Abstract
Background:
Azadirachta indica (neem), a Meliaceae family tree, has been used in India for several decades for the treatment of several diseases in medicine and dentistry. Neem has been considered to have antiseptic activity, but still its use for the treatment of gingivitis and periodontitis is not very clear. Hence, the purpose of the present study was to assess the efficacy of neem based mouth rinse regarding its antigingivitis effect.
Materials and Methods:
Forty five subjects with plaque induced gingivitis were selected for the study. They were equally divided into three groups. Group I patients were asked to rinse with 15 ml of neem mouthwash twice daily, group II with 15 ml of chlorhexidine mouthwash twice daily, and group III with 15 ml of saline twice daily. The three groups were asked to perform the routine oral hygiene procedures thought out the study period. Bleeding on probing and gingivitis were evaluated by Muhlemann and Son's Sulcus bleeding index (1971) and Loe and Sillness gingival index (1963), respectively, at base line, after every week till one month.
Results:
Our result showed that an A. indica mouthrinse is equally effective in reducing periodontal indices as Chlorhexidine. The results demonstrated a significant reduction of gingival, bleeding, and plaque indices in both groups over a period of 21 days as compared to placebo.
Conclusion:
A. indica-based mouth rinse is equally efficacious with fewer side effects as compared to chlorhexidine and may be used as an adjunct therapy in treating plaque induced gingivitis.
Keywords: Azadirachta indica, chlorhexidin gluconate, plaque index
INTRODUCTION
Periodontal disease is characterized by inflammation and/or destruction of supporting tissue of the teeth. The most effective method of prevention and maintenance of periodontal disease is mechanical as well as chemical plaque control.[1] Several chemical plaque control agents have been evaluated for their effectiveness on supragingival plaque including bis biguinaides, essential oils, enzymes, and even herbal extract. Some of these substances have been associated with various side effects incapacitating their long term use, so new formulation of equal efficacy and fewer side effects are required to be evaluated. For thousands of years, humans have sought to fortify their health and cure various aliments with herbal remedies. Many herbal extracts are commercially available. Azadirachta indica also known as neem has been used in India and South Asia for thousands of years as a perfect tool for maintaining healthy periodontium. Neem has been long considered to have an astringent, antiseptic, insecticidal, antiulcer and for medical properties. It is used for periodontitis and other dental diseases. The antibacterial activity of neem has been evaluated and known from ancient times.[2,3] Other than this, the leaf extract of neem has also shown superior antiviral and antihyperglycemic activity in vitro and in vivo on animals.[4] Neem leaves have been used in the treatment of gingivitis and periodontitis. The possible mechanism of anti inflammatory action of neem is by inhibiting prostaglandin E and 5 HT and thus reducing the inflammation. The antibacterial action can be explained by “Azadiachtin” that is known to destroy bacterial cell wall and thus inevitably inhibit the growth of bacteria,[5] also the breakdown of cell wall disturb osmotic pressure and leads to cell death.[6] But still, its use of treatment for gingivitis and perodontitis is not very clear. Hence, aim of the present study was “to assess the anti-gingivitis and antiplaque properties of an A. indica based mouthrinse in plaque induced gingivitis.”
MATERIALS AND METHODS
The present study was a double-blind, randomized, parallel group, placebo clinical trial approved by the ethical committee of the IDS, Bareilly. Patients were selected from the out-patient department of periodontics, Institute of Dental Science, Bareilly. Total 45 subjects were enrolled in this study design. Study was approved by Ethical Committee of institute of dental sciences, Bareilly. Patients were informed about the study and written consent was obtained. Eligible subjects were randomly assigned into three groups.
Group I subjects received 0.19% A. indica mouthrinse and were asked to rinse 15 ml of mouth rinse twice daily for 1 min [Figures 1–3].
Figure 1.
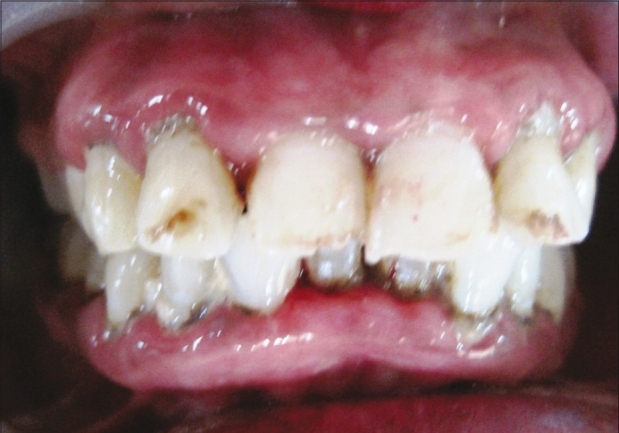
Neem patient at baseline
Figure 3.
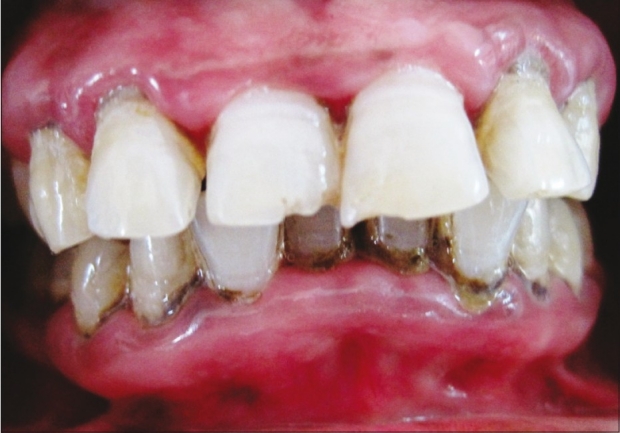
Neem patient at 21st day
Figure 2.
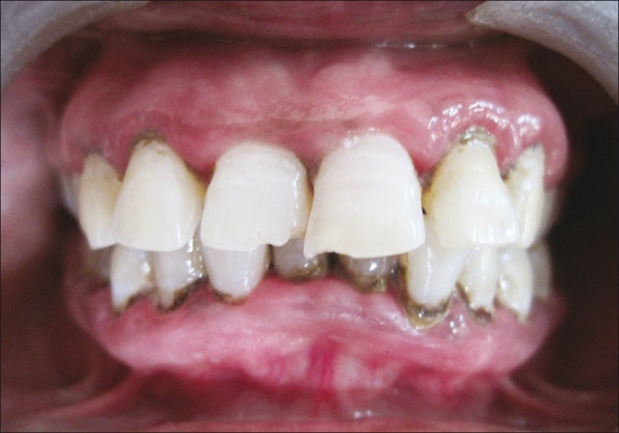
Neem patient at 7th day
Group II subjects were received 0.2% CHX and were asked to rinse 15 ml of mouthrinse twice daily for 1 min [Figures 4–6].
Figure 4.
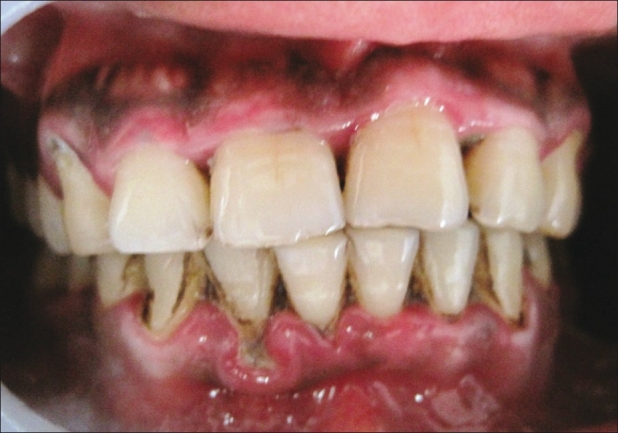
Chlorhexidine patient at baseline
Figure 6.
Chlorhexidine patient at 21st day
Figure 5.
Chlorhexidine patient at 7th day
Group III subjects were received saline and were asked to rinse 15ml of mouthrinse twice daily for 1 min. Subjects were asked not to refrain from oral hygiene procedures.
Following indices were recorded
Loe and Sillness Gingival index (1963) Muhlemann and Son's Sulcus;
Bleeding index (1971) Sillness and Loe; and
Plaque index[7].
Recordings were made at 7th day and 21st day for all the subjects and were compared to baseline. Inclusion criteria were subjects between the age group of 18-65 years, minimum of 10 teeth were present, Mean plaque index of at least 1.05, mean gingival index of at least 1.0. Pregnant/lactating women. Smokers. Subjects that had used any type of antibacterial mouth rinse within four weeks of commencement of the study. No periodontal treatment or antibiotic therapy for last three months prior to the study. Patients unwilling to complete the treatment protocol were excluded from the study.
Statistical analysis
Paired ‘t’ test was applied for Intragroup comparison. Unpaired ‘t’ test applied for Intergroup comparison and P value ≤0.05 was considered statistically significant.
RESULT
Figure 7 shows a bleeding index scores at baseline as well as 7th and 21st day after treatment. Within subjects data demonstrated a statistically significant decrease in mean bleeding, for both group at 7th and 21st day as compared to baseline except in saline group.
Figure 7.
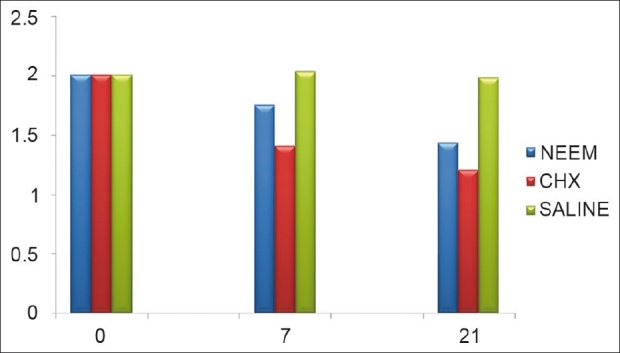
Bleeding index
Figure 8 shows a gingival index scores at baseline as well as 7th and 21st day after treatment and the data demonstrated a significant decrease in mean gingival index as compared to base line except in saline group.
Figure 8.

Gingival index
Figure 9 shows a plaque index scores at baseline, as well as 7th and 21st day after treatment and data demonstrated same decrease in mean plaque index as compared to baseline except in saline group.
Figure 9.
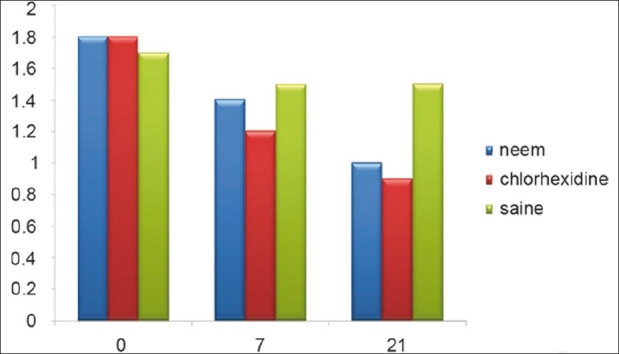
Plaque index
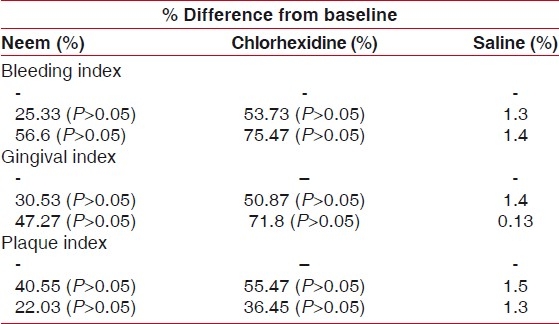
Table 1 shows that significant difference between groups for baseline, 7th, 21st was applied 5% of level of significance. A significant difference was observed from baseline to 7th days and baseline to 21st days. But there was no statistically significant difference between Azadirachta indica based mouthrinse and chlorhexidine for any clinical parameters throughout the study.
Table 1.
Shows that significant difference between groups for baseline, 7th 21st was applied 5% of level of significance
DISCUSSION
Though chlorhexidine was discovered in 1950s, it is still considered one of the most effective antiplaque agents in dentistry. However, long term use of chlorhexidine is limited by its disagreeable taste and propensity to stain the teeth brown[8] Therefore, the effectiveness of Azadirachta indica leaf extract against plaque formation was assessed. Almost every part of the tree was used in indigenous systems of medicine for the treatment of a variety of human ailments, particularly against diseases of bacterial and fungal origin.[9] Therefore, new formulation with similar or superior efficacy and possible few long effect need to be investigated. In the present study, A. indica extract mouthrinse was formulated along with the sweetener and flavor so as to increase the patient compliance. Our data shows that an A. indica mouthrinse is equally effective in reducing periodontal indices as chlorhexidine. The treatment doses were applied after breakfast and after lunch. In order to eliminate the bias, decreased flow of the saliva during overnight that could be attributed to was also successful in reducing the plaque index compared to the control group. The results demonstrated a significant reduction of gingival, bleeding, and plaque indices in both groups over a period of 21 day as compared to placebo. The results of our study are consistent with the study done by Botelho et al.[10] reported that A. indica-based mouth rinse is highly efficacious and that it may be used as an alternative therapy in the treatment of periodontal disease-based mouth rinse is highly efficacious and that it may be used as an alternative therapy in the treatment of periodontal disease . However, certain studies have proved that neem extract leads to even better reduction in plaque index and bacterial load. Patel and Ventakrishna (1988) reported a significant reduction in probing depth and gain in clinical attachment level by its use.[11]
CONCLUSION
Within limitations of the study, it was found that 0.19% Azadirachta indica (neem) has significant anti-inflammatory property. Thus, it can be used as an adjunct to mechanical therapy for treating plaque induced gingivitis. Present study has an important impact in order to create an effective and inexpensive oral health intervention for low socio-economic communities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Botelho MA, BezerraFilho JG, Correa LL, Heukelbach J. Effect of a novel essential oil mouthrinse without alcohol on gingivitis: A double-blinded randomized controlled trial. J Appl Oral Sci. 2007;15:175–80. doi: 10.1590/S1678-77572007000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaurasia SC, Jain PC. Antibacterial activity of essential oils of four medicinal plants. Indian J Hospital Pharm. 1978;23:166–7. [Google Scholar]
- 3.Chawla AS, Kumar M, Bansal I. Chemical constituents and biological activity of neem-a review. Indian Drugs. 1994;32:57–62. [Google Scholar]
- 4.Chattopadhyay RR. Possible mechanism of antihyperglycemic effect of Azadirachta indica leaf extract: Part V. J Ethnopharmacol. 1999;67:367–72. doi: 10.1016/s0378-8741(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 5.Trewari DN. DehraDun, India: Int. Book Distributors; 1992. Monograph on Neem (Azadirachta indica) p. 179. [Google Scholar]
- 6.Robinson T. 6th ed. Bandung: Penerbit ITB; 1995. The organic constituens of higher plants; pp. 191–2. [Google Scholar]
- 7.Silness J, Löe H. Periodontal disease index. Ann Periodontol. 1964;4:655–69. [Google Scholar]
- 8.Fardal D, Turnbull RS. A review of the literature on use of chlorhexidine in dentistry. J Am Dent Assoc. 1986;112:863–9. doi: 10.14219/jada.archive.1986.0118. [DOI] [PubMed] [Google Scholar]
- 9.Randhawa NS, Parmar BS. 2nd ed. India: New age International Pvt. Ltd. Publishers; 1996. Neem; pp. 77–111. [Google Scholar]
- 10.Botelho MA, Santos RC Jose Galberto Martins JG, Cintia Oliveira Carvalho Co1, Mabel Calina Paz Mc, Cláudio Azenha, Ronaldo Sousa Ruela, Dinalva BritoQueiroz, Wagner Sousa Ruela, Gloria Marinho, Francisca Isabel Ruela. Efficacy of a mouthrinse based on leaves of the neem tree (Azadirachta indica) in the treatment of patients with chronic gingivitis: A double-blind, randomized, controlled trial. J Med Plants Res. 2008;2:341–6. [Google Scholar]
- 11.Patel VK, Venkatakrishna-Bhatt H. Folklore therapeutic indigenous plants in periodontal disorders in India (review, experimental and clinical approach) Int J Clin Pharmacol Ther Toxicol. 1988;26:176–84. [PubMed] [Google Scholar]


