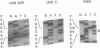Abstract
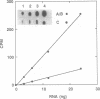
Three distinct ribosomal RNA (rRNA) transcription units (rDNA units), designated A, B and C, were identified in the intraerythrocytic protozoan parasite, Babesia bigemina. These rDNA units were cloned, and restriction maps were constructed showing the approximate location of the small and large rRNA coding regions. The arrangement of the genes in the genome and copy number analysis suggests the presence of a single copy of each rDNA unit per haploid genome. The complete nucleotide sequence of the small subunit rRNA coding region (1693 bp) and parts of the 5' and 3' flanking regions were determined for all three units. Units A and B have identical sequences, but unit C differs from units A and B at ten nucleotide positions, two in the small subunit rRNA coding region and four each in the adjacent 5' and 3' flanking regions. The differences in the coding region are confirmed in genomic DNA and RNA from two different isolates of B.bigemina. The RNA of both sequence types is transcribed in parasites from erythrocyte culture, however, the products of gene units A + B accumulate at a ratio of approximately 4:1 compared with the product of unit C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. M., Enomoto T., Perantoni A. O., Riggs C. W., Kovatch R. M., Reed C. D., Giner-Sorolla A., Rice J. M. N-nitrosocimetidine as an initiator of murine skin tumors with associated H-ras oncogene activation. Carcinogenesis. 1989 Nov;10(11):2009–2013. doi: 10.1093/carcin/10.11.2009. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dalrymple B. P. Cloning and characterization of the rRNA genes and flanking regions from Babesia bovis: use of the genes as strain discriminating probes. Mol Biochem Parasitol. 1990 Nov;43(1):117–124. doi: 10.1016/0166-6851(90)90136-a. [DOI] [PubMed] [Google Scholar]
- Dame J. B., McCutchan T. F. Cloning and characterization of a ribosomal RNA gene from Plasmodium berghei. Mol Biochem Parasitol. 1983 Jul;8(3):263–279. doi: 10.1016/0166-6851(83)90048-8. [DOI] [PubMed] [Google Scholar]
- Dame J. B., McCutchan T. F. Identification of 5 S and 5.8 S ribosomal RNA molecules and their genes in Plasmodium berghei. Mol Biochem Parasitol. 1984 Apr;11:301–307. doi: 10.1016/0166-6851(84)90074-4. [DOI] [PubMed] [Google Scholar]
- Dame J. B., McCutchan T. F. The four ribosomal DNA units of the malaria parasite Plasmodium berghei. Identification, restriction map, and copy number analysis. J Biol Chem. 1983 Jun 10;258(11):6984–6990. [PubMed] [Google Scholar]
- Dame J. B., Sullivan M., McCutchan T. F. Two major sequence classes of ribosomal RNA genes in Plasmodium berghei. Nucleic Acids Res. 1984 Jul 25;12(14):5943–5952. doi: 10.1093/nar/12.14.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gunderson J. H., McCutchan T. F., Sogin M. L. Sequence of the small subunit ribosomal RNA gene expressed in the bloodstream stages of Plasmodium berghei: evolutionary implications. J Protozool. 1986 Nov;33(4):525–529. doi: 10.1111/j.1550-7408.1986.tb05656.x. [DOI] [PubMed] [Google Scholar]
- Gunderson J. H., Sogin M. L., Wollett G., Hollingdale M., de la Cruz V. F., Waters A. P., McCutchan T. F. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987 Nov 13;238(4829):933–937. doi: 10.1126/science.3672135. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Weiser B., Woese C. R., Noller H. F. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. doi: 10.1016/s0079-6603(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol Gen Genet. 1987 May;207(2-3):196–203. doi: 10.1007/BF00331578. [DOI] [PubMed] [Google Scholar]
- Langsley G., Hyde J. E., Goman M., Scaife J. G. Cloning and characterisation of the rRNA genes from the human malaria parasite Plasmodium falciparum. Nucleic Acids Res. 1983 Dec 20;11(24):8703–8717. doi: 10.1093/nar/11.24.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., de la Cruz V. F., Lal A. A., Gunderson J. H., Elwood H. J., Sogin M. L. Primary sequences of two small subunit ribosomal RNA genes from Plasmodium falciparum. Mol Biochem Parasitol. 1988 Feb;28(1):63–68. doi: 10.1016/0166-6851(88)90181-8. [DOI] [PubMed] [Google Scholar]
- Neefs J. M., Van de Peer Y., Hendriks L., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2237–2317. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C. D., Dahlberg A. E. A single base change at 726 in 16S rRNA radically alters the pattern of proteins synthesized in vivo. EMBO J. 1990 Jan;9(1):289–294. doi: 10.1002/j.1460-2075.1990.tb08107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C. D., Göringer H. U. A single mutation in 16S rRNA that affects mRNA binding and translation-termination. Nucleic Acids Res. 1990 Sep 25;18(18):5381–5386. doi: 10.1093/nar/18.18.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippen-Lentz D., Afroze T., Vezza A. C. Heterogeneity and expression of the Plasmodium falciparum 5.8S ribosomal RNA genes. Mol Biochem Parasitol. 1990 Jan 1;38(1):113–120. doi: 10.1016/0166-6851(90)90211-4. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H. Structural diversity of eukaryotic small subunit ribosomal RNAs. Evolutionary implications. Ann N Y Acad Sci. 1987;503:125–139. doi: 10.1111/j.1749-6632.1987.tb40603.x. [DOI] [PubMed] [Google Scholar]
- Unnasch T. R., Wirth D. F. The avian malaria Plasmodium lophurae has a small number of heterogeneous ribosomal RNA genes. Nucleic Acids Res. 1983 Dec 10;11(23):8443–8459. doi: 10.1093/nar/11.23.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C. A., Buening G. M., Green T. J., Carson C. A. In vitro cultivation of Babesia bigemina. Am J Vet Res. 1985 Feb;46(2):416–420. [PubMed] [Google Scholar]
- Vega C. A., Buening G. M., Rodriguez S. D., Carson C. A. Concentration and enzyme content of in vitro-cultured Babesia bigemina-infected erythrocytes. J Protozool. 1986 Nov;33(4):514–518. doi: 10.1111/j.1550-7408.1986.tb05653.x. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Isolation and characterization of DNA from Tritrichomonas foetus and Trichomonas vaginalis. Mol Biochem Parasitol. 1985 Mar;14(3):323–335. doi: 10.1016/0166-6851(85)90060-x. [DOI] [PubMed] [Google Scholar]
- Waters A. P., Syin C., McCutchan T. F. Developmental regulation of stage-specific ribosome populations in Plasmodium. Nature. 1989 Nov 23;342(6248):438–440. doi: 10.1038/342438a0. [DOI] [PubMed] [Google Scholar]