial cells react to certain fibrogenic stimuli to (re)engage in the process of EMT.13–16 Fibrosis, ranging from mild inflammation to severe sclerosing peritonitis and encapsulating sclerosing peritonitis, is also responsible for negative changes in the peritoneal membrane; this preservation is crucial for long-term survival in peritoneal dialysis. Glucose and glucose degradation products stimulate the profibrotic factor TGF-β by mesothelial cells and induce EMT, the pivotal triggering mechanism of peritoneal membrane fibrosis in peritoneal dialysis patients.17–19 Accordingly, the quality of life on renal replacement therapy is much impaired, and the life expectancy of these patients is substantially shorter when compared to the general population.20
On the other hand, the EMT developmental process has gained much attention in oncology because some aspects of embryonic EMT are instrumentally employed by human cancer cells to facilitate metastasis. Tumor cells undergoing the EMT acquire the capacity to migrate and invade the surrounding stroma; they subsequently spread via blood and lymphatic vessels to repopulate distant sites as metastases.21–25 More importantly, it has recently been discovered that tumor cells undergoing EMT acquire stem cell–like characteristics, thus showing a link between EMT and pathways involved in promoting cellular stemness.26–31 Induction of nontumorigenic, immortalized human mammary epithelial cells to the EMT phenotype likewise results in the loss of the epithelial phenotype (e.g., downregulation and relocation of the epithelial marker E-cadherin) and the acquisition of the mesenchymal phenotype (e.g., gain of the mesenchymal marker vimentin) concomitant with the acquisition of the CD44high/CD24lowimmunophenotype, a molecular signature associated with stem cells and increased tumor-initiating capacity in breast cancer disease.26,31 Because EMT-phenotypic tumor cells acquire stem-like cell signatures characterized by increased metastatic capacity and self-renewal ability, the stem-like cells or cancer stem cells (CSCs) generated from (e.g., TGF-β driven) EMT induction provide a resource for cancer to recur because these cells are well known to be highly drug resistant.32–35
A definitive understanding of the cellular and molecular events driving excessive fibrosis causally underlying end-state organ failures is crucial to designing effective and specifically targeted therapeutic interventions aimed to impede “fibrogenic EMT.” Similarly, a better molecular understanding and biological characterization of EMT phenotypic cells, cancer stem-like cells, and CSCs should allow us to screen for potential drugs that could cause selective killing of these cells that are the “root cause” of tumor development, maintenance, recurrence, and metastasis.36–38 Indeed, it is reasonable to suggest that molecular characterization of agents able to impede the generation of EMT-phenotypic tumor cells (i.e., “oncogenic EMT”) will allow not only the development of newer therapies for complete eradication of tumors, which will certainly improve the overall survival of patients diagnosed with cancers, but also to ameliorate progressive tissue fibrosis in various age-related chronic diseases.2,39
Supporting the hypothesis that EMT-focused studies can promise the generation of new anticancer and antifibrosis drugs, it should be noted that the gerosuppressant drug rapamycin (i.e., a blocker of the nutrient-sensing mammalian target of rapamycin [mTOR] pathway that, by slowing down organismal aging, efficiently delays cancer)40–42 has been found to significantly enhance the expression of a protective gene for EMT (i.e., E-cadherin) to impede TGF-β–induced EMT in cultured human peritoneal mesothelial cells.43,44 Similarly, the gerosuppressant agent metformin appears to molecularly behave as a bona fide antiaging modality owing its ability to prevent TGF-β–driven EMT in cultured Madin–Darby canine kidney (MDCK) cells (an in vitro mouse system model to study the critical involvement of the EMT phenomenon in renal fibrosis) and in cultured MCF-7 breast cancer epithelial cells (an in vitro human system model to study the loss of E-cadherin expression/function as the central molecular process required for EMT-driven acquisition of malignant and stem cell traits because post-EMT MCF-7/TGF-β mesenchymal phenotypic cells display increased tumorsphere-initiating stem cell–like features compared to their pre-EMT parental MCF-7 epithelial phenotypic cells).45,46 Accordingly, metformin treatment decreases both the self-renewal and the proliferation of breast CSC populations and efficiently prevents EMT-promoted ontogenesis of the breast CSC molecular signature because it ablates the ability of TGF-β to increase the population of breast cancer cells that can form mammospheres in suspension—a feature endowed by CSCs.47–50
Because crude phenolic extracts directly obtained from extra virgin olive oil (EVOO) and naturally enriched in secoiridoids (i.e., a family of complex polyphenols characteristics of Oleaceae plants)51–54 can efficiently attack drug-resistant EMT-type breast cancer cells intrinsically enriched with CSC-like phenotypes,55,56 we recently envisioned that EVOO phenolics can negatively impact TGF-β–triggered fibrogenic and oncogenic EMT. Here we reveal for the first time that an EVOO-derived crude phenolic fraction is sufficient to efficiently impede fibrogenic and oncogenic EMT in cultured MDCK and MCF-7 cells, respectively. Our current study strongly suggests that Oleaceae secoiridoids might constitute a novel valuable phytochemical platform for the discovery of previously unrecognized antiaging biomolecules.
Materials and Methods
Olive oil
The EVOO employed in this study was from the monovariety Picual obtained from Córdoba (Andalusia, Spain) in 2008. Picual olives were processed by continuous industrial plants equipped with a hammer crusher, a horizontal malaxator, and a two-phase decanter. Samples were stored in bottles without headspace at room temperature and in darkness before analysis.
Isolation of EVOO phenolic fraction
To isolate the phenolic fraction of Picual EVOO, we used solid-phase extraction (SPE) with Diol-cartridges. In all, 60 grams of Picual EVOO was dissolved in methanol and loaded onto the column. The cartridge was washed with 15 mL of hexane, which was then discarded to remove the nonpolar fraction of the EVOO. Finally, the sample was recovered by passing it through 40 mL of methanol; the solvent was evaporated under vacuum. The residue was dissolved with 2 mL of methanol and filtered through a 0.25-mm filter before characterization of the phenolic profile.
Qualitative and quantitative characterization of phenolic compounds
To characterize the phenolic profile in the Picual EVOO phenolic extract, rapid resolution liquid chromatography (RRLC) coupled to electrospray interface time-of-flight mass spectrometry (ESI-TOF-MS) was performed in an Agilent 1200-RRLC system (Agilent Technologies, Waldbronn, Germany) of the Series Rapid Resolution equipped with a vacuum degasser, an autosampler, a binary pump, and a UV-Vis detector. The chromatographic separation was carried out on a Zorbax Eclipse Plus C18 analytical column (4.6 mm×150 mm, 1.8-μm particle size). The flow rate was 0.80 mL/min, and the temperature of the column was maintained at 25°C. The mobile phases used were water with 0.25% acetic acid as eluant A and methanol as eluant B. The total run time was 27 min.53 Compounds were monitored in sequence first with diode array detection (DAD; 240 and 280 nm) and then with a mass analyzer (MA) detector. MS was performed using the microTOF (Bruker Daltonik, Bremen, Germany), which was coupled to the RRLC system. At this stage, the use of a splitter was required to the coupling with the MS detector because the flow that arrived to the TOF detector had to be 0.2 mL/min to obtain reproducible results and stable spray. The TOF mass spectrometer was equipped with an ESI (model G1607A from Agilent Technologies, Palo Alto, CA) operating in negative ion mode. External mass spectrometer calibration was performed with sodium formiate clusters (5 mM sodium hydroxide in water/2-propanol 1/1 [vol/vol], with 0.2% of formic) in quadratic þ high-precision calibration (HPC) regression mode.
The calibration solution was injected at the beginning of the run, and all of the spectra were calibrated prior to identification of EVOO polyphenols. The optimum values of the source and transfer parameters were get for a good sensitivity and reasonable resolution of the mass range for compounds of interest (50–1000 m/z) to improve ionization performance.53 The accurate mass data for the molecular ions were processed using the software Data Analysis 3.4 (Bruker Daltonik), which provided with a list of possible elemental formulas by using the Generate Molecular Formula Editor. The latter employs a CHNO algorithm that provides standard functionalities such as minimum/maximum elemental range, electron configuration, and ring-plus double bonds equivalent, as well as a sophisticated comparison of the theoretical with the measured isotopic pattern (sigma value) for increased confidence in the suggested molecular formula. The widely accepted accuracy threshold for confirmation of elemental compositions has been established at 5 ppm for most of the compounds.
The individual quantification of the identified phenolic compounds was carried out by RRLC-ESI-TOF using the validated method described above.53 Ten standard calibration graphs for the quantification of the principal compounds found in the samples were prepared using the following commercial available standards. Hydroxytyrosol, tyrosol, vanillin, luteolin, apigenin, p-coumaric acid, ferulic acid, and vanillic acid were purchased from Sigma-Aldrich (St. Louis, MO), and (+)-pinoresinol was acquired from Arbo Nova (Turku, Finland). Oleuropein (Ole) was purchased from Extrasynthèse (Lyon,France).
As complementary information, the total phenolic content of the crude EVOO phenolic extract was determined by a spectrophotometric method based on the Folin–Ciocalteau technique.57 The absorbance of the solution was measured at a wavelength of 725 nm in a Spectronic Genesys™ 5 spectrophotometer (Spectronic Instruments Inc. Rochester, NY). The extracts were diluted 1:10 with methanol. After this, a 50-μL aliquot of the diluted methanolic extract of EVOO was used in this determination. Total polyphenols were expressed as caffeic acid equivalents. A calibration curve of freshly prepared caffeic acid solution was carried out. Three replicates of each analysis and for each calibration point were performed to obtain reproducible results.
Cell lines and culture conditions
MDCK cells were obtained from Dr. Manel Esteller (Cancer Epigenetics and Biology Program-PEBC, Bellvitge Biomedical Research Institute–IDIBELL, L'Hospitalet, Barcelona, Spain) and cultured in Dulbecco modified Eagle medium (DMEM; BioSource International; Invitrogen S.A., Barcelona, Spain) supplemented with 10% fetal bovine serum (FBS), L-glutamine (2 mmol/L), and antibiotics (all from Biowhittaker, Madrid, Spain). MCF-7 human breast cancer cells were obtained from the American Type Culture Collection (ATCC) and they were routinely grown in improved MEM (IMEM) supplemented with 5% FBS and L-glutamine (2 mmol/L). Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were screened periodically for Mycoplasma contamination.
TGF-β1
Recombinant human (carrier-free) TGF-β1 was purchased from R&D Systems Inc. (Minneapolis, MA).
Immunofluorescence staining and high-content confocal imaging
Cells were seeded at approximately 5,000 cells/well in 96-well, clear-bottomed imaging tissue culture plates (Becton Dickinson Biosciences; San Jose, CA) optimized for automated imaging applications. Triton X-100 permeabilization and blocking, primary antibody staining (1:50 dilution), secondary antibody staining using Alexa Fluor 488/594 goat anti-rabbit/mouse immunoglobulin Gs (IgGs) (Invitrogen, Molecular Probes, Eugene, OR), and counterstaining (using Hoechst 33258; Invitrogen) were performed by following BD Biosciences protocols. Images were captured in different channels for Alexa Fluor 488 (pseudocolored green) and Hoechst 33258 (pseudocolored blue) on a BD Pathway™ 855 Bioimager System (Becton Dickinson Biosciences, San Jose, CA) with 20× or 40× objectives (NA 075 Olympus). Merged images were obtained according to the recommended assay [rocedure using BD Attovision™ software.
Quantitative, real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from MCF-7 cell cultures supplemented with TGF-β (100 ng/mL), EVOO phenolics, or TGF-β plus EVOO phenolics as specified using a Qiagen RNeasy kit and QIAshredder columns according to the manufacturer's instructions (Qiagen, Valencia, CA). One microgram of total RNA was reverse-transcribed to cDNA using the Reaction Ready™ First Strand cDNA Synthesis Kit (SABiosciences, Frederick, MD) and applied to EMT PCR Arrays (cat. no. PAHS-090; 96-well format) following SABiosciences RT-PCR manual. Plates were processed in an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA), using automated baseline and threshold cycle detection. Data were interpreted by using SABiosciences' web-based PCR array analysis tool.
Results
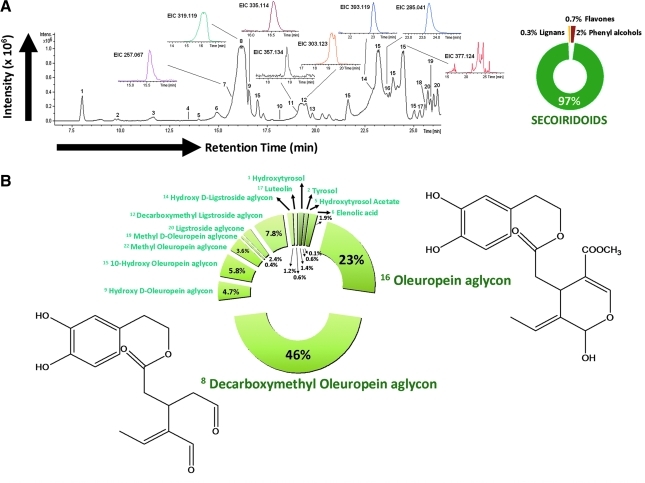
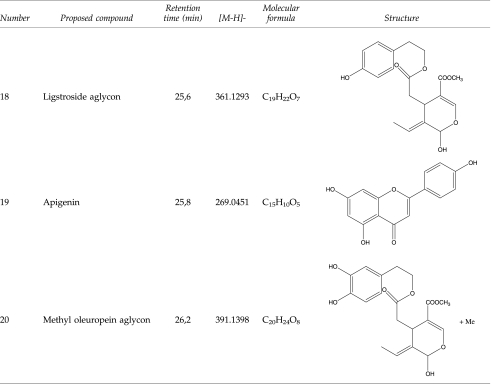
Qualitative and quantitative characterization of phenolic compounds in EVOO phenolic extract was performed in a previously reported analytical approach.53 Identification of phenolic compounds was performed by comparing both retention times and MS spectral data from EVOO methanolic extract and standards, as detailed in Materials and Methods. The remaining compounds, for which no commercial standards were available, were characterized by interpretation of their ultraviolet (UV) absorbance maxima, mass spectra provided by the TOF-MS, and other analytical information reported elsewhere because most of these compounds have been previously described in OO samples. Table 1 summarizes the main compounds identified in the Picual EVOO variety, including their structure and the information generated by TOF analyzer—retention time, calculated m/z, and molecular formula. Twenty compounds from different families (i.e., simple phenols, flavonoids, lignans, and secoiridoids) were identified. Figure 1A shows the resulting chromatogram of EVOO phenolic extract. Several isomers of oleuropein aglycon were tentatively characterized in this sample. This compound and its isomers, for which there are no available commercial standards, have been previously described in OO,58 and the accuracy of MS data provided enough confidence during their identification. The analysis of the true isotopic pattern by ESI-TOF-MS in combination with excellent mass resolution and mass accuracy is the perfect choice for molecular formula determination using the Generate Molecular Formula Editor as detailed in Materials and Methods.
Table 1.
Main Phenolic Compounds Identified in Extra Virgin Olive Oil Phenolic Extract by HPLC-DAD-ESI-TOF
|
FIG. 1.
(A) (Left) Base peak chromatogram (BPC) of extra virgin olive oil (EVOO) phenolic extract obtained by high-performance liquid chromatograph–electrospray ionization–time-of-flight mass spectrometry (HPLC-ESI-TOF) with the extracted ion chromatogram (EIC) of the main phenolic compounds. (Right) Percent distribution of main phenolic families identified in Picual EVOO phenolic extract. (B) Percent distribution of identified phenolic compounds in Picual EVOO phenolic extract. The figure shows chemical structures of oleuropein aglycone and its derivative decarboxymethyl oleuropein aglycone, the major components of the Picual EVOO phenolic extract. (Color image available online at www.liebertpub.com/rej)
Concentrations of individual phenolics were determined using the areas of each individual compound (three replicates) obtained by peak integration in their extracted ion chromatogram (EIC) and by interpolation in the corresponding calibration curve. EIC results of the main phenolic compounds are also shown in Fig. 1A. The phenolic compounds hydroxytyrosol, tyrosol, luteolin, and apigenin were quantified by their respective commercial standards prepared as previously described.53 Other phenolic compounds, which had no commercial standards, were tentatively quantified on the basis of other compounds having similar structures. Secoiridoid and lignan groups were quantified with oleuropein and (+)-pinoresinol standards, respectively. Elenolic acid derivates, which cannot be considered as true phenolic compounds, were expressed as oleuropein. It should be considered that response of the standards can be different from each one of the EVOO analytes included in the Picual variety, and consequently the quantification of these compounds was only an estimation of their actual concentrations.
Table 2 summarizes the amount of each phenolic from Picual EVOO, with the main components belonging to the secoiridoids group. Among these complex polyphenols, two derivatives of hydroxytyrosol (3,4-DHEPA) linked to the elenolic acid and its decarboxymethylated form, namely oleuropein aglycon (3,4-DHEPA-EA) and decarboxymethyl oleuropein aglycon (3,4-DHEPA-DEA), were the most abundant compounds as they constituted up to 23% and 46% of total phenolics, respectively (Fig. 1B). Regarding other phenolics belonging to the secoiridoid family, the relative contents of the decarboxymethylated form of ligstroside aglycon as well as the hydroxylated and methylated forms of oleuropein aglycon were significantly higher than other phenolic compounds. Simple phenols (e.g., tyrosol, hydroxytyrosol), lignans (e.g., siringaresinol, [+]-pinoresinol), and flavones (e.g., luteolin and apigenin) were notably underrepresented in the phenolic mixture. The average total polyphenols content was 683.579±4.169 expressed as milligrams of analyte/kg of EVOO, as tentatively calculated as the sum of the individual phenolic compound concentrations.
Table 2.
Quantitative Results Expressed in mg Analyte/ kg of Picual Extra Virgin Olive Oil Phenolic Extract
| Phenolic compounds | Concentration | Percent |
|---|---|---|
| Simple phenols | 14.467±0.285 | 2.116 |
| Hydroxytyrosol | 9.625±0.208 | 1.408 |
| Tyrosol | 4.162±0.109 | 0.609 |
| Hydroxytyrosol acetate | 0.680±0.003 | 0.099 |
| Secoiridoids | 662.673±4.782 | 96.942 |
| Elenolic acid | 13.123±0.125 | 1.920 |
| Hydroxy elenolic acid | 0.097±0.002 | 0.014 |
| Oleuropein aglycon | 157.917±1.257 | 23.102 |
| Decarboxymethyl oleuropein aglycon | 314.429±2.189 | 45.998 |
| Hydroxy d-oleuropein aglycon | 32.373±1.584 | 4.736 |
| 10-Hydroxy oleuropein aglycon | 39.479±0.806 | 5.775 |
| Methyl oleuropein aglycon | 24.349±0.516 | 3.562 |
| Methyl d-oleuropein aglycon | 2.903±0.079 | 0.425 |
| Ligstroside aglycon | 16.261±0.709 | 2.379 |
| Decarboxymethyl ligstroside aglycon | 53.652±0.354 | 7.849 |
| Hydroxy d-ligstroside aglycon | 8.087±0.353 | 1.183 |
| Lignans | 1.587±0.061 | 0.232 |
| Pinoresinol | 0.815±0.032 | 0.119 |
| Syringaresinol | 0.772±0.029 | 0.113 |
| Flavones | 4.852±0.084 | 0.710 |
| Luteolin | 4.041±0.065 | 0.591 |
| Apigenin | 0.811±0.040 | 0.119 |
| Total phenolic contents | 683.579±4.169 | 100.000 |
Value=X±standard deviation (SD). Percent distribution of identified phenolic compounds.
Because an inherent problem with using crude natural extracts in cell-based screens is that many of them are toxic to cells at the dilutions that might generate optimal hit rates because of the high concentrations of salts and other materials they contain, the EVOO phenolic extract was not cytotoxic at tested concentration to avoid unacceptable false-positive results. On the basis of earlier studies using crude EVOO phenolic extracts in human breast cancer cell lines,53,54,56 we first confirmed that EVOO phenolics did not significantly alter viability at 200 ng/mL (∼0.01% vol/vol), the concentration employed during 72 hr to test modulation of the EMT process in MDCK and MCF-7 cells.
EVOO phenolics impede “fibrogenic EMT”
We first employed MDCK cells to evaluate the impact of the EVOO-derived crude phenolic extract on several TGF-β–altered EMT parameters including cell morphology. Thus, we established TGF-β–induced EMT and then we measured the response to EVOO phenolics (Fig. 2). Untreated MDCK control cells displayed a typical epithelial-like morphology with respect to their light-microscopic appearance. Upon treatment with TGF-β (50 ng/mL), the cells acquired a spindle-type morphology and a reduced number of cell–cell contacts. These alterations started after 24 hr and they were maximal after 72 hr of treatment, when TGF-β supplementation fully induced a complete conversion of parental MDCK epithelial cells to a fibroblast-like, spindle-shaped, elongated mesenchymal morphology. The changes of morphology were partially reversible when TGF-β treatment was carried out in the presence of the EVOO-derived crude phenolic extract. Whereas EVOO phenolics as sole treatment had no significant effects on EMT-related morphological changes, TGF-β–treated MDCK cells changed from scattered and fibroblast-like shapes in the absence of EVOO phenolics to a more packed cobblestone morphology in their presence. Although MDCK cells co-treated with TGF-β and the EVOO-derived crude phenolic extract failed to maintain entirely the compact cuboidal appearance of parental MDCK control cells, EVOO phenolics notably prevented TGF-β–induced cell scattering and reduced the number of TGF-β–induced spindle-shaped cells and pseudopodia.
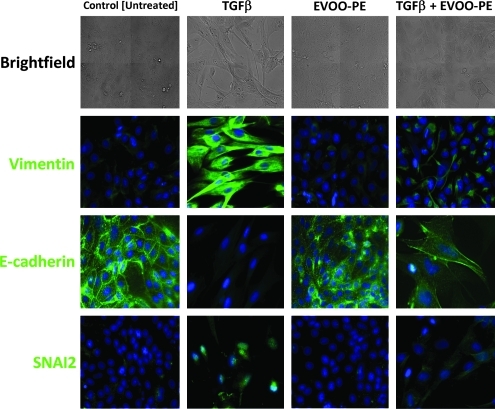
FIG. 2.
Effects of extra virgin olive oil (EVOO) phenolics on transforming growth factor-β (TGF-β–induced changes in the morphology and expression of epithelial/mesenchymal markers of Madin–Darby canine kidney (MDCK) cells. MDCK cells were grown until 80% confluence, serum-starved for 24 hr, and then treated for 3 days with a minimal amount (0.1%) of fetal bovine serum (FBS) supplemented with vehicle (control [untreated]), TGF-β1 alone (10 ng/mL), extra virgin olive oil phenolic extract (EVOO-PE) alone (200 ng/mL), or TGF-β1 plus EVOO-PE. Phase-contrast and immunofluorescence images were obtained and merged on a BD Pathway™ 855 Bioimager System according to the recommended assay procedure using BD Attovision™ software. Images show representative portions of MDCK cultures showing subcellular distribution of vimentin, E-cadherin, and SNAI2 from two to three independent experiments.
Because the above-mentioned findings suggested that exposure to EVOO phenolics triggered molecular changes that were consistent with a partial reversion of the EMT phenomenon, we carried out immunostaining studies to assess the expression status of marker proteins of either the epithelial (i.e., E-cadherin) or the mesenchymal (i.e., vimentin) phenotype in MDCK cell populations (Fig. 2). Compared with untreated MDCK control cells, immunostaining studies showed the reduced expression of E-cadherin in TGF-β1–treated MDCK cells, especially in the adherens junctions in which the expression of E-cadherin was not detected by immunofluorescence microscopy. The intermediate filament vimentin accumulated profusely in the cytoplasm of TGF-β1–treated MDCK cells. The EVOO-derived crude phenolic extract significantly prevented TGF-β1–induced disintegration of E-cadherin at cell–cell contacts. Moreover, EVOO phenolics largely prevented the strong activation of the post-EMT marker vimentin that occurred upon treatment with TFG-β1. We finally evaluated whether the EVOO-derived crude phenolic extract could impact the expression of EMT-promoting transcription factors such as SNAI2 (Slug), which not only functions as a bona fide epithelial repressor that inhibits E-cadherin gene expression through its direct binding to E-boxes within the E-cadherin promoter but also interacts with mesenchymal activators such as β-catenin to induce vimentin expression.34 Likewise, SNAI2 protein was significantly upregulated during TGF-β1–driven mesenchymal conversion of MDCK cells. Of note, the EVOO-derived crude phenolic extract negatively regulated nuclear accumulation of SNAI2 in response to TGF-β1.
EVOO phenolics impede “oncogenic EMT”
We first confirmed that cellular changes (morphology and size) observed in MCF-7 breast cancer epithelial cells supplemented exogenously with TGF-β1 resembled those of tumor cells undergoing EMT. Immunoreactivity of the epithelial cell marker E-cadherin was likewise found at sites of cell–cell contact in untreated MCF-7 control cells. This classical expression of E-cadherin in the basolateral membrane of epitheloid cells was significantly altered upon exposure to the EMT promoter TGF-β1, which promoted a drastic distribution of E-cadherin from a conspicuous, highly membranous staining to a more diffuse, barely detectable staining in the cytoplasm (Fig. 3). The EVOO-derived crude phenolic extract as sole treatment appeared to significantly accumulate E-cadherin protein expression at sites of cell–cell contact, thus promoting a more cuboidal appearance of MCF-7 cell cultures, which grew in more compact, homogeneously structured monolayers. Of note, TGF-β1 failed to downregulate membranous E-cadherin when MCF-7 epithelial breast cancer cells were concurrently co-exposed to EVOO phenolics.
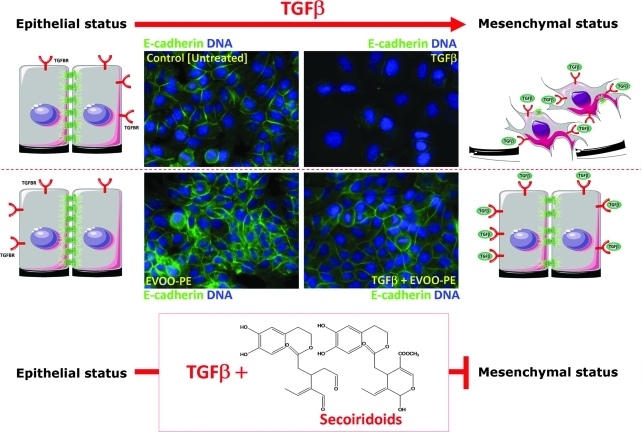
FIG. 3.
Effect of extra virgin olive oil (EVOO) phenolics on distribution of the epithelial marker E-cadherin in MCF-7 cells. Subcellular distribution of E-cadherin was evaluated by indirect immunofluorescence in MCF-7 cells grown until 80% confluence, serum-starved for 24 hr, and then treated for 3 days with a minimal amount (0.1%) of fetal bovine serum (FBS) supplemented with vehicle (control [untreated]), transforming growth factor-β1 (TGF-β1) alone (10 ng/mL), EVOO phenolic extract (EVOO-PE) alone (200 ng/mL), or TGF-β1 plus EVOO-PE. Images show representative portions (n=3) of MCF-7 cell cultures treated as specified for 3 days and captured in different channels for E-cadherin (green) and DNA (blue) on a BD Pathway™ 855 Bioimager System. The figure also shows a schematic summarizing EVOO phenolics' preventative activity against TGF-β–promoted downregulation or complete loss of the metastasis suppressor protein E-cadherin. Secoiridoids-targeted E-cadherin expression could efficiently prevent TGF-β–induced conversion of epithelial into migratory mesenchymal cells and may thus be a useful strategy to impede formation of migratory cancer stem cells (CSCs).
To make a preliminary evaluation of whether EVOO phenolics negatively impacted formation and activation of EMT-promoting SMAD complexes (i.e., SMAD proteins [intracellular transcription factors and transducers of TGF-β signaling] have low affinity for DNA and need to interact and form a complex with EMT-promoting transcription factors, including SNAIL and ZEB, to target the gene promoter of E-cadherin during TGF-β–driven EMT in breast epithelial cells), we employed immunofluorescence microscopy to monitor the expression status of SMAD4, SNAI2, and E-cadherin proteins of MCF-7 epithelial cells cultured with TGF-β1 in the absence or presence of EVOO phenolics (Fig. 4). TGF-β1 treatment of MCF-7 cells for 72 hr resulted in enhanced expression of SMAD4 and SNAI2, which paralleled loss of E-cadherin at intercellular junctions. The EVOO-derived crude phenolic extract significantly prevented the ability of TGF-β to upregulate SMAD4 and SNAI2.
FIG. 4.
Effects of extra virgin olive oil (EVOO) phenolics in transforming growth factor-β–regulated epithelial-to-mesenchymal transition (EMT) signaling cascade. Subcellular distribution of SMAD4, SNAI2, and E-cadherin was evaluated strictly in parallel by indirect immunofluorescence in MCF-7 cells grown until 80% confluence, serum-starved for 24 hr, and then treated for 3 days with a minimal amount (0.1%) of fetal bovine serum (FBS) supplemented with vehicle (control [untreated]), transforming growth factor-β1 (TGF-β1) alone (10 ng/mL), EVOO-phenolic extract (EVOO-PE) alone (200 ng/mL), or TGF-β1 plus EVOO-PE. Images show representative portions (n=3) of MCF-7 cell cultures treated as specified for 3 days and captured in different channels for E-cadherin (green) and DNA (blue) on a BD Pathway™ 855 Bioimager System. This figure also shows a schematic summarizing EVOO phenolics' preventative activity against TGF-β–driven EMT (1: Input layer [ligand/receptor/adaptors]; 2: Signal-processing layer [signaling cascade/transcription factors]; 3: Output layer [epithelial/mesenchymal markers]. TGF-β binding to its receptor (TGFBR) results in activation and nuclear translocation of SMAD transcription factors (e.g., SMAD4), which achieve target gene (e.g., E-cadherin) specificity through interaction with transcriptional cofactors (e.g., SNAI2). Epithelial-to-mesenchymal transition (EMT)-promoting transcription factors, including epithelial repressors such as SNAILs, ZEB, or TWIST, interact with SMADs, thus resulting in the formation of EMT-promoting SMAD complexes that can drive EMT by repressing epithelial genes such as E-cadherin. E, E-boxes; SBE, SMAD-binding elements.
EVOO phenolics prevent TGF-β1–induced activation of the EMT transcriptional program
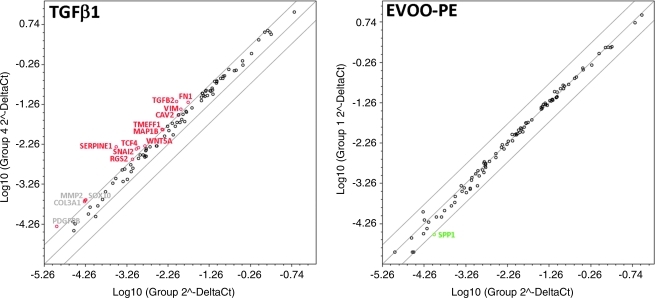
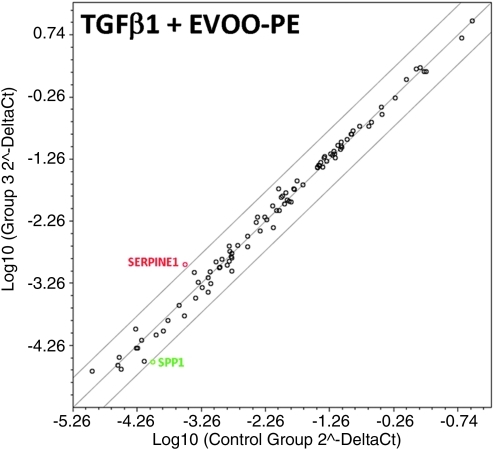
Finally, we decided to explore whether EVOO phenolics actively regulated a programmed series of genes events controlling TGF-β1–induced EMT, as suggested by the immunofluorescence studies. Total RNA from untreated MCF-7 control cells and MCF-7 cells treated with TGF-β 1, EVOO phenolics, or TGF-β1 plus EVOO phenolics were evaluated using qRT-PCR of genes specifically associated with EMT and the reciprocal mesenchymal-to-epithelial transition (MET) (Fig. 5). When a three-fold or greater difference in mRNA expression levels was used as the cutoff to determine significant regulatory effects on genes involved in the EMT genetic program, TGF-β1 treatment was found to significantly upregulate a total of 11 of the 84 EMT gene regulators assessed in the experiment. MCF-7 cells treated with TGF-β1 for 72 hr transcriptionally enhanced the expression of developmental EMT genes that have been repeatedly demonstrated to play pivotal roles in malignant progression, including SNAI2 and TCF4 (approximately four-fold increases).59,60 Accordingly, MCF-7/TGF-β1 cells notably increased the expression of plasminogen activator inhibitor type-1 (PAI-1; SERPINE1), a prominent member of the subset of TGF-β–initiated EMT-related signaling events,61,62 and the mesenchymal markers vimentin (VIM;∼4-fold) and fibronectin (FN;∼4-fold). Supporting the notion that EVOO phenolics appear to negatively regulate oncogenic EMT, co-treatment of MCF-7 cells with the EVOO-derived crude phenolic extract efficiently prevented the occurrence of most of TGF-β1–induced EMT transcriptional events, including up-regulation of SNAI2, TCF4, VIM, and FN. TGF-β1–induced accumulation of SERPINE1 decreased from ∼15 times in the absence of EVOO phenolics to ∼4 times in their presence. Regardless the presence of the EMT inducer TGF-β1, the EVOO-derived crude phenolic extract significantly decreased by ∼4-fold the baseline expression of the mesenchymal marker osteopontin (SPP1, secreted phosphoprotein 1)63–65 independently of the presence of TGF-β1.
FIG. 5.
Epithelial-to-mesenchymal transition (EMT) expression profiling of MCF-7 cells treated with transforming growth factor-β (TFG-β) in the absence or presence of extra virgin olive oil (EVOO)phenolics. This figure shows representative scatter plots of the difference in relative abundance of 84 mRNAs whose levels, in published results, are significantly altered during activation/deactivation of the EMT genetic program (≥3-fold). (Green and red symbols) Downregulation and upregulation versus basal expression levels in untreated MCF-7 cells, respectively; (grey symbols) fold-change results that need to be validated with a sufficient number of biological replicates (i.e., fold-change results may have greater variations of p value>0.05 or the p value for the fold-change is either unavailable or relatively high [p>0.05]) or they are uninterpretable because the gene's average threshold cycles were either not determined or greater than the define cut-off value (default 35) in both samples. (Color image available online at www.liebertpub.com/rej)
Discussion
EVOO, the juice of the olive obtained solely by pressing and consumed without any further refining process, is unique among other vegetable oils because of its high level of naturally occurring phenolic compounds. Here, we have tested the hypothesis that prevention of TGF-β–induced EMT may represent a previously unrecognized mechanism through which phenolic molecules naturally occurring in EVOO might actively regulate the pathophysiology of many age-related human diseases, including end-state organ failures, due to excessive EMT-related fibrosis and cancer metastasis, given that the EMT genetic program is sufficient to generate migrating CSCs by directly linking the acquisition of cellular motility with the maintenance and enhancement of tumor-initiating capacity.
Using canine MDCK kidney cells and human MCF-7 breast cancer cells, we directly evaluated the impact of a crude EVOO phenolic extract on several TGF-β–altered EMT parameters, including cell morphology and expression levels of epithelial and mesenchymal markers. EVOO phenolics significantly reduced TGF-β1–induced cell scattering and TGF-β1–increased intercellular spaces in MDCK cell cultures. The inhibitory effects of EVOO phenolics on TGF-β1–induced conversion of MDCK epithelial cells to the spindle-like mesenchymal morphology was accompanied by a significant prevention of the cytoplasmic accumulation of the mesenchymal marker vimentin occurring in the TGF-β1–induced mesenchymal state of MDCK cells. Perhaps more importantly, treatment with EVOO phenolics notably prevented the occurrence of hallmarks of TGF-β–induced acquisition of the mesenchymal phenotype, including the ability of TGF-β to cause loss of plasma membrane staining for E-cadherin and its ability to enhance cytoplasmic punctate and diffuse vesicle E-cadherin staining. Disintegration of cell–cell contacts (tight junctions, adherens junctions, and desmosomes) is a key feature of EMT.
More importantly, several lines of evidence have also substantiated that cell–cell contacts are not passive targets but they are active regulators of EMT. Loss of the adherens junctions' component E-cadherin is well established to promote EMT in developmental biology and tumor models, whereas forced expression of E-cadherin can restore (noninvasive) epithelial phenotypes in (invasive) mesenchymal tumor cells. In terms of “fibrotic EMT,” E-cadherin has been shown to play a central role in the EMT of renal tubular cells. In terms of “oncogenic EMT,” activation of EMT in response to short hairpin (sh)RNA-mediated knockdown of E-cadherin expression has been shown to increase the tumorigenic CD44high/CD24low mesenchymal population in transformed breast cancer cells. Indeed, combined immunohistochemical staining of positive (e.g., SNAIL) and negative (e.g., E-cadherin) EMT markers together with SMAD3/4 has been proven to be an efficient approach to identify CSC-like EMT phenotypic cells in samples of invasive human ductal breast carcinomas.34 In these studies, loss of E-cadherin at intercellular junctions significantly correlated with nuclear expression of SNAIL and SMAD3/4 at invasive regions, thus supporting a role for SNAIL-SMAD3/4 complexes in the repression of junction proteins during breast cancer cell invasion. In our hands, TGF-β treatment of MCF-7 breast epithelial cancer cells in vitro notably recapitulated the ability of EMT to promote ontogenesis of the breast cancer stem cell molecular signature in vivo because TGF-β–promoted loss of E-cadherin took place upon activation of critical mediators of TGFβ signaling, such as SMAD4, and epithelial repressors, such as the SMAD transcriptional cofactor SNAIL2. Remarkably, EVOO phenolics largely nullified upstream activation of TGF-β signaling to impede downstream formation of EMT-promoting SMAD repressor complexes. By weakening the ability of TGF-β to fully induce mesenchymal cell states, our current findings illustrate for the first time that the anti-EMT activity in vitro of EVOO phenolics might translate into antifibrotic and anti-CSCs in vivo.
From a mechanistic perspective, and considering that EVOO phenolics remarkably repressed the ability of TGF-β ability to transcriptionally reprogram MCF-7 breast cancer epithelial cells to express pivotal drivers and effectors of the EMT genetic program including SNAI2, TCF4, VIM, and FN, whereas they failed to fully prevent TGF-β–induced EMT, as determined by cellular morphology in MDCK cells, it might be reasonable to suggest that EVOO phenolics efficiently impede an important subset of TGF-β–regulated EMT changes but fail to complete the EMT reversal program (i.e., MET). This scenario agrees with the hypothesis that reversal of EMT not only requires re-establishment of epithelial gene transcription but further a regain of structural components of the epithelial cytoskeleton.66
Our current description of a significant anti-EMT activity of EVOO phenolics supports and expands further earlier studies showing that the green tea polyphenol epigallocatechin-3 gallate (EGCG) can significantly reduce the invasive breast cancer phenotype by upregulating the epithelial marker E-cadherin while downregulating the proinvasive SNAIL gene.67,68 Indeed, the common ability of a naturally occurring complex polyphenols to alter the expression of key regulators in the EMT pathway may suggest that they share an identical target that activation/deactivation prevents or reverses TGF-β–driven EMT. Activation of the energy sensor adenosine monophosphate–activated protein kinase (AMPK) is a plausible mechanism through which polyphenolic phytochemicals may reduce the ability of TGF-β to activate and maintain the mesenchymal molecular signature. On the one hand, AMPK activation has been shown to abrogate TGF-β–induced SMAD3-dependent transcription in cultured human primary mesangial cells and adult mouse cardiac fibroblasts.69,70 On the other hand, naturally occurring polyphenols such as EGCG significantly induce increases in the phosphorylation status of AMPK and in AMPK activity.71–73 If EVOO complex polyphenols behave molecularly in an EGCG-like manner, it might be tempting to suggest that, by acting as AMPK agonists, they can blunt TGF-β–activated EMT-related events (e.g., phosphorylation of signal transducer and activator of transcription 3 [STAT3], which upregulates expression of several factors important for EMT induction).74–76 Another plausible mechanism through which EVOO phenolics might inhibit EMT and reactivate an epithelial phenotype might relate to their ability to molecularly mimic the anti-EMT effects of the polyphenol resveratrol, which has recently been found to inhibit the expression of SNAIL, SLUG, and ZEB1 in pancreatic CSCs.77
Although it remains to be elucidated whether a resveratrol-like up-regulation of silent information regulator T1 (SIRT1) deacetylase activity may counterintuitively regulate EVOO phenolics-induced enhancement and/or maintenance of epithelial versus mesenchymal phenotypes (i.e., inhibition of SIRT1 increases acetylation of histone H3 at the E-cadherin gene promoter, thus increasing E-cadherin expression,78 whereas activation of SIRT1 reverses acetylation of SMAD3, thus inhibiting TGF-β–induced renal fibrosis),79,80 it should be noted that the dietary bioflavonoid quercetin also inhibits the expression of EMT markers (e.g., vimentin) as well as the transcription factors SNAIL and SLUG to inhibit CSC characteristics, including invasion, migration, and self-renewal.81,82 Polyphenolic phytochemicals, therefore, could all cause inhibition of fibrogenic and oncogenic EMT-driven phenomena. It is noteworthy that all of them had been reported to be either inhibitors of the mitochondrial respiratory chain or inhibitors of the mitochondrial adensoine triphosphate (ATP) synthesis, thus suggesting the hypothesis that these compounds can inhibit mitochondrial ATP production and therefore activate AMPK by increasing the intracellular AMP/ATP ratio.83–85 In addition, numerous lines of evidence suggest that most dietary polyphenols, including EGCG, resveratrol, and EVOO phenolics, have the capacity to mitigate cellular damage induced via metabolic production of reactive oxygen species (ROS) by mitochondrial complex I.86–90 Given that complex I can control the nicotinamide adenine dinucleotide (NAD+)-to-NADH ratio, thus allowing the activation of SIRT1,91 it might tempting to suggest that EVOO phenolics–targeted functioning of mitochondrial complex I might connect the master energy regulatory proteins AMPK (which senses the AMP/ATP ratio) and sirtuins (which require NAD to deacetylate protein substrates) molecularly. In this regard, it has been recently demonstrated that AMPK and SIRT1 can be connected in a linear pathway because AMPK activation upregulates the gene encoding the NAD synthetic enzyme Nampt, thus providing the crucial link to the downstream activation of SIRT1.92–94
We should express caution, however, when interpreting in vitro data to actual actions of EVOO polyphenols in the body, especially if no data have been collected regarding the action of physiological metabolites of tested EVOO polyphenols on the same cell systems. To produce their beneficial effects, other than on the gastrointestinal (GI) tract itself, EVOO polyphenols must be absorbed into the body after oral ingestion and be carried by the bloodstream from the absorption site to target tissues and organs. Obviously, our in vitro assays do not take into account the in vivo bioavailability issue and can lead to false-positive interpretations (i.e., the in vitro active compound within the EVOO phenolic extract is quickly metabolized or has limited bioavailability to the target organ). Thus, if there is no evidence for the absorption of a particular EVOO polyphenol (e.g., oleuropein aglycon, decarboxymethyl oleuropein aglycon), it could make no biological sense to gain mechanistical insights (e.g., anti-EMT effects) by exposing it to cultured cells obtained from the kidney and/or the breast. We should acknowledge that, similar to most pharmaceuticals, the body will treat consumed EVOO polyphenols as xenobiotics, or foreign substances, and EVOO polyphenols will be therefore subjected to the same protective xenobiotic-metabolizing and efflux mechanisms, which result not only in major changes in biological activity but also in increased rates of excretion from the body.95–97 Because parental EVOO polyphenols contain suitable functional groups (e.g., hydroxyl groups), they can undergo conjugation reactions with endogenous compounds to yield more polar and water-soluble derivatives. The principal conjugation reaction is the formation of β-glucuronides catalyzed by uridine diphosphoglucuronosyl transferases (UGTs), but conjugation with a sulfo moiety or glutathione catalyzed by sulfotransferases (SULTs) and glutathione-S-transferases, respectively, also occurs. Less-polar conjugates may also be formed by methylation, catalyzed by catechol-O-methyltransferase (COMT).
Although absorption and bioavailability studies have revealed that single phenols tyrosol and hyxdroxytyrosol can be retrieved in plasma and urine after OO consumption,98 there is an urgent need for data regarding the plasma/urine concentration of the free forms of various secoiridoid aglycones. For instance, after ingestion of flavonoids, conjugates of the aglycon, such as glucuronides, sulfates, and methylated metabolites, have been found to predominate in the blood circulation rather than the original plant glycoside or aglycon.99 Indeed, all of the conjugation mechanisms are highly efficient, and free aglycones are either absent or present in low concentrations in plasma after consumption of nutritional doses. Because in studies with green tea polyphenols, the metabolites mostly had reduced biological activity, it might be tempting to suggest that limited bioavailability of EVOO complex polyphenols and their conversion into less-active metabolites (e.g., glucuronidated or sulfated forms) could negatively affect an anti-EMT effect, if any, in vivo. In some systems, however, polyphenols-derived metabolites were found to have the equivalent or even greater activity than the parental polyphenols.100 Thus, whereas some studies have suggested that methylations can increase the bioavailability of polyphenols, other studies have indicated a decrease in the anticancer benefits of methylated polyphenols.101
Kinetics of penetration and elimination of EVOO polyphenols in tissues is largely unknown and data on actual bioavailability are scarce, thus it is plausible that cellular metabolites may differ from those obtained in plasma. Whether EVOO polyphenols could accumulate in specific organs remains to be ascertained, and the presence of cellular-specific mechanisms to incorporate polyphenols is still highly controversial.102–105 Recently, we employed a nano liquid chromatography–electrospray ionization–time-of-flight mass spectrometry (nanoLC-ESI-TOF MS) method to evaluate both the cellular uptake and the metabolism of EVOO phenolics in human breast carcinomas cells highly sensitive to the growth inhibitory effects of secoiridoid-rich EVOO phenolic extracts (Rocío García-Villalba, Alegría Carrasco-Pancorbo, Javier A. Menéndez, Antonio Segura-Carretero, Alberto Fernández-Gutiérrez, submitted manuscript). Most of free EVOO phenolics disappeared from the culture medium (i.e., the extracellular milieu) in different extents and at different times according to the type of phenolic we tested. When the time course of cellular uptake of parental phenolics was expressed as percent of the quantity originally supplemented in culture medium, we concluded that, as early as 15 min, oleuropein aglycone and decarboxymethyl oleuropein aglycon were rapidly incorporated into the cell. Secoiridoids were also the phenolic group most extensively metabolized, and methylation was the preferential pathway for conjugation of oleuropein aglycone and decarboxymethyl oleuropein aglycon, which was followed or preceded, in some cases, by hydrogenation reactions. We observed extremely low intracellular accumulations of parental secoiridoids, with only traces of some compounds being detected in the cytoplasm and membranous structures. In addition, the amounts of methylated forms increased with time strictly in parallel with the disappearance of parental secoiridoids in the extracellular milieu, a process that reached a maximum after 2 hr of incubation, thus suggesting a crucial role for COMT at metabolizing EVOO polyphenols at target tissues such as breast carcinomas.
These findings might be crucial when designing synergy strategies aimed to improve the bioavailability and hence bioactivity of EVOO polyphenols.95,96,106 First, because metabolism of dietary polyphenols by gut bacteria constitutes a significant barrier to their bioavailability, modulation of intestinal microflora population can be modulated by antibiotics or other natural products (e.g., catechins).107 Second, efflux transporter inhibitors and metabolism enzyme modulators can significantly affect phenolic absorption and metabolism, which consequently can improve their bioavailabilities. Third, pharmacological inhibition of glucuronidation using piperine108,109 can be expected to increase bioavailability of EVOO phenolics. In vivo studies have revealed that curcumin bioavailability can be drastically increased in human volunteers (up to 2000%) when curcumin is administered together with piperine.110 Piperine has been found to also increase the bioavailability of green tea polyphenols in mice.111 Recently, Johnson et al.112 examined the hypothesis that piperine will enhance the pharmacokinetic parameters of resveratrol via inhibiting its glucuronidation, thereby slowing its rapid metabolism and elimination. By using animal models, the degree of exposure to resveratrol was enhanced to 229% and the maximum serum concentration was increased to 1544% with the addition of piperine.112 Fourth, several nutraceuticals, including curcumin, green tea polyphenols, coenzyme Q, quercetin, and others, have been packaged as nanoparticles and proven to be useful in “nanochemoprevention” and “nanochemotherapy.”113 Efforts are currently underway in our laboratories to develop biocompatible, biodegradable, and nontoxic nano-size liposomal formulations that offer the possibility of carrying and delivering EVOO polyphenols in the prevention or treatment of several pathological conditions.
In summary, excessive fibrosis due to a sustained and unresolved EMT occurs in many age-related human diseases, including heart failure, sclerosis, nonalcoholic steatohepatitis, and progressive renal fibrosis. CSCs generated from EMT induction provide a resource for cancer to recur and metastasize because EMT-phenotypic tumor cells acquire stem-like increased metastatic capacity and self-renewal ability. Our current findings reveal for the first time that naturally occurring phenolics in EVOO efficiently impede EMT, the common link in the life-threatening progression of organ fibrosis and cancer toward organ failure and metastasis, respectively. Forthcoming studies should definitely establish whether, by targeting mitochondrial complex I, EVOO-derived complex polyphenols such as secoiridoids can mimic an energy limitation situation that enhances the net effect of ATP and NAD to activate AMPK and SIRT1 and lastly restrain the EMT differentiation process.
Because poor in vivo bioavailability of EVOO phenolics is being considered a major obstacle in translating in humans their multitude of health-promoting properties found in preclinical studies, future studies should focus on how to increase the bioavailabilities of EVOO so we can rapidly accelerate the development of this class of compounds into effective chemopreventing, anticancer, and/or antiaging agents.53,54,56,114–118 Nevertheless, Oleaceae secoiridoids, such as oleuropein aglycone and decarboxymethyl oleuropein aglycon, certainly merit consideration for longevity studies and, perhaps, for ulterior design of more pharmacologically active second-generation anti-EMT molecules.
Acknowledgments
Work at the laboratory of Javier A. Menendez is supported by the Instituto de Salud Carlos III (Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria [FIS], Spain, grants CP05-00090 and PI06-0778 and RD06-0020-0028), the Fundación Científica de la Asociación Española Contra el Cáncer (AECC, Spain), and by the Ministerio de Ciencia e Innovación (SAF2009-11579, Plan Nacional de I+D+I, MICINN, Spain). Alejandro Vazquez-Martin is the recipient of a “Sara Borrell” postdoctoral contract (CD08/00283, Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria [FIS], Spain). Sílvia Cufí is the recipient of a Research Fellowship (Formación de Personal Investigador [FPI]) by the Ministerio de Ciencia e Innovación (MICINN, Spain).
References
- 1.Moustakas A. Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiery JP. Acloque H. Huang RY. Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R. Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging (Albany NY) 2009;1:281–288. doi: 10.18632/aging.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stahl PJ. Felsen D. Transforming growth factor-beta, basement membrane and epithelial-mesenchymal transdifferentiation: implications for fibrosis in kidney disease. Am J Pathol. 2001;159:1187–1192. doi: 10.1016/s0002-9440(10)62503-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwano M. Plieth D. Danoff TM. Xue C. Okada H. Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg M. Yang C. Martino M. Duncan MB. Rieder F. Tanjore H. Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 8.Syn WK. Jung Y. Omenetti A. Abdelmalek M. Guy CD. Yang L. Wang J. Witek RP. Fearing CM. Pereira TA. Teaberry V. Choi SS. Conde-Vancells J. Karaca GF. Diehl AM. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488.e8. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato M. Muragaki Y. Saika S. Roberts AB. Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh YC. Wei WC. Wang YK. Lin SC. Sung JM. Tang MJ. Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol. 2010;177:1743–1754. doi: 10.2353/ajpath.2010.091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meindl-Beinker NM. Dooley S. Transforming growth factor-beta and hepatocyte transdifferentiation in liver fibrogenesis. J Gastroenterol Hepatol. 2008;23(Suppl 1):S122–S127. doi: 10.1111/j.1440-1746.2007.05297.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh AK. Bradham WS. Gleaves LA. De Taeye B. Murphy SB. Covington JW. Vaughan DE. Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation. 2010;122:1200–1209. doi: 10.1161/CIRCULATIONAHA.110.955245. [DOI] [PubMed] [Google Scholar]
- 13.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–549. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedi S. Vidyasagar A. Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev (Orlando) 2008;22:1–5. doi: 10.1016/j.trre.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djamali A. Samaniego M. Fibrogenesis in kidney transplantation: potential targets for prevention and therapy. Transplantation. 2009;88:1149–1156. doi: 10.1097/TP.0b013e3181bcccea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strutz F. Pathogenesis of tubulointerstitial fibrosis in chronic allograft dysfunction. Clin Transplant. 2009;23(Suppl 21):26–32. doi: 10.1111/j.1399-0012.2009.01106.x. [DOI] [PubMed] [Google Scholar]
- 17.Aguilera A. Yáñez-Mo M. Selgas R. Sánchez-Madrid F. López-Cabrera M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr Opin Investig Drugs. 2005;6:262–268. [PubMed] [Google Scholar]
- 18.Selgas R. Bajo A. Jiménez-Heffernan JA. Sánchez-Tomero JA. Del Peso G. Aguilera A. López-Cabrera M. Epithelial-to-mesenchymal transition of the mesothelial cell—its role in the response of the peritoneum to dialysis. Nephrol Dial Transplant. 2006;21(Suppl 2):ii2–ii7. doi: 10.1093/ndt/gfl183. [DOI] [PubMed] [Google Scholar]
- 19.Aroeira LS. Aguilera A. Sánchez-Tomero JA. Bajo MA. del Peso G. Jiménez-Heffernan JA. Selgas R. López-Cabrera M. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: Pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18:2004–2013. doi: 10.1681/ASN.2006111292. [DOI] [PubMed] [Google Scholar]
- 20.Li C. Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513–519. doi: 10.1038/nrneph.2009.113. [DOI] [PubMed] [Google Scholar]
- 21.Brabletz T. Jung A. Spaderna S. Hlubek F. Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 22.Lee JM. Dedhar S. Kalluri R. Thompson EW. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christiansen JJ. Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 24.Moustakas A. Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guarino M. Rubino B. Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 26.Mani SA. Guo W. Liao MJ. Eaton EN. Ayyanan A. Zhou AY. Brooks M. Reinhard F. Zhang CC. Shipitsin M. Campbell LL. Polyak K. Brisken C. Yang J. Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollier BG. Evans K. Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 28.Singh A. Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May CD. Sphyris N. Evans KW. Werden SJ. Guo W. Mani SA. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011;13:202. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong D. Li Y. Wang Z. Sarkar FH. Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: Are they cousin or twins? Cancers. 2011;3:716–729. doi: 10.3390/cancers30100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morel AP. Lièvre M. Thomas C. Hinkal G. Ansieau S. Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blick T. Hugo H. Widodo E. Waltham M. Pinto C. Mani SA. Weinberg RA. Neve RM. Lenburg ME. Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 33.Caja L. Bertran E. Campbell J. Fausto N. Fabregat I. The transforming growth factor-beta (TGF-β) mediates acquisition of a mesenchymal stem cell-like phenotype in human liver cells. J Cell Physiol. 2011;226:1214–1223. doi: 10.1002/jcp.22439. [DOI] [PubMed] [Google Scholar]
- 34.Fuxe J. Vincent T. de Herreros AG. Transcriptional crosstalk between TGFbeta and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–2374. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 35.Phinney DG. Twist, epithelial-to-mesenchymal transition, and stem cells. Stem Cells. 2011;29:3–4. doi: 10.1002/stem.553. [DOI] [PubMed] [Google Scholar]
- 36.Gupta PB. Onder TT. Jiang G. Tao K. Kuperwasser C. Weinberg RA. Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z. Li Y. Ahmad A. Azmi AS. Kong D. Banerjee S. Sarkar FH. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug Resist Updat. 2010;13:109–118. doi: 10.1016/j.drup.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y. VandenBoom TG., 2nd Kong D. Wang Z. Ali S. Philip PA. Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Novoa JM. Nieto MA. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anisimov VN. Zabezhinski MA. Popovich IG. Piskunova TS. Semenchenko AV. Tyndyk ML. Yurova MN. Antoch MP. Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle. 2010;9:3151–3156. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- 42.Blagosklonny MV. Rapamycin and quasi-programmed aging: Four years later. Cell Cycle. 2010;9:1859–1862. doi: 10.4161/cc.9.10.11872. [DOI] [PubMed] [Google Scholar]
- 43.Aguilera A. Aroeira LS. Ramirez-Huesca M. Perez-Lozano ML. Cirugeda A. Bajo MA. Del Peso G. Valenzuela-Fernandez A. Sanchez-Tomero JA. Lopez-Cabrera M. Selgas R. Effects of rapamycin on the epithelial-to-mesenchymal transition of human peritoneal mesothelial cells. Int J Artif Organs. 2005;28:164–169. doi: 10.1177/039139880502800213. [DOI] [PubMed] [Google Scholar]
- 44.Lamouille S. Derynck R. Emergence of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin axis in transforming growth factor-β-induced epithelial-mesenchymal transition. Cells Tissues Organs. 2011;193:8–22. doi: 10.1159/000320172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menendez JA. Cufí S. Oliveras-Ferraros C. Vellon L. Joven J. Vazquez-Martin A. Gerosuppressant Metformin: Less is more. Aging (Albany NY) 2011;4:348–362. doi: 10.18632/aging.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cufí S. Vazquez-Martin A. Oliveras-Ferraros C. Martin-Castillo B. Joven J. Menendez JA. Metformin against TGFβ-induced epithelial-to-mesenchymal transition (EMT): From cancer stem cells to aging-associated fibrosis. Cell Cycle. 2010;9:4461–4468. doi: 10.4161/cc.9.22.14048. [DOI] [PubMed] [Google Scholar]
- 47.Oliveras-Ferraros C. Cufí S. Vazquez-Martin A. Torres-Garcia VZ. Del Barco S. Martin-Castillo B. Menendez JA. Micro(mi)RNA expression profile of breast cancer epithelial cells treated with the anti-diabetic drug metformin: Induction of the tumor suppressor miRNA let-7a and suppression of the TGFβ-induced oncomiR miRNA-181a. Cell Cycle. 2011;10:1144–1151. doi: 10.4161/cc.10.7.15210. [DOI] [PubMed] [Google Scholar]
- 48.Vazquez-Martin A. Oliveras-Ferraros C. Cufí S. Del Barco S. Martin-Castillo B. Menendez JA. Metformin regulates breast cancer stem cell ontogeny by transcriptional regulation of the epithelial-mesenchymal transition (EMT) status. Cell Cycle. 2010;9:3807–3814. [PubMed] [Google Scholar]
- 49.Vazquez-Martin A. Oliveras-Ferraros C. Del Barco S. Martin-Castillo B. Menendez JA. The anti-diabetic drug metformin suppresses self-renewal and proliferation of trastuzumab-resistant tumor-initiating breast cancer stem cells. Breast Cancer Res Treat. 2011;126:355–364. doi: 10.1007/s10549-010-0924-x. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez-Martin A. Oliveras-Ferraros C. Cufí S. Martin-Castillo B. Menendez JA. Metformin and energy metabolism in breast cancer: from insulin physiology to tumour-initiating stem cells. Curr Mol Med. 2010;10:674–691. doi: 10.2174/156652410792630625. [DOI] [PubMed] [Google Scholar]
- 51.Jensen SR. Franzyk H. Wallander E. Chemotaxonomy of the Oleaceae: Iridoids as taxonomic markers. Phytochemistry. 2002;60:213–231. doi: 10.1016/s0031-9422(02)00102-4. [DOI] [PubMed] [Google Scholar]
- 52.Bendini A. Cerretani L. Carrasco-Pancorbo A. Gómez-Caravaca AM. Segura-Carretero A. Fernández-Gutiérrez A. Lercker G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules. 2007;12:1679–1719. doi: 10.3390/12081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lozano-Sánchez J. Segura-Carretero A. Menendez JA. Oliveras-Ferraros C. Cerretani L. Fernández-Gutiérrez A. Prediction of extra virgin olive oil varieties through their phenolic profile. Potential cytotoxic activity against human breast cancer cells. J Agric Food Chem. 2010;58:9942–9955. doi: 10.1021/jf101502q. [DOI] [PubMed] [Google Scholar]
- 54.García-Villalba R. Carrasco-Pancorbo A. Oliveras-Ferraros C. Vázquez-Martín A. Menéndez JA. Segura-Carretero A. Fernández-Gutiérrez A. Characterization and quantification of phenolic compounds of extra-virgin olive oils with anticancer properties by a rapid and resolutive LC-ESI-TOF MS method. J Pharm Biomed Anal. 2010;51:416–429. doi: 10.1016/j.jpba.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 55.Oliveras-Ferraros C. Vazquez-Martin A. Martin-Castillo B. Cufí S. Del Barco S. Lopez-Bonet E. Brunet J. Menendez JA. Dynamic emergence of the mesenchymal CD44(pos)CD24(neg/low) phenotype in HER2-gene amplified breast cancer cells with de novo resistance to trastuzumab (Herceptin) Biochem Biophys Res Commun. 2010;397:27–33. doi: 10.1016/j.bbrc.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 56.Oliveras-Ferraros C. Fernández-Arroyo S. Vazquez-Martin A. Lozano-Sánchez J. Cufí S. Joven J. Micol V. Fernández-Gutiérrez A. Segura-Carretero A. Menendez JA. Crude phenolic extracts from extra virgin olive oil circumvent de novo breast cancer resistance to HER1/HER2-targeting drugs by inducing GADD45-sensed cellular stress, G2/M arrest and hyperacetylation of Histone H3. Int J Oncol. 2011;38:1533–1547. doi: 10.3892/ijo.2011.993. [DOI] [PubMed] [Google Scholar]
- 57.Singleton YL. Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungtic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 58.Fu SP. Segura-Carretero A. Arráez-Román D. Menéndez JA. De La Torre A. Fernández-Gutiérrez A. Tentative Characterization of Novel Phenolic Compounds in Extra Virgin Olive Oils by Rapid-Resolution Liquid Chromatography Coupled with Mass Spectrometry. J Agric Food Chem. 2009;57:11140–11147. doi: 10.1021/jf901590n. [DOI] [PubMed] [Google Scholar]
- 59.Sobrado VR. Moreno-Bueno G. Cubillo E. Holt LJ. Nieto MA. Portillo F. Cano A. The class I bHLH factors E2-2A and E2-2B regulate EMT. J Cell Sci. 2009;122:1014–1024. doi: 10.1242/jcs.028241. [DOI] [PubMed] [Google Scholar]
- 60.Jayachandran A. Königshoff M. Yu H. Rupniewska E. Hecker M. Klepetko W. Seeger W. Eickelberg O. SNAI transcription factors mediate epithelial-mesenchymal transition in lung fibrosis. Thorax. 2009;64:1053–1061. doi: 10.1136/thx.2009.121798. [DOI] [PubMed] [Google Scholar]
- 61.Freytag J. Wilkins-Port CE. Higgins CE. Higgins SP. Samarakoon R. Higgins PJ. PAI-1 mediates the TGF-beta1+EGF-induced “scatter” response in transformed human keratinocytes. J Invest Dermatol. 2010;130:2179–2190. doi: 10.1038/jid.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Senoo T. Hattori N. Tanimoto T. Furonaka M. Ishikawa N. Fujitaka K. Haruta Y. Murai H. Yokoyama A. Kohno N. Suppression of plasminogen activator inhibitor-1 by RNA interference attenuates pulmonary fibrosis. Thorax. 2010;65:334–340. doi: 10.1136/thx.2009.119974. [DOI] [PubMed] [Google Scholar]
- 63.Chagraoui J. Lepage-Noll A. Anjo A. Uzan G. Charbord P. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood. 2003;101:2973–2982. doi: 10.1182/blood-2002-05-1341. [DOI] [PubMed] [Google Scholar]
- 64.Saika S. Shirai K. Yamanaka O. Miyazaki K. Okada Y. Kitano A. Flanders KC. Kon S. Uede T. Kao WW. Rittling SR. Denhardt DT. Ohnishi Y. Loss of osteopontin perturbs the epithelial-mesenchymal transition in an injured mouse lens epithelium. Lab Invest. 2007;87:130–138. doi: 10.1038/labinvest.3700508. [DOI] [PubMed] [Google Scholar]
- 65.Rödder S. Scherer A. Raulf F. Berthier CC. Hertig A. Couzi L. Durrbach A. Rondeau E. Marti HP. Renal allograftswith IF/TA displaydistinctexpressionprofiles of metzincins and related genes. Am J Transplant. 2009;9:517–526. doi: 10.1111/j.1600-6143.2008.02512.x. [DOI] [PubMed] [Google Scholar]
- 66.Das S. Becker BN. Hoffmann FM. Mertz JE. Complete reversal of epithelialto mesenchymal transitionrequiresinhibition of both ZEB expression and the Rho pathway. BMC Cell Biol. 2009;10:94. doi: 10.1186/1471-2121-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belguise K. Guo S. Sonenshein GE. Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor alpha expression reversing invasive phenotype of breast cancer cells. Cancer Res. 2007;67:5763–5770. doi: 10.1158/0008-5472.CAN-06-4327. [DOI] [PubMed] [Google Scholar]
- 68.Belguise K. Guo S. Yang S. Rogers AE. Seldin DC. Sherr DH. Sonenshein GE. Green tea polyphenols reverse cooperation between c-Rel and CK2 that induces the aryl hydrocarbon receptor, slug, and an invasive phenotype. Cancer Res. 2007;67:11742–11750. doi: 10.1158/0008-5472.CAN-07-2730. [DOI] [PubMed] [Google Scholar]
- 69.Xiao H. Ma X. Feng W. Fu Y. Lu Z. Xu M. Shen Q. Zhu Y. Zhang Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res. 2010;87:504–513. doi: 10.1093/cvr/cvq066. [DOI] [PubMed] [Google Scholar]
- 70.Mishra R. Cool BL. Laderoute KR. Foretz M. Viollet B. Simonson MS. AMP-activated protein kinase inhibitstransforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem. 2008;283:10461–10469. doi: 10.1074/jbc.M800902200. [DOI] [PubMed] [Google Scholar]
- 71.Collins QF. Liu HY. Pi J. Liu Z. Quon MJ. Cao W Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem. 2007;282:30143–30149. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murase T. Misawa K. Haramizu S. Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem Pharmacol. 2009;78:78–84. doi: 10.1016/j.bcp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 73.Huang CH. Tsai SJ. Wang YJ. Pan MH. Kao JY. Way TD. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol Nutr Food Res. 2009;53:1156–1165. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- 74.Nerstedt A. Johansson A. Andersson CX. Cansby E. Smith U. Mahlapuu M. AMP-activated protein kinase inhibits IL-6-stimulated inflammatory response in human liver cells by suppressing phosphorylation of signal transducer and activator of transcription 3 (STAT3) Diabetologia. 2010;53:2406–2416. doi: 10.1007/s00125-010-1856-z. [DOI] [PubMed] [Google Scholar]
- 75.Lo HW. Hsu SC. Xia W. Cao X. Shih JY. Wei Y. Abbruzzese JL. Hortobagyi GN. Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan NJ. Sasser AK. Axel AE. Vesuna F. Raman V. Ramirez N. Oberyszyn TM. Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shankar S. Nall D. Tang S-N. Meeker D. Passarini J. Sharma J. Srivastava RK. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One. 2011;6:e16530. doi: 10.1371/journal.pone.0016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tryndyak VP. Beland FA. Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126:2575–2583. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- 79.Li J. Qu X. Ricardo SD. Bertram JF. Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: Potential role in deacetylation of Smad3. Am J Pathol. 2010;177:1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li C. Cai F. Yang Y. Zhao X. Wang C. Li J. Jia Y. Tang J. Liu Q. Tetrahydroxystilbene glucoside ameliorates diabetic nephropathy in rats: involvement of SIRT1 and TGF-β1 pathway. Eur J Pharmacol. 2010;649:382–389. doi: 10.1016/j.ejphar.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Tang SN. Singh C. Nall D. Meeker D. Shankar S. Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration, epithelial-mesenchymal transition. J Mol Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Srivastava RK. Tang SN. Zhu W. Meeker D. Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed) 2011;3:515–528. doi: 10.2741/e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner N. Li JY. Gosby A. To SW. Cheng Z. Miyoshi H. Taketo MM. Cooney GJ. Kraegen EW. James DE. Hu LH. Li J. Ye JM. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: A mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–1418. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- 84.Gledhill JR. Montgomery MG. Leslie AG. Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci USA. 2007;104:13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hwang JT. Kwon DY. Yoon SH. AMP-activated protein kinase: A potential target for the diseases prevention by natural occurring polyphenols. New Biotechnol. 2009;26:17–22. doi: 10.1016/j.nbt.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 86.Lee KW. Lee HJ. The roles of polyphenols in cancer chemoprevention. Biofactors. 2006;26:105–121. doi: 10.1002/biof.5520260202. [DOI] [PubMed] [Google Scholar]
- 87.Rahman I. Biswas SK. Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 88.Page MM. Robb EL. Salway KD. Stuart JA. Mitochondrial redox metabolism: Aging, longevity and dietary effects. Mech Ageing Dev. 2010;131:242–252. doi: 10.1016/j.mad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 89.Katsiki M. Chondrogianni N. Chinou I. Rivett AJ. Gonos ES. The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts. Rejuvenation Res. 2007;10:157–172. doi: 10.1089/rej.2006.0513. [DOI] [PubMed] [Google Scholar]
- 90.Paiva-Martins F. Fernandes J. Rocha S. Nascimento H. Vitorino R. Amado F. Borges F. Belo L. Santos-Silva A. Effects of olive oil polyphenols on erythrocyte oxidative damage. Mol Nutr Food Res. 2009;53:609–616. doi: 10.1002/mnfr.200800276. [DOI] [PubMed] [Google Scholar]
- 91.Stefanatos R. Sanz A. Mitochondrial complex I: A central regulator of the aging process. Cell Cycle. 2011:10. doi: 10.4161/cc.10.10.15496. [DOI] [PubMed] [Google Scholar]
- 92.Fulco M. Cen Y. Zhao P. Hoffman EP. McBurney MW. Sauve AA. Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guarente L. Connecting the dots: Linking sirtuins and AMPK in metabolism and aging. Dev Cell. 2011;20:e1. [Google Scholar]
- 94.Wang Y. Liang Y. Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: A critical review and a working model. FEBS Lett. 2011;585:986–994. doi: 10.1016/j.febslet.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 95.Scheepens A. Tan K. Paxton JW. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010;5:75–87. doi: 10.1007/s12263-009-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vauzour D. Rodriguez-Mateos A. Corona G. Oruna-Concha MJ. Spencer JPE. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Visioli F. de la Lastra CA. Andres-Lacueva X. Aviram M. Calhau C. Cassano A. D'Archivio M. Faria A. Favé G. Fogliano V. Llorach R. Vitaglione P. Zoratti M. Edeas M. Polyphenols and human health: A prospectus. Crit Rev Food Sci Nutr. 2011;51:524–546. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- 98.Vissers MN. Zock PL. Roodenburg AJC. Leenen R. Katan MB. Olive oil phenols are absorbed in humans. J Nutr. 2002;132:409–417. doi: 10.1093/jn/132.3.409. [DOI] [PubMed] [Google Scholar]
- 99.Zhang L. Zuo Z. Lin G. Intestinal and hepatic glucuronidation of flavonoids. Mol Pharm. 2007;4:833–845. doi: 10.1021/mp700077z. [DOI] [PubMed] [Google Scholar]
- 100.Lambert JD. Sang SM. Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharmaceutics. 2007;4:819–825. doi: 10.1021/mp700075m. [DOI] [PubMed] [Google Scholar]
- 101.Landis-Piwowar KR. Dou QP. Polyphenols: Biological activities, molecular targets, and the effect of methylation. Curr Mol Pharmacol. 2008;1:233–243. doi: 10.2174/1874467210801030233. [DOI] [PubMed] [Google Scholar]
- 102.Chang HC. Churchwell MI. Delclos KB. Newbold RR. Doerge DR. Mass spectrometric determination of Genistein tissue distribution in diet exposed Sprague-Dawley rats. J Nutr. 2000;130:1963–1970. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- 103.Datla KP. Christidou M. Widmer WW. Rooprai HK. Dexter DT. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson's disease. Neuroreport. 2001;12:3871–3875. doi: 10.1097/00001756-200112040-00053. [DOI] [PubMed] [Google Scholar]
- 104.Schramm D. Collins H. German B. Flavonoid transport by mammalian endothelial cells. J Nutr Biochem. 1999;10:193–197. doi: 10.1016/s0955-2863(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 105.Suganuma M. Okabe S. Oniyama M. Tada Y. Ito H. Fujiki H. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 106.Gao S. Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev Med Chem. 2010;10:550–567. doi: 10.2174/138955710791384081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Selma MV. Espin JC. Tomas-Barberan FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 108.Singh J. Dubey RK. Atal CK. Piperine-mediated inhibition of glucuronidation activity in isolated epithelial cells of the guinea-pig small intestine: evidence that piperine lowers the endogeneous UDP-glucuronic acid content. J Pharmacol Exp Ther. 1986;236:488–493. [PubMed] [Google Scholar]
- 109.Reen RK. Jamwal DS. Taneja SC. Koul JL. Dubey RK. Wiebel FJ. Singh J. Impairment of UDP-glucose dehydrogenase and glucuronidation activities in liver and small intestine of rat and guinea pig in vitro by piperine. Biochem Pharmacol. 1993;46:229–238. doi: 10.1016/0006-2952(93)90408-o. [DOI] [PubMed] [Google Scholar]
- 110.Shoba G. Joy D. Joseph T. Majeed M. Rajendran R. Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 111.Lambert JD. Hong J. Kim DH. Mishin VM. Yang CS. Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J Nutr. 2004;134:1948–1952. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 112.Johnson JJ. Nihal M. Siddiqui IA. Scarlett CO. Bailey HH. Mukhtar H. Ahmad N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol Nutr Food Res. 2011;55:1169–1176. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nair HB. Sung B. Yadav VR. Kannappan R. Chaturvedi MM. Aggarwal BB. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem Pharmacol. 2010;80:1833–1843. doi: 10.1016/j.bcp.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goulas V. Exarchou V. Troganis AN. Psomiadou E. Fotsis T. Briasoulis E. Gerothanassis IP. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol Nutr Food Res. 2009;53:600–608. doi: 10.1002/mnfr.200800204. [DOI] [PubMed] [Google Scholar]
- 115.Menendez JA. Vazquez-Martin A. Colomer R. Brunet J. Carrasco-Pancorbo A. Garcia-Villalba R. Fernandez-Gutierrez A. Segura-Carretero A. Olive oil's bitter principle reverses acquired autoresistance to trastuzumab (Herceptin) in HER2-overexpressing breast cancer cells. BMC Cancer. 2007;7:80. doi: 10.1186/1471-2407-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Menendez JA. Vazquez-Martin A. Oliveras-Ferraros C. Garcia-Villalba R. Carrasco-Pancorbo A. Fernandez-Gutierrez A. Segura-Carretero A. Analyzing effects of extra-virgin olive oil polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int J Mol Med. 2008;22:433–439. [PubMed] [Google Scholar]
- 117.Menendez JA. Vazquez-Martin A. Oliveras-Ferraros C. Garcia-Villalba R. Carrasco-Pancorbo A. Fernandez-Gutierrez A. Segura-Carretero A. Extra-virgin olive oil polyphenols inhibit HER2 (erbB-2)-induced malignant transformation in human breast epithelial cells: relationship between the chemical structures of extra-virgin olive oil secoiridoids and lignans and their inhibitory activities on the tyrosine kinase activity of HER2. Int J Oncol. 2009;34:43–51. [PubMed] [Google Scholar]
- 118.Menendez JA. Vazquez-Martin A. Garcia-Villalba R. Carrasco-Pancorbo A. Oliveras-Ferraros C. Fernandez-Gutierrez A. Segura-Carretero A. Anti-HER2 (erbB-2) oncogene effects of phenolic compounds directly isolated from commercial Extra-Virgin Olive Oil (EVOO) BMC Cancer. 2008;8:377. doi: 10.1186/1471-2407-8-377. [DOI] [PMC free article] [PubMed] [Google Scholar]