Abstract
An acoustic pointing task was used to measure extents of laterality produced by combinations of ongoing envelope-based interaural temporal disparities (ITDs) and interaural intensitive disparities (IIDs) of 4-kHz-centered raised-sine stimuli [Bernstein and Trahiotis, J. Acoust. Soc. Am. 125, 3234–3242 (2009),] while varying, parametrically, their peakedness, depth of modulation, and frequency of modulation. The study was designed to assess whether IIDs act as “weights” within the putative “binaural display” at high spectral frequencies (where the envelopes convey ITD-information) as appears to be the case at low spectral frequencies (where the waveforms, i.e., fine-structure and envelopes, convey ITD-information). The data indicate that envelope-based IIDs do principally act as weights and that they appear to exert their influence on lateral position independently of the influence of ITDs. Quantitative analyses revealed that an augmented form of the cross-correlation-based “position-variable” model of Stern and Shear [J. Acoust. Soc. Am. 100, 2278–2288 (1996)] accounted for 94% of the variance in the data. This success notwithstanding, for a small subset of the data, predictions could be improved by assuming that the listeners utilized information within auditory filters having center frequencies above 4 kHz.
INTRODUCTION
We recently reported measurements of extents of laterality produced by interaural temporal disparities (ITDs) conveyed by the envelopes of high-frequency “raised-sine stimuli” centered at 4 kHz (Bernstein and Trahiotis, 2011b). Raised-sine stimuli were employed because they allow one to vary, independently, frequency of modulation, depth of modulation, and relative “peakedness” or “sharpness” of the envelope of waveforms centered at high spectral frequencies (e.g., Bernstein and Trahiotis, 2009, 2010). The data revealed that increasing depth of modulation and increasing peakedness generally resulted in larger extents of laterality. In contrast, increasing frequency of modulation led to non-monotonic changes in extent of laterality. Specifically, increasing frequency of modulation from 32 Hz up to 128 Hz produced increases in extents of laterality but a further increase of frequency of modulation to 256 Hz produced modest decreases in extents of laterality. The data were successfully accounted for quantitatively using a variant of the cross-correlation-based “position-variable” model developed by Stern and Shear (1996) to account for ITD-based extents of laterality obtained at low spectral frequencies. In order to account for the non- monotonic effect of frequency of modulation on extent of laterality, the model was augmented to include a stage of “internal” envelope low-pass filtering of the type initially reported and discussed by Kohlrausch et al. (2000) and confirmed by others (e.g., Moore and Glasberg, 2001; Bernstein and Trahiotis, 2002).
The purposes of the present study were both empirical and theoretical. Empirically, the goal was to measure extents of laterality produced by envelope-based ongoing ITDs while also manipulating, independently and parametrically, interaural intensitive differences (IIDs). An important aspect of the experimental design was the inclusion of both “positive” values of IID, which favored the ear leading in time, and “negative” values of IID, which favored the ear lagging in time. The advantage of parametrically varying IID and ITD in this manner is that it allows one to determine whether and to what degree the effects on lateralization produced by variations of ITD and IID are independent or interactive in nature. Imposing similar combinations of ITDs and IIDs on low-frequency stimuli centered at 500 Hz has been shown to produce both types of effects (see Domnitz and Colburn, 1977; Buell et al., 1994). Currently, to our knowledge, there exist no published data and no mathematical formulations that allow one to discern and to predict (1) how ITDs and IIDs combine to produce extents of laterality when the two types of interaural disparities are conveyed by the envelopes of high-frequency stimuli and (2) how the effects produced by combinations of ITDs and IIDs depend on fundamental features of the temporal signatures of the envelopes such as frequency of modulation, depth of modulation, and peakedness. Theoretically, the goal was to test the generality of the previously discussed augmented position-variable model of Stern and his colleagues.
As will be seen, data with this set of parametrically varied stimuli indicate that, for the most part, envelope-based IIDs exert their influence on lateral position independently of the influence of ITDs. In addition, it will be shown that predictions of extent of laterality derived from the augmented position-variable model capture quite well the patterning of the data, both qualitatively and quantitatively.
EXPERIMENT
Generation of raised-sine stimuli
A raised-sine stimulus is constructed by raising a DC-shifted sine-wave to a power (exponent) greater than or equal to 1.0 prior to multiplication with a carrier. The equation used to generate such stimuli was originally described by John et al. (2002) and is
| (1) |
where fc is the frequency of the carrier, fm is the frequency of the modulator, and m is the index of modulation. The exponent, n, determines the peakedness or “sharpness” of the individual lobes of the envelope. As described in Bernstein and Trahiotis (2010), an equivalent (save for a quarter-period shift of the modulator) and more compact form of Eq. 1 is
| (2) |
Examples of raised sine waveforms generated with m = 1.0 and values of n of from 1.0 to 8.0 can be found in Bernstein and Trahiotis (2009).
Stimuli and procedure
Extents of laterality were measured for three adult listeners, one male and two female, whose ages spanned the range between about 35 and 55 years, and who had audiometrically normal hearing.1 An acoustic pointing task was employed in which the listeners varied the IID of an otherwise diotic 200-Hz-wide band of Gaussian noise centered at 500 Hz (the pointer) so that it matched the intracranial position of the raised-sine target. This procedure has been used previously in several studies (e.g., Trahiotis and Stern, 1989; Buell et al., 1991; Heller and Trahiotis, 1996; Bernstein and Trahiotis, 2003, 2011a,b) and is described fully in Bernstein and Trahiotis (1985).
Extents of laterality were measured for raised-sine “targets” while varying, parametrically, their exponent, depth (index) of modulation, rate of modulation, and IID. The values of the exponents (n) were 1.0 (equivalent to a SAM tone) or 8.0; the depths of modulation (m) were 0.25, 0.50, 0.75, or 1.00; the rates of modulation (fm) were 32, 64, 128, or 256 Hz; the IIDs were (−8, −4, 0, 4, or 8 dB). Positive values of IID favored the left ear; negative values of IID favored the right ear.
All 160 raised-sine targets were centered at 4 kHz. They were generated digitally as two-second-long buffers using a sampling rate of 20 kHz (TDT AP2). Ongoing ITDs (0, 200, 400, 600, 800, and 1000 μs, left ear leading) were imposed by applying linear phase-shifts to the representation of the targets in the frequency domain and then transforming them to the time domain. Prior to presentation, a 100-ms-long segment of the stimuli destined for each ear was chosen randomly from the buffer, after which coincident 10-ms cos2 rise/decay ramps were applied. Target-IIDs were implemented via TDT PA4 programmable attenuators. A given value of IID was produced by symmetrically increasing and decreasing (in dB) the intensities across the ears by half of the magnitude of that IID. Targets were low-pass filtered at 8.5 kHz (TDT FLT2) and were presented via Etymotic ER-2 insert earphones at a level of 68 dB SPL, assuming an IID of zero. The pointer was generated digitally and presented in a manner similar to that described above and its overall level, when presented diotically (IID=0), was 60 dB SPL.
Listeners adjusted the intracranial position of the pointer by rotating a knob. Rotation of the knob produced symmetric changes of the IID of the pointer (i.e., increases in level at one ear and decreases in level at the other ear) produced by multiples of 0.1 dB changes of attenuation in each channel. The IID adjusted by the listener served as a metric of the intracranial position of the target. A value of IID was randomly chosen from a uniform distribution between ±10 dB (in 0.2 dB steps) and was inserted in the pointer prior to each match. This served to randomize the initial position of the pointer with respect to the absolute position of the knob. Each sequence of stimuli consisted of three presentations of the target (each separated by 150 ms), a pause of 200 ms, three presentations of the pointer (each separated by 150 ms), and a pause of 600 ms. Targets and pointers were repeated until the listeners indicated that they had matched the intracranial positions of the target and pointer. Prior to completing a match, listeners had the option of halting, and then restarting, the sequence in order to check their adjustments after a period of silence.
Collection of data began with the set of targets having non-zero IIDs. Those conditions were visited in random order. Having chosen a particular stimulus condition as the target, the following procedure was used so that listeners made three independent matches for each of the six values of ITD to be tested. Effectively, three instances of each of the six values of ITD were placed in a table. Then, values of ITD were drawn randomly from that set of 18, without replacement. After three matches were made at each ITD for all of the stimulus conditions, the entire experiment was re-run with the conditions visited in reverse order.
Included among the 18 values of ITDs composing the set to be tested for each particular stimulus condition were three diotic instances of the target (i.e., both the ITD and IID of the target were set to zero). This was done in order to determine a “correction factor” that was calculated as the mean IID inserted by the listener to match the diotic targets. The purpose of the correction factor was to adjust the listeners’ matches so that a pointer IID of 0 dB indicated the intracranial position of the target presented diotically. In order to accomplish this, the correction factor, which was typically less than 3 dB, was subtracted from the pointer-IIDs resulting from all of the other matches in the run. In the event that the correction factor for a particular run of a stimulus condition was greater than or equal to 5 dB, that run was discarded and was repeated until that criterion was not exceeded.
Following this, using the same type of data-collection scheme, matches were obtained for targets having a rate of modulation of 64 Hz and an IID of zero. Those data were combined with other “target-IID-zero” data obtained earlier from the same three listeners when the rates of modulation of 32, 128, and 256 Hz (Bernstein and Trahiotis, 2011b).
Results and discussion
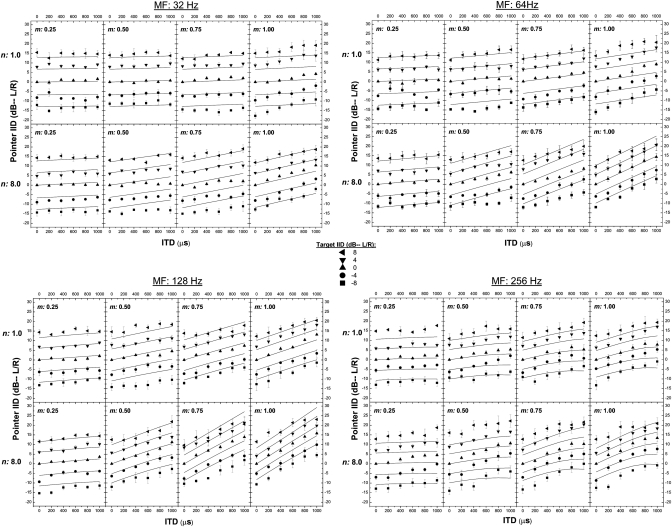
For each combination of target-IID and target-ITD, the mean “corrected” value of IID of the pointer was computed across the six “valid” matches (three from each run) made by each listener and then averaged across the three listeners. Figure 1 displays the across-subject mean IIDs of the 500-Hz-centered pointer that were required to match the intracranial positions of the 4-kHz-centered targets. The four quadrants of the figure contain, respectively, data obtained at each of the four rates of modulation (32, 64, 128, and 256 Hz). Within each quadrant, the upper and lower rows contain the data obtained when the exponent of the raised sine was 1.0 and 8.0, respectively. Each column contains the data obtained at a common depth of modulation: 0.25, 0.50, 0.75, or 1.0. The parameter within each panel is the IID of the target, as indicated in the legend placed in the center of the figure. Within each of the 32 panels, the symbols represent the mean IID of the pointer (taken across the three listeners) and are plotted as a function of the ITD imposed on the target. Positive values of IID are those favoring the left ear. Error bars represent plus and minus one standard error of the mean. The lines within each of the 32 panels represent corresponding predictions of pointer-IID derived from the augmented position-variable model of Stern and Shear (1996). For ease of exposition and depending on the context, the data will be discussed either in terms of extent of laterality or in terms of the IID of the pointer, per se, required to match the intracranial position of the target (see Bernstein and Trahiotis, 1985, 2003; Schiano et al., 1986).
Figure 1.
The four quadrants of the figure display data obtained when the frequency of modulation was 32, 64, 128, and 256 Hz, respectively. The panels along the top and bottoms rows of each quadrant display data obtained when the exponent of the raised sine, n, was 1.0 and 8.0, respectively. Panels within the columns of each quadrant contain data obtained for depths of modulation, m, of 0.25, 0.50, 0.75, and 1.00, respectively. Each panel displays the IID of the pointer (in dB) required to match the intracranial position of the target as a function of the ongoing ITD (left ear leading) imposed on a 200-Hz-wide band of Gaussian noise centered at 500 Hz. Symbols represent the mean values computed across the three listeners. The error bars represent ±1 standard error of the mean. The parameter within each plot is the IID of the target. As discussed in the text, the data plotted for conditions in which the target-IID was zero for rates of modulation of 32, 128, and 256 Hz were obtained in an earlier study (Bernstein and Trahiotis, 2011b) from the same listeners who provided the rest of the data. The lines represent predictions from the position-variable model (see text).
The general patterning of the data within each of the 32 plots reveals that imposing a given IID on the target shifts the position of the intracranial image (indexed by the IID of the pointer) by a relatively constant amount. The direction of that shift is dependent upon the ear favored by the IID, but the amount of the shift appears to be largely independent of both the value of ITD and the ear favored by the ITD. Visually, these effects are manifested by the parallel nature of the functions relating pointer-IID to target-ITD within each panel.
Several aspects of the current results are consistent with our earlier study (Bernstein and Trahiotis, 2011b): (1) increases in depth of modulation, exponent of the raised sine, and rate of modulation up to 128 Hz all resulted in increases in extent of laterality produced by a given value of ITD; (2) further increasing the rate of modulation to 256 Hz resulted in modest decreases in extent of laterality as compared to those obtained when the rate of modulation was 128 Hz; (3) when the rate of modulation was 256 Hz, the relation between pointer-IID and target-ITD was generally curvilinear as compared to the essentially linear trends of the data obtained at the three lower rates of modulation. The results of the current experiment further reveal that those effects appear to occur independently of the value of IID and whether that IID favors the ear that leads or lags in time.
Note that the vertical displacements in pointer-IID produced by each of the non-zero values of IID are somewhat larger than the magnitude of the IID imposed on the target. For example, consider the data obtained when the rate of modulation was 32 Hz, the exponent of the raised sine was either 1.0 or 8.0, and the depth of modulation was 0.25. For these conditions, the listeners required pointer-IIDs of about ±15 and about ±8 dB in order to match the intracranial position of targets on which were imposed IIDs of ±8 and ±4 dB, respectively. Such an outcome means that, in order to match the intracranial position of the target, the listeners had to insert a larger IID in the 500-Hz-centered noise pointer than was imposed on the 4-kHz-centered raised-sine target. This outcome replicates the one recently reported by Bernstein and Trahiotis (2011a), who presented data and analyses confirming that this type of outcome results from true across-frequency differences in the potency of IID.
The data in Fig. 1 were subjected to a five-factor (four frequencies of modulation × four depths of modulation × two values of exponent × five values of IID × six values of ITD), within-subjects analysis of variance (ANOVA). The error terms for the main effects and for the interactions were the interaction of the particular main effect (or the particular interaction) with the subject “factor” (Keppel, 1991). In addition to testing for significant effects, the proportions of variance accounted for (ω2) were determined for each significant main effect and interaction (Hays, 1973).
Overall, the statistical analysis revealed that 93% of the variability in the IIDs of the pointer calculated across the three listeners was accounted for by the stimulus variables. Thus, only 7% of the variance in the data is attributable to experimental “error” which, within this design, includes both differences among the three listeners and errors of measurement. Each of the five main effects was significant (assuming an α of 0.05) and, in aggregate, they accounted for 90% of the variance: (1) frequency of modulation [F(3,6)= 29.1, p = 0.001], accounting for 2% of the variance; (2) depth of modulation [F(3,6) = 30.2, p = 0.001], accounting for 3% of the variance; (3) value of exponent [F(1,2) = 25.9, p = 0.037], accounting for 1% of the variance; (4) value of ITD [F(5,10) = 186.9, p < 0.001], accounting for 4% of the variance, and (5) value of IID [F(4,8) = 59.6, p < 0.001], accounting for 80% of the variance. Of the 26 interactive effects, 10 were significant and, in aggregate, they accounted for 3% of the variance, with the interaction between depth of modulation and ITD [F(15,30) = 26.8, p < 0.001] accounting for 1% of the variance. This interaction is visually apparent in the substantially different patterns of data across the four columns of each quadrant of Fig. 1. Specifically, within each quadrant of Fig. 1 (i.e., for each rate of modulation), the extent of lateralization produced by a given ITD increased with depth of modulation.
The remainder of the 93% of the variance accounted for by the stimulus variables resulted from the 16 interactive effects that, individually, were not statistically significant.
QUANTITATIVE PREDICTIONS
An augmented form of the position-variable model recently described and employed by Bernstein and Trahiotis (2011b) was used to make predictions for the data in Fig. 1. Within the model, bandpass filtering was accomplished by passing the respective stimuli through a pair of (left/right) gammatone filter banks (see Patterson et al., 1995) spanning center frequencies of 2000 to 8000 Hz. The output of each gammatone filter was subjected to half-wave, cube-law rectification and low-pass filtering at 1200 Hz in accord with and as described by Stern and Shear (1996). This was followed by 150-Hz low-pass filtering that served in previous studies to explain and to emulate the apparent low-pass filtering of the envelope of high-frequency stimuli found in previous behavioral and physiological experiments (e.g., Kohlrausch et al., 2000; Bernstein and Trahiotis, 2002; Griffin et al., 2005). Then, the correlogram was computed for ITDs ranging from −2.0 to +2.0 ms. The correlogram was then modified using Stern and Shear’s frequency-dependent centrality function [p(τ)] (where τ represents internal delay) with the proviso that the “frequency” used to calculate that function be the frequency of the envelope, rather than the 4000-Hz center frequency of the stimulus (see Bernstein and Trahiotis, 2011b, for further explanation).
In order to account for the influence of IIDs on extents of laterality, the centrality weighted correlogram was then multiplied by the same “intensity function” described in detail by Stern and Colburn (1978). That intensity function is a Gaussian having a standard deviation or “width parameter” of 1778 μs along the interaural delay axis. The positioning of the intensity function along the interaural delay axis as a function of IID was determined according to the relation depicted in Stern and Colburn’s Fig. 4.2
Finally, the centroid of the across-filter-averaged activity was computed along the interaural delay axis and linearly scaled in order to convert ITD to IID of the pointer. The predictions, in units of IID (dB), were made assuming that one dB of IID of the pointer equals 10.0 μs of ITD along the internal delay axis.3 The computations were carried out via Dr. Michael Akeroyd’s “Binaural Toolbox” for MATLAB®.
The lines in Fig. 1 represent the predictions of the model. The six predictions across ITD for a given target condition are, for reasons of clarity, plotted as connected solid lines without symbols. As is visually evident, with few exceptions, the predictions of the model are highly accurate. The amount of variance accounted for was calculated in two quite different ways. One calculation was r2, a quantity not affected by constant differences between data and their predictions. The other calculation was 100 × (1 − [Σ(Oi − Pi)2]/[Σ(Oi − )2]), where Oi and Pi represent individual observed and predicted values of threshold, respectively, and represents the mean of the observed values of threshold (e.g., Bernstein and Trahiotis, 1994). That quantity is affected by constant differences between data and their predictions. According to both calculations, the position-variable model accounted for 94% of the variance in the data, thereby validating what was concluded based on visual inspection.
What was surprising to us was the model’s general ability to predict correctly the vertical displacements separating the data within each panel which, as discussed earlier, suggest that a given value of IID produces a greater lateral displacement at 4 kHz (the spectral region of the target) than it does at 500 Hz (the spectral region of the pointer). Rather than relying on intuition and speculation to suggest any particular explanation for this outcome, it seems prudent to await future research targeted toward determining both (1) the generality of this finding for several types and classes of stimuli and (2) the mechanism(s) that operate to produce differential effects of IID as a function of spectral frequency.
Nevertheless, it appears worthwhile to report that, based on a number of analyses of the operation of the position-variable model, there may be several factors which, independently and possibly in combination, could be responsible. The analyses suggested that one way the differential effects of IID could come about concerns the shape of the final correlogram upon which the centroid is computed. For example, assuming a given IID, let us consider a comparison of the final shape of the correlogram of a 100% modulated SAM tonal target centered at 4-kHz having a frequency of modulation of 32 Hz to the final shape of the correlogram of our 500 Hz-centered noise pointer. That comparison revealed that the correlogram of the 4-kHz target was more “skewed” toward the side favored by the IID than was the correlogram of the 500-Hz centered pointer.
The difference in “skewing” appeared to result from differing frequency-specific interactions among the patterns of the interaural cross correlation functions of the processed stimuli, the p(τ) function, and the intensity function. More specifically, the analyses revealed that modest changes in the p(τ) function, [which Stern and Shear (1996) suggested could be frequency-dependent] and modest frequency-specific changes in the width of the intensity function affected the relative differences in the amount of “skewing” of the shapes of the final correlograms. Thus, there appear to be many ways in which frequency-specific differences in left/right asymmetry of the final correlogram could actually come about. For purposes of this discussion, the important concept is that, independent of how it arises, a greater left/right asymmetry would result in the value of the centroid being “pulled” relatively farther toward the side favored by the IID, thereby producing a relative magnification of the effect of IID.
The overall success of the position-variable model notwithstanding, careful inspection of the data obtained when the frequency of modulation was 256 Hz, the exponent of the raised sine was 8.0, and the depth of modulation was less than 1.0, reveals a general tendency of the model to under-predict the magnitude of the IID of the pointer required to match the intracranial position of the target. This discrepancy is reminiscent of the under-prediction by an interaural cross-correlation-based model of listeners’ sensitivities to changes in envelope-based ITDs for the same stimuli (Bernstein and Trahiotis, 2010). Of relevance was the finding that the empirical threshold-ITDs and the model’s predictions of them were brought into close register when it was assumed that the listeners utilized information in auditory filters centered above the 4 kHz center-frequency of the targets. It was shown that employing such a strategy would benefit performance because it would, effectively, enhance the depth of modulation of the internal representation of the stimulus for high, but not for low, rates of modulation (see Figs. 5–6 of Bernstein and Trahiotis, 2010).
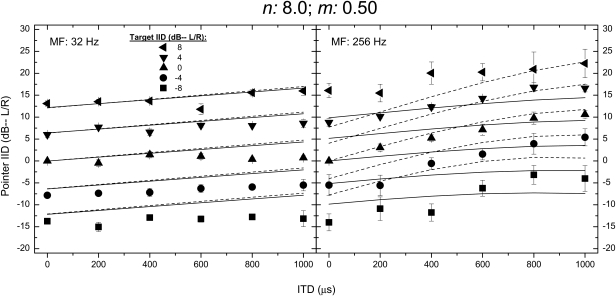
The data in Fig. 1, in addition to the findings of Bernstein and Trahiotis (2011b), demonstrate that, in general, envelope-based ITDs conveyed by stimuli having larger depths of modulation lead to larger extents of laterality. In order to determine whether the under-predictions of extents of laterality discussed above would be redressed by assuming that listeners utilize information in “off-frequency” auditory filters that would contain larger depths of modulation (see Bernstein and Trahiotis, 2010), new predictions were generated after the filterbank in the model was truncated so that it only extended from 5000 to 8000 Hz. Figure 2 contains data and predictions for raised-sine targets having an exponent of 8.0, a depth of modulation of 0.50, and rates of modulation of either 32 or 256 Hz. The data (symbols) and the original predictions (solid lines) are re-plotted from Fig. 1. The dashed lines represent the predictions obtained with the modified filterbank. For the rate of modulation of 256 Hz, note that, in general, employing the modified model led to an increase in the accuracy of the predictions. That improvement notwithstanding, it is visually apparent that there remain sizable discrepancies between the data and the predictions of them for the lower range of ITDs tested. The amount of variance accounted for (using the more stringent calculation described above) increased from 75% to 86% when the modified filterbank was employed.
Figure 2.
Data and predictions for raised-sine targets having an exponent of 8.0, a depth of modulation of 0.50, and rates of modulation of either 32 or 256 Hz. The data (symbols) and the original predictions (solid lines) are re-plotted from Fig. 1. The dotted lines represent the predictions obtained when the filterbank within the model was modified to include only the outputs of filters centered between 5000 and 8000 Hz (see text).
When the rate of modulation was 32 Hz, the predictions proved to be essentially identical to those obtained with the original filterbank. Consistent with that observation, the amount of variance accounted for by the two versions of the model was essentially the same (94% vs 93%). For archival reasons, it appears worth noting that virtually identical predictions were generated by using only the information within the auditory filter centered at 5 kHz, rather than a bank of auditory filters spanning 5–8 kHz. Based on these analyses, it appears that, for certain stimuli like those described above, listeners’ lateralization judgments, as well as listeners’ abilities to discriminate changes in ITD are consistent with the use of information within “off-frequency” filters that convey enhanced internal (i.e., neural) depths of modulation.
SUMMARY
An acoustic pointing task was used to measure extents of laterality produced by combinations of ongoing envelope-based interaural temporal disparities (ITDs) and interaural intensitive disparities (IIDs) imposed on 4-kHz-centered raised-sine stimuli while varying, parametrically, their peakedness, depth of modulation, and frequency of modulation. The data indicate that envelope-based IIDs principally act as weights and that they appear to exert their influence on lateral position independently of the influence of ITDs. Quantitative analyses revealed that an augmented form of the cross-correlation-based “position-variable” model of Stern and Shear (1996) accounted for 94% of the variance in the data. This success notwithstanding, for a small subset of the data, predictions could be improved by assuming that the listeners utilized information within auditory filters having center frequencies above 4 kHz. This outcome is consistent with the report of Bernstein and Trahiotis (2010) that, for similar stimuli, empirical threshold-ITDs and cross-correlation-based predictions of them were brought into closer register when it was assumed that the listeners utilized such “off-frequency” information.
ACKNOWLEDGMENTS
The authors thank Carmelo Rizza, our laboratory research assistant, for his dedication and care while collecting the behavioral data and performing preliminary analyses of them. The authors also thank Dr. H. Steven Colburn for his detailed and helpful review. This research was supported by research grant NIH DC-04147 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Footnotes
Data were also obtained from a fourth listener whose matches were highly inconsistent. Upon querying the listener regarding her strategy for matching the intracranial positions of the target and pointer, it was discovered that she had misunderstood the instructions for the task. Therefore, her data were excluded.
Apparently, Stern and Colburn (1978) determined the position of the intensity function along the interaural delay axis by entering the value of the IID (in dB) imposed on their target stimuli into the relation shown in their Fig. 4. We wished to incorporate the determination of the IID of the target into the processing stages of the model. To that end, the desired IID was imposed on the stimuli that served as inputs to the model. The IID was then calculated or “recovered” subsequent to the stages of the model designed to emulate peripheral auditory processing. Because half-wave, cube-law rectification was included in the model, the value of IID (in dB) so recovered was three times that imposed on the “external” stimuli. The value was divided by three and was used according to the relation described in Fig. 4 of Stern and Colburn (1978) in order to determine the position of the intensity function along the interaural axis. Thus, in both this study and in that of Stern and Colburn, the independent variable in the relation was the IID (in dB) imposed on the external target stimulus.
This value, used to convert the position of the centroid in units of interaural delay (in μs) to units of pointer-IID (in dB), differs slightly from the value of 11.7 μs employed by Bernstein and Trahiotis (2011b). In that earlier study, extents of ITD-based laterality were measured for 4 kHz-centered raised sine stimuli all having an IID of 0 dB. No intensity function was incorporated into the position-variable model that was used to account for those data. It was necessary, therefore, to determine what effect, if any, the inclusion of the intensity function (centered at 0 μs) would have on the predictions of the model reported by Bernstein and Trahiotis. It was found that the inclusion of the intensity function produced a slight reduction in the magnitudes of the predicted values of pointer IID for targets containing an ITD but no IID. Reducing the “scaling” value from 11.7 μs to 10 μs was found to minimize the rms error between the original predictions reported by Bernstein and Trahiotis and those calculated with the inclusion of the intensity function within the position-variable model. Therefore, the model used in the study reported here was “calibrated” to the predictions reported by Bernstein and Trahiotis (2011b). Thus, the scaling value employed here is not ad hoc because it is not the value determined to yield the largest percent of variance accounted for the set of data reported here.
References
- Bernstein, L. R.,and Trahiotis, C. (1985). “Lateralization of sinusoidally-amplitude-modulated tones: Effects of spectral locus and temporal variation,” J. Acoust. Soc. Am. 78, 514–523. 10.1121/1.392473 [DOI] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (1994). “The effect of non-simultaneous on-frequency and off-frequency cues on the detection of a tonal signal masked by narrow-band noise,” J. Acoust. Soc. Am. 95, 920–930. 10.1121/1.408401 [DOI] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (2002). “Enhancing sensitivity to interaural delays at high frequencies by using transposed stimuli,” J. Acoust. Soc. Am. 112, 1026–1036. 10.1121/1.1497620 [DOI] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (2003). “Enhancing interaural-delay-based extents of laterality at high frequencies by using ‘transposed stimuli’,” J. Acoust. Soc. Am. 113, 3335–3347. 10.1121/1.1570431 [DOI] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (2009). “How sensitivity to ongoing interaural temporal disparities is affected by manipulations of temporal features of the envelopes of high-frequency stimuli,” J. Acoust. Soc. Am. 125, 3234–3242. 10.1121/1.3101454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (2010). “Accounting quantitatively for sensitivity to envelope-based interaural temporal disparities at high frequencies,” J. Acoust. Soc. Am. 128, 1224–1234. 10.1121/1.3466877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (2011a). “Lateralization produced by interaural intensitive disparities appears to be larger for high- vs low-frequency stimuli,” J. Acoust. Soc. Am. 129, EL15–EL20. 10.1121/1.3528756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (2011b). “Lateralization produced by envelope-based interaural temporal disparities of high-frequency, raised-sine stimuli: Empirical data and modeling,” J. Acoust. Soc. 129, Am. 1501–1508. 10.1121/1.3552875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell, T. N., Trahiotis, C., and Bernstein, L. R. (1991). “Lateralization of low-frequency tones: Relative potency of gating and ongoing interaural delay,” J. Acoust. Soc. Am. 90, 3077–3085. 10.1121/1.401782 [DOI] [PubMed] [Google Scholar]
- Buell, T. N., Trahiotis, C., and Bernstein, L. R. (1994). “Lateralization of bands of noise as a function of combinations of interaural intensitive differences, interaural temporal differences and bandwidth,” J. Acoust. Soc. Am. 95, 1482–1489. 10.1121/1.410028 [DOI] [PubMed] [Google Scholar]
- Domnitz, R. H., and Colburn, H. S. (1977). “Lateral position and interaural discrimination,” J. Acoust. Soc. Am. 61, 1586–1598. 10.1121/1.381472 [DOI] [PubMed] [Google Scholar]
- Griffin, S. J., Bernstein, L. R., Ingham, N. J., and McAlpine, D. (2005). “Neural sensitivity to interaural envelope delays in the inferior colliculus of the guinea pig,” J. Neurophys. 93, 3463–3478. 10.1152/jn.00794.2004 [DOI] [PubMed] [Google Scholar]
- Hays, W. L. (1973). Statistics for the Social Sciences (Holt, Rinehart, and Winston, New York: ), pp. 417–419. [Google Scholar]
- Heller, L. M., and Trahiotis, C. (1996). “Extents of laterality and binaural interference effects,” J. Acoust. Soc. Am, 99, 3632–3637. 10.1121/1.414961 [DOI] [PubMed] [Google Scholar]
- John, M. S., Dimitrijevic, A., and Picton, T. (2002). “Auditory steady-state responses to exponential modulation envelopes,” Ear Hear. 23, 106–117. 10.1097/00003446-200204000-00004 [DOI] [PubMed] [Google Scholar]
- Keppel, G. (1991). Design and Analysis: A Researchers Handbook (Prentice-Hall, Englewood Cliffs, NJ: ), pp. 494. [Google Scholar]
- Kohlrausch, A., Fassel, R., and Dau, T. (2000). “The influence of carrier level and frequency on modulation and beat-detection thresholds for sinusoidal carriers,” J. Acoust. Soc. Am. 108, 723–734. 10.1121/1.429605 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J., and Glasberg, B. R. (2001). “Temporal modulation transfer functions obtained using sinusoidal carriers with normally hearing and hearing-impaired listeners,” J. Acoust. Soc. Am. 110, 1067–1073. 10.1121/1.1385177 [DOI] [PubMed] [Google Scholar]
- Patterson, R. D., Allerhand, M. H., and Giguere, C. (1995). “Time-domain modeling of peripheral auditory processing: A modular architecture and a software platform,” J. Acoust. Soc. Am. 98, 1890–1894. 10.1121/1.414456 [DOI] [PubMed] [Google Scholar]
- Schiano, J. L., Trahiotis, C., and Bernstein, L. R. (1986). “Lateralization of low-frequency tones and narrow bands of noise,” J. Acoust. Soc. Am. 79, 1563–1570. 10.1121/1.393683 [DOI] [PubMed] [Google Scholar]
- Stern, R. M., and Colburn, H. S. (1978). “Theory of binaural interaction based on auditory-nerve data. IV. A model for subjective lateral position,” J. Acoust. Soc. Am. 64, 127–140. 10.1121/1.381978 [DOI] [PubMed] [Google Scholar]
- Stern, R. M., and Shear, G. D. (1996). “Lateralization and detection of low-frequency binaural stimuli: Effects of distribution of internal delay,” J. Acoust. Soc. Am. 100, 2278–2288. 10.1121/1.417937 [DOI] [Google Scholar]
- Trahiotis, C., and Stern, R. M. (1989). “Lateralization of bands of noise: Effects of bandwidth and differences of interaural time and phase,” J. Acoust. Soc. Am. 86, 1285–1293. 10.1121/1.398743 [DOI] [PubMed] [Google Scholar]