Abstract
Plants are the tremendous source for the discovery of new products with medicinal importance in drug development. Today several distinct chemicals derived from plants are important drugs, which are currently used in one or more countries in the world. Secondary metabolites are economically important as drugs, flavor and fragrances, dye and pigments, pesticides, and food additives. Many of the drugs sold today are simple synthetic modifications or copies of the naturally obtained substances. The evolving commercial importance of secondary metabolites has in recent years resulted in a great interest in secondary metabolism, particularly in the possibility of altering the production of bioactive plant metabolites by means of tissue culture technology. Plant cell and tissue culture technologies can be established routinely under sterile conditions from explants, such as plant leaves, stems, roots, and meristems for both the ways for multiplication and extraction of secondary metabolites. In vitro production of secondary metabolite in plant cell suspension cultures has been reported from various medicinal plants, and bioreactors are the key step for their commercial production. Based on this lime light, the present review is aimed to cover phytotherapeutic application and recent advancement for the production of some important plant pharmaceuticals.
KEY WORDS: Cell suspension culture, medicinal plants, plant pharmaceuticals, secondary metabolites
Medicinal plants are the most exclusive source of life-saving drugs for majority of the world's population. The utilization of plant cells for the production of natural or recombinant compounds of commercial interest has gained increasing attention over past decades.[1] The secondary metabolites are known to play a major role in the adaptation of plants to their environment and also represent an important source of pharmaceuticals.[2] The metabolism is defined as the sum of all the biochemical reactions carried out by an organism. Primary metabolic pathways converge too few end products, while secondary metabolic pathways diverge too many products. Primary requires the cell to use nutrients in its surroundings such as low-molecular weight compounds for cellular activity. There are three potential pathways for primary metabolism: the Embden Meyerhof-Parnas Pathway (EMP), the Entner-Dourdorof pathway, and the hexose monophosphate (HMP) pathway. The EMP produces two molecules of pyruvate via triose phosphate intermediates. This pathway occurs most widely in animal, plant, fungal, yeast, and bacterial cells. Many microorganisms, however, use this pathway solely for glucose utilization. During primary metabolism, hexoses such as glucose are converted to single cell protein by yeasts and fungi. This is generally done by using a combination of EMP and HMP pathways, followed by the tricarboxylic acid (TCA) cycle and respiration. Plants produce a vast and diverse assortment of organic compounds, the great majority of which do not appear to participate directly in growth and development. These substances, traditionally referred to as secondary metabolites, often are differentially distributed among limited taxonomic groups within the plant kingdom. Their functions, many of which remain unknown, are being elucidated with increasing frequency. The primary metabolites, in contrast, such as phytosterols, acyl lipids, nucleotides, amino acids, and organic acids, are found in all plants and perform metabolic roles that are essential and usually evident. Although noted for the complexity of their chemical structures and biosynthetic pathways, natural products have been widely perceived as biologically insignificant and have historically received little attention from most plant biologists. Pharmaceutical organic chemists, however, have long been interested in these novel phytochemicals and have investigated their chemical properties extensively since the 1850s. Studies of natural products stimulated development of the separation techniques, spectroscopic approaches to structure elucidation, and synthetic methodologies that now constitute the foundation of contemporary organic chemistry. Interest in natural products was not purely academic but rather was prompted by their great utility as dyes, polymers, fibers, glues, oils, waxes, flavoring agents, perfumes, and drugs. Recognition of the biological properties of myriad natural products has fueled the current focus of this field, namely, the search for new drugs, antibiotics, insecticides, and herbicides. Based on their biosynthetic origins, plant natural products can be divided into three major groups: the terpenoids, the alkaloids, and the phenolic compounds. All terpenoids, including both primary metabolites and more than 25,000 secondary compounds, are derived from the five-carbon precursor isopentenyl diphosphate (IPP). The 12,000 or so known alkaloids, which contain one or more nitrogen atoms, are biosynthesized principally from amino acids. The 8000 or so phenolic compounds are formed by way of either the shikimic acid pathway or the malonate/acetate pathway.[3] In this review, we provide an overview of recent trends on production of secondary plant metabolites.
Rationale of the Study
The objectives of this study were to get an overview of various works that have been done and could be done in the field of metabolic engineering of plant secondary metabolites by using yeast and to search the possibility of using methods and mechanisms for the production of various human health promoting plant secondary metabolites in the coming future. The principle advantage of recent technologies is that it may provide continuous, reliable source of plant pharmaceuticals and could be used for the large-scale culture of plant cells from which these metabolite can be extracted. Plant cell and tissue cultures hold great promise for controlled production of myriad of useful secondary metabolites on demand. The current yield and productivity cannot fulfill the commercial goal of plant cell-based bioprocess for the production of most secondary metabolites. In order to stretch the boundary, recent advances, new directions, and opportunities in plant cell-based processes are being critically examined. However, most often trials with plant cell cultures fail to produce the desired products. In such cases, strategies to improve the production of secondary metabolites must be considered. One of the main problems encountered is the lack of basic knowledge of the biosynthetic routes and mechanisms responsible for the production of plant metabolites. Where the productivity of the desired metabolites is limited by the lack of particular precursors, biotransformation using an exogenous supply of biosynthetic precursors, genetic manipulation, and metabolic engineering may improve the accumulation of compounds. Elicitors, compounds triggering the formation of secondary metabolites, can be abiotic or biotic. Natural elicitors include polysaccharides such as pectin and chitosan, which are also used in the immobilization and permeabilization of plant cells. Immobilization with suitable bioreactor system provides several advantages, such as continuous process operation, but for the development of an immobilized plant cell culture process, natural or artificially induced secretion of the accumulated product into the surrounding medium is necessary.
Advancements in the Production of Secondary Metabolites
Plant cell and tissue cultures hold great promise for controlled production of myriad of useful secondary metabolites on demand. Discoveries of cell cultures capable of producing specific medicinal compounds at a rate similar or superior to that of intact plants have accelerated in the last few years has been summarized in Table 1.[4] In order to obtain high yields suitable for commercial exploitation, efforts have been focused on isolating the biosynthetic activities of cultured cells, achieved by optimizing the cultural conditions, selecting high-producing strains and employing precursor feeding, transformation methods, and immobilization techniques.[5] Transgenic hairy root cultures have revolutionized the role of plant tissue culture in secondary metabolite production. They are unique in their genetic and biosynthetic stability, faster in growth, and more easily maintained. Using this methodology, a wide range of chemical compounds has been synthesized.[6] Advances in tissue culture, combined with improvement in genetic engineering of pharmaceuticals, nutraceuticals, and other beneficial substances.[7] Recent advances in the molecular biology, enzymology, and fermentation technology of plant cell cultures suggest that these systems will become a viable source of important secondary metabolites.[8] Genome manipulation is resulting in relatively large amounts of desired compounds produced by plants infected with an engineered virus, whereas transgenic plants can maintain constant levels of production of proteins without additional intervention.[9] Large-scale plant tissue culture is found to be an attractive alternative approach to traditional methods of plantation as it offers controlled supply of biochemical's independent of plant availability.[10]
Table 1.
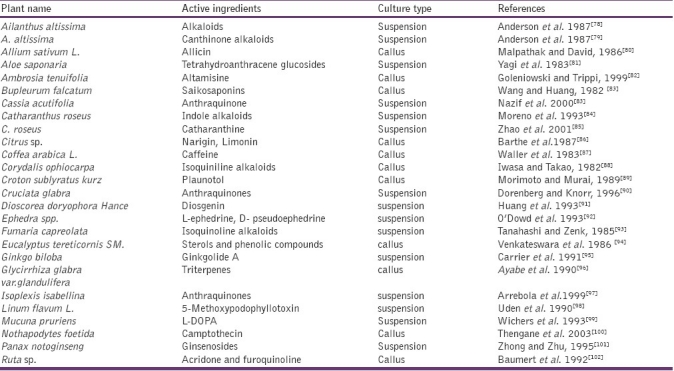
Production of Secondary Metabolites from Medicinal Plants by Plant Tissue Cultures
Due to these advances, research in the area of tissue culture technology for production of plant chemicals has bloomed beyond expectations.[4] The major advantages of a cell culture system over the conventional cultivation of whole plants are as follows:
Useful compounds can be produced under controlled conditions independent of climatic changes or soil conditions.
Cultured cells would be free of microbes and insects.
The cells of any plants, tropical or alpine, could easily be multiplied to yield their specific metabolites.
Automated control of cell growth and rational regulation of metabolite processes would reduce labor costs and improve productivity.
Organic substances are extractable from callus cultures.
Trends in Production of Secondary Plant Metabolites from Higher Plants
Plant cell and tissue cultures can be established routinely under sterile conditions from explants, such as plant leaves, stems, roots, and meristems for multiplication and extraction of secondary metabolites. Strain improvement, methods for the selection of high-producing cell lines, and medium optimizations can lead to an enhancement in secondary metabolite production. The capacity for plant cell, tissue, and organ cultures to produce and accumulate many of the same valuable chemical compounds as the parent plant in nature has been recognized almost since the inception of in vitro technology. The strong and growing demand in today's marketplace for natural, renewable products has refocused attention on in vitro plant materials as potential factories for secondary phytochemical products and has paved the way for new research exploring secondary product expression in vitro.[11] There is a series of distinct advantages to producing a valuable secondary product in plant cell culture, rather than in vivo in the whole crop plant.
These include the following:
Production can be more reliable, simpler, and more predictable.
Isolation of the phytochemical can be rapid and efficient, when compared with extraction from complex whole plants.
Compounds produced in vitro can directly parallel compounds in the whole plant.
Interfering compounds that occur in the field-grown plant can be avoided in cell cultures.
Tissue and cell cultures can yield a source of defined standard phytochemicals in large volumes.
Tissue and cell cultures are a potential model to test elicitation.
Cell cultures can be radiolabeled, such that the accumulated secondary products, when provided as feed to laboratory animals, can be traced metabolically.
While research to date has succeeded in producing a wide range of valuable secondary phytochemicals in unorganized callus or suspension cultures, in other cases production requires more differentiated micro plant or organ cultures.[12] This situation often occurs when the metabolite of interest is only produced in specialized plant tissues or glands in the parent plant. A prime example is ginseng (Panax ginseng). Because saponin and other valuable metabolites are specifically produced in ginseng roots, root culture is required in vitro. Similarly, herbal plants such as Hypericum perforatum (St. John's wort), which accumulates the hypericins and hyperforins in foliar glands, have not demonstrated the ability to accumulate phytochemicals in undifferentiated cells.[13] As another example, biosynthesis of lysine to anabasine occurs in tobacco (Nicotiana tabacum) roots, followed by the conversion of anabasine to nicotine in leaves. Callus and shoot cultures of tobacco can produce only trace amounts of nicotine because they lack the organ-specific compound anabasine. In other cases, at least some degree of differentiation in a cell culture must occur before a product can be synthesized (e.g., vincristine or vinblastine from Catharanthus roseus). Reliance of a plant on a specialized structure for production of a secondary metabolite, in some cases, is a mechanism for keeping a potentially toxic compound sequestered. Intensive activities have been centered on production of natural drugs or chemoprotective compounds from plant cell culture by one or more of the following strategies:
Organ Cultures for Secondary Metabolite Production
Fritillaria unibracteata can be rapidly propagated, directly from small cuttings of the bulb by the technique of organ culture. The cultured bulb can be harvested after a 50-day culture period in MS media supplemented with 4.44 - M BA and 5.71 - M IAA. The growth rate was about 30–50 times higher than that under natural wild growth conditions. The content of alkaloid and beneficial microelements in the cultured bulbs was higher than found in the wild bulb.[14]
In vitro shoot multiplication of Frangula alnus was obtained on woody plant medium with indole-3-acetic acid and 6-benzylaminapurine, the highest metabolite production (1731 mg/100 g of total anthraquinone was in the shoots grown on the MS medium with addition of 1-naphthilaceneacetic (NAA) (0.1 mg/l) and thidiazuron (TDZ) (0.1 mg/l).[15]
Precursor Addition for Improvement of Secondary Metabolite Production
The treatment of plant cells with biotic and/or abiotic elicitors has been a useful strategy to enhance secondary metabolite production in cell cultures.[11] The most frequently used elicitors in previous studies were fungal carbohydrates, yeast extract, M,J and chitosan. MJ, a proven signal compound, is the most effective elicitor of taxol production in Taxus chinensis Roxb.[16] and gonsenoside production in P. ginseng C.A. Meyercell/organ culture.[17,18,19]
The involvement of amino acids in the biosynthesis of hyperforin and adhyperforin was reported in H. perforatum shoot cultures. Valine and isoleucine, upon administration to the shoot cultures, were incorporated into acyl side chain of hyperforin and adhyperforin, respectively. Feeding the shoot cultures with unlabelled lisoleucine at a concentration of 2 mM induced a 3-7-fold increase in the production of a hyperforin.[20] Production of triterpenes in leaf-derived callus and cell suspension cultures of Centella asiatica was enhanced by the feeding of amino acids. In the callus culture, manifold increase of asiaticoside accumulation was reported with the addition of leucine.[21]
Elicitation of In vitro products
Plants and/or plant cells in vitro show physiological and morphological responses to microbial, physical, or chemical factors which are known as “elicitors.” Elicitation is a process of inducing or enhancing synthesis of secondary metabolites by the plants to ensure their survival, persistence, and competitiveness.[11,22] The study was applied in several abiotic elicitors to enhance growth and ginseng saponin biosynthesis in the hairy roots of P. ginseng.[23] Generally, elicitor treatments were found to inhibit the growth of the hairy roots, although simultaneously enhancing ginseng saponin biosynthesis. Tannic acid profoundly inhibited the hairy root growth during growth period.
The production of secondary metabolites in callus, cell suspension, and hairy roots of Ammi majus L. is by exposing them to elicitors: benzo (1,2,3)-thiadiazole-7-carbothionic acid S-methyl ester and autoclaved lysate of cell suspension of bacteria- Enterobacter sakazaki.[24] GC and GC-MS analysis of chloroform and methanol extracts indicated a higher accumulation of umbelliferone in the elicited tissues than in the control ones. Chitosan was the biotic elicitor polysaccharide and it is eliciting the manifold increase of anthraquinone production in Rubia akane cell culture.[25]
Hairy Root Cultures as a Source of Secondary Metabolites
The hairy root system based on inoculation with Agrobacterium rhizogenes has become popular in the two last decades as a method of producing secondary metabolites synthesized in plant roots.[11,26] The hairy root phenotype is characterized by fast hormone-independent growth, lack of geotropism, lateral branching, and genetic stability. The secondary metabolites produced by hairy roots arising from the infection of plant material by A. rhizogenes are the same as those usually synthesized in intact parent roots, with similar or higher yields.[27] This feature, together with genetic stability and generally rapid growth in simple media lacking phytohormones, makes them especially suitable for biochemical studies not easily undertaken with root cultures of an intact plant. During the infection process, A. rhizogenes transfers a part of the DNA (transferred DNA, T-DNA) located in the root-inducing plasmid Ri to plant cells, and the genes contained in this segment are expressed in the same way as the endogenous genes of the plant cells. Some A. rhizogenes, such as strain A4, have the T-DNA divided into two sections: the TR-DNA and TL-DNA, each of which can be incorporated separately into the plant genome. Two sets of pRi genes are involved in the root induction process: the aux genes located in the TR region of the pRi T-DNA and the rol (root loci) genes of the TL region.[28] The hairy roots are normally induced on aseptic, wounded parts of plants by inoculating them with A. rhizogenes.
Genetic Manipulation in Hairy Root Culture for Secondary Metabolite Production
Transformed roots provide a promising alternative for the biotechnological exploitation of plant cells. A. rhizogenes-mediated transformation of plants may be used in a manner analogous to the well-known procedure employing Agrobacterium tumifaciens. A. rhizogenes-mediated transformation has also been used to produce transgenic hairy root cultures and plantlets have been regenerated.[11] None of the other T-DNA sequences are required for the transfer with the exception of the border sequences. The rest of the T-DNA can be replaced with the foreign DNA and introduced into cells from which whole plants can be regenerated. These foreign DNA sequences are stably inherited in a Mendelian manner.[29] The A. rhizogenes-mediated transformation has the advantage of being able to transfer any foreign gene of interest placed in binary vector to the transformed hairy root clone. An example of a gene of interest with regard to secondary metabolism that was introduced into hairy roots is the 6-hydroxylase gene of Hyoscyamus muticus which was introduced to hyocyamin-rich Atropa belladonna by a binary vector system using A. rhizogenes.[30] Engineered roots showed an increased amount of enzyme activity and a five-fold higher concentration of scopolamine.
Role of Endophytes in In vitro Production of Secondary Metabolites
There are three schools of thought on the origins of secondary metabolism in plants.[11,31] There is the argument that both plants and endophytic microbes coevolved with pathways to produce these natural products. Another thought is that an ancient horizontal gene transfer took place between plants and microbes. The third suggests that either plants or endophytic fungi produce these secondary metabolites and transfer them to the other symbiont. Biosynthetic pathway studies using radiolabeled precursor amino acids reveal that plants and endophytic fungi have similar but distinct metabolic pathways for production of secondary metabolites.[32] The question is whether bioactive phytochemicals of plants are produced by the plant itself or as a consequence of a mutualistic relationship with beneficial organisms in their tissue. The fact that a combination of inducing factors from both plants and endophytic fungi increased the accumulation of secondary metabolites in plants and fungi, respectively,[33,34] suggest that the fungal endophyte may play important roles in the biosynthesis of secondary metabolites. Therefore, the symbiotic association and effects of plants and endophytes on each other during the production of other important pharmacological bioactive natural products such as camptothecin, vinblastine, and podophyllotoxin need to be explored. This could provide the framework for future natural product production through genetic and metabolic engineering.[35]
Bioreactors Scaling up of Production of Secondary Metabolites
This is the application of bioreactor system for large-scale cultivation of plant cells for the production of valuable bioactive compounds in an active field. Plant cells in liquid suspension offer a unique combination of physical and chemical environments that must be accommodated in large-scale bioreactor process.
Immobilization Scaling up of Secondary Metabolite Accumulation
Advances in scale-up approaches and immobilization techniques contribute to a considerable increase in the number of applications of plant cell cultures for the production of compounds with a high added value. Plant-derived compounds with cancer chemotherapeutic or antioxidant properties use rosmarinic acid and taxol as representative examples.
Cell cultures of Plumbago rosea were immobilized in calcium alginate and cultured in Murashige and Skoog's basal medium containing 10 mM CaCl2 for the production of plumbagin, an important medicinal compound.[36] Studies were carried to find out the impact of immobilization on the increased accumulation of this secondary metabolite. Immobilization in calcium alginate enhanced the production of plumbagin by three-, two-, and one-folds compared with that of control, un-crosslinked alginate and CaCl2 -treated cells, respectively.
Tissue Cultures Producing Pharmaceutical Products of Interest
Research in the area of plant tissue culture technology has resulted in the production of many pharmaceutical substances for new therapeutics. Advances in the area of cell cultures for the production of medicinal compounds have made possible the production of a wide variety of pharmaceuticals such as alkaloids, terpenoids, steroids, saponins, phenolics, flavanoids, and amino acids. Successful attempts to produce some of these valuable pharmaceuticals in relatively large quantities by cell cultures are illustrated.[37,9]
Taxol
Taxol (paclitaxel), a complex diterpene alkaloid found in the bark of the Taxus tree, is one of the most promising anticancer agents known due to its unique mode of action on the microtubular cell system Figure 1.[38] At present, production of taxol by various Taxus species cells in cultures has been one of the most extensively explored areas of plant cell cultures in recent years owing to the enormous commercial value of taxol, the scarcity of the Taxus tree, and the costly synthetic process.[39,40] In order to increase the taxoid production in these cultures, the addition of different amino acids to the culture medium was studied, and phenylalanine was found to assist in maximum taxol production in Taxus cuspidata cultures.[41] The influence of biotic and abiotic elicitors was also studied to improve the production and accumulation of taxol through tissue cultures.[42–44]
Figure 1.
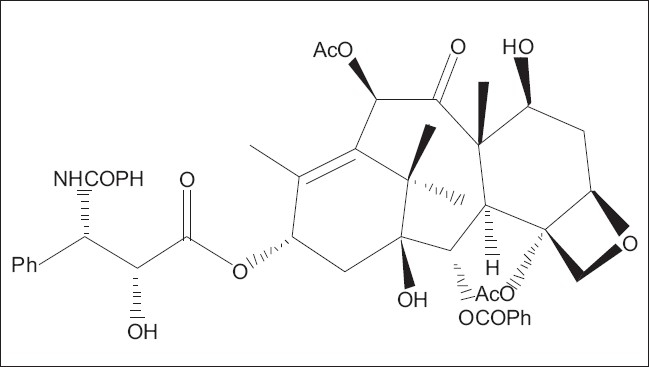
Chemical structure of taxol
Morphine and codeine
Latex from the opium poppy, Papaver somniferum, is a commercial source of the analgesics, morphine, and codeine. Callus and suspension cultures of P. somniferum are being investigated as an alternative means for the production of these compounds [Figure 2]. Production of morphine and codeine in morphologically undifferentiated cultures has been reported.[45,46] Without exogenous hormones, maximum codeine and morphine concentrations were 3.0 mg/g dry weight and 2.5 mg/g dry weight, respectively, up to three times higher than in cultures supplied with hormones. Biotransformation of codeinone to codeine with immobilized cells of P. somniferum has been reported by Furuya et al. (1972).[47] The conversion yield was 70.4%, and about 88% of the codeine converted was excreted into the medium.
Figure 2.
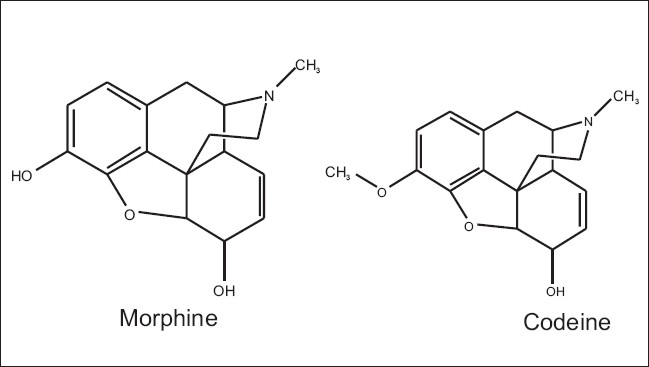
Chemical structure of morphine and codeine
L-DOPA
L-3, 4-dihydroxyphenylalanine, is an important intermediate of secondary metabolism in higher plants and is known as a precursor of alkaloids, betalain, and melanine, isolated from Vinca faba,[48] Mucuna, Baptisia, and Lupinus.[49] It is also a precursor of catecholamines in animals and is being used as a potent drug for Parkinson's disease, a progressive disabling disorder associated with a deficiency of dopamine in the brain [Figure 3]. The widespread application of this therapy created a demand for large quantities of L-DOPA at an economical price level and this led to the introduction of cell cultures as an alternative means for enriched production. Brain[49] found that the callus tissue of Mucuna pruriense accumulated 25 mg/l DOPA in the medium containing relatively high concentrations of 2, 4-D. Teramoto and Komamine (1988) induced callus tissues of Mucuna hassjoo, M. Pruriense, and Mucuna deeringiana and optimized the culture conditions. The highest concentration of DOPA was obtained when M. hassjoo cells were cultivated in MS medium with 0.025 mg/l 2.4-D and 10 mg/l kinetin.
Figure 3.
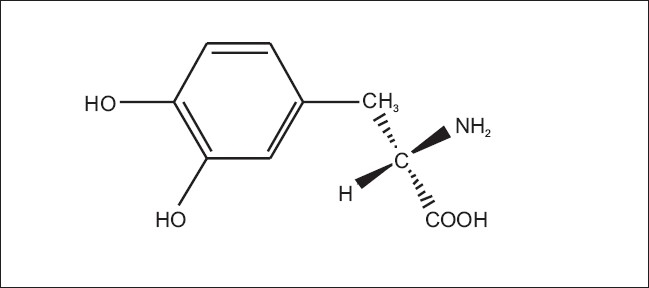
Chemical structure of 3-(3’, 4’-dihydroxyphenyl)-L-alanine (L-DOPA)
Diosgenin
Diosgenin is a precursor for the chemical synthesis of steroidal drugs and is tremendously important to the pharmaceutical industry.[50] In 1983, Tal et al.[51] reported on the use of cell cultures of Dioscorea deltoidea for the production of diosgenin [Figure 4]. They found that carbon and nitrogen levels greatly influenced diosgenin accumulation in one cell line. Ishida (1988) established Dioscorea immobilized cell cultures, in which reticulated polyurethane foam was shown to stimulate diosgenin production, increasing the cellular concentration by 40% and total yield by 25%. Tal et al.[50] have been able to obtain diosgenin levels as high as 8% in batch-grown D. deltoidea cell suspensions.
Figure 4.
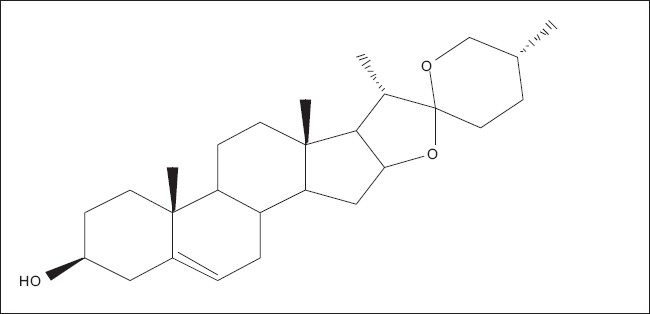
Chemical structure of diosgenin
Capsaicin
Capsaicin, an alkaloid, is used mainly as a pungent food additive in formulated foods.[52] It is obtained from fruits of green pepper (Capsicum spp.). Capsaicin is also used in pharmaceutical preparations as a digestive stimulant and for rheumatic disorders.[53] Suspension cultures of Capsicum frutescens produce low levels of capsaicin, but immobilizing the cells in reticulated polyurethane foam can increase production approximately 100-fold. Further improvements in productivity can be brought about by supplying precursors such as isocapric acid. Lindsey[54] reported that treatments, which suppress cell growth and primary metabolism, seem to improve capsaicin synthesis. A biotechnological process has been developed for the production of capsaicin from C. frutescens cells. Holden et al.[55] have reported elicitation of capsaicin in cell cultures of C. frutescens by spores of Gliccladium deliquescens. The effects of nutritional stress on capsaicin production in immobilized cell cultures of Capsicum annum were studied thoroughly by Ravishankar and Ramachandra Rao.[56] Biotransformation of externally fed protocatechuic aldehyde and caffeic acid to capsaicin in freely suspended cells and immobilized cells cultures of Capsicum frutescens has also been reported.[57]
Camptothecin
Campothecin, a potent antitumor alkaloid, was isolated from Camptotheca acuminata.[58] Sakato and Misawa[59] induced C. acuminata callus on MS medium containing 0.2 mg/l 2,4-D and l mg/l kinetin and developed liquid cultures in the presence of gibberellin, l-tryptophan, and conditioned medium, which yielded camptothecin at about 0.0025% on a dry weight basis. When the cultures were grown on MS medium containing 4 mg/l NAA, accumulation of camptothecin reached 0.998 mg/l.[60]
Berberine
Berberine is an isoquinoline alkaloid found in the roots of Coptis japonica and cortex of Phellondendron amurense. This antibacterial alkaloid has been identified from a number of cell cultures, notably those of C. japonica,[61,62,63] Thalictrum spp.,[64,65] and Berberis spp.[62] The productivity of berberine was increased in cell cultures by optimizing the nutrients in the growth medium and the levels of phytohormones.[63,66,67] By selecting high-yielding cell lines, Mitsui group produced berberine on a large scale with a productivity of 1.4 g/l over 2 weeks. Other methods for increasing yields include elicitation of cultures with a yeast polysaccharide elicitor, which has been successful with a relatively low-producing Thalictrum rugosum culture.[68] The influence of spermidine on berberine production in Thalictrum minus cell cultures has been reported by Hara et al.[69]
Metabolic Engineering and Production of Secondary Metabolites
Metabolic engineering involves the targeted and purposeful alteration of metabolic pathways found in an organism to achieve better understanding and use of cellular pathways for chemical transformation, energy transduction, and supramolecular assembly.[70] This technique applied to plants will permit endogenous biochemical pathways to be manipulated and results in the generation of transgenic crops in which the range, scope, or nature of a plant's existing natural products are modified to provide beneficial commercial, agronomic, and/or postharvest processing characteristics.[71]
As in many cases production is too low for commercialization, metabolic engineering can provide various strategies to:
Improve productivity, such as increasing the number of producing cells.
Increasing the carbon flux through a biosynthetic pathway by overexpression of genes.
Codify for rate-limiting enzymes or blocking the mechanism of feedback inhibition and competitive pathways.
Decrease catabolism.
Several genes in the biosynthetic pathways for scopolamine, nicotine, and berberine have been cloned, making the metabolic engineering of these alkaloids possible. Expression of two branching-point enzymes was engineered: putrescine N-methyltransferase (PMT) in transgenic plants of Atropa belladonna and Nicotiana sylvestris and (S)-scoulerine 9-O-methyltransferase (SMT) in cultured cells of C. japonica and Eschscholzia californica. Overexpression of PMT increased the nicotine content in N. sylvestris, whereas suppression of endogenous PMT activity severely decreased the nicotine content and induced abnormal morphologies. Ectopic expression of SMT caused the accumulation of benzylisoquinoline alkaloids in E. californica.[72]
Metabolic Engineering of Yeast for the Production of Plant Secondary Metabolites
Metabolic engineering is the alteration of cellular activities by the manipulation of enzymatic, transport, and regulatory functions of the cell by using recombinant DNA technology. Difficulty to obtain sufficient amounts of desired plant, slow growth of plants, varying composition and concentration depending on the geographical position and climatic conditions, and low yield of isolated compounds are some of the limitations of commercial extraction of these compounds by using plant as a single resource. On the other hand, some of the bottlenecks of chemical synthesis may include higher energy requirements, pollution, low reaction load due to unwanted chemical reactions, cost and availability of starting materials, and cost of separating and purifying the end products. For the metabolic engineering of plant secondary metabolites, it is necessary to know the biosynthetic pathways of those compounds, rate-limiting steps, and enzymes involved [Figures 5 and 6].
Figure 5.
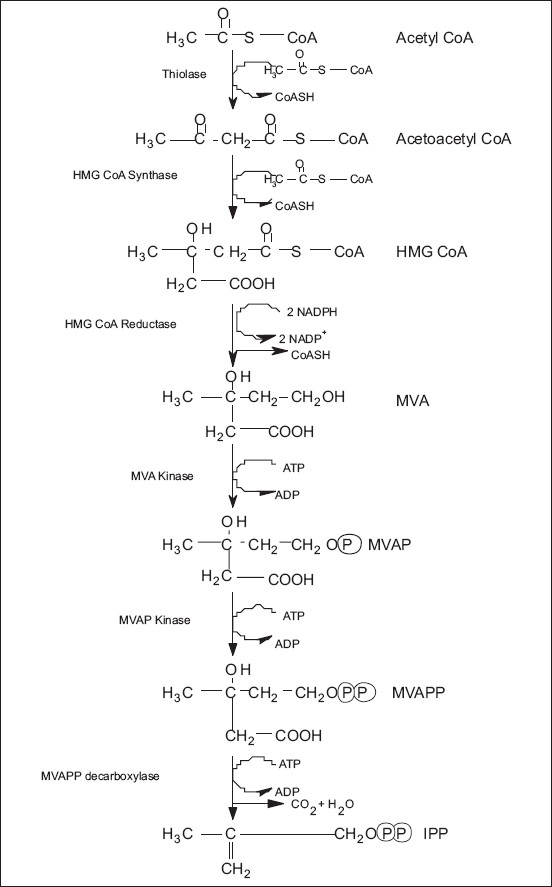
The acetate/mevalonate pathway for the formation of IPP, the basic five-carbon unit of terpenoid biosynthesis. Synthesis of each IPP unit requires three molecules of acetyl-CoA
Figure 6.
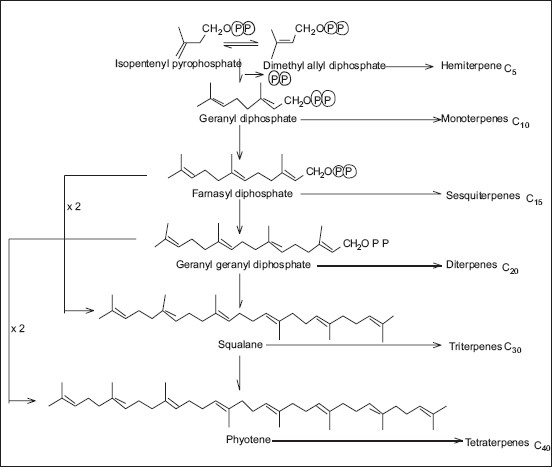
The major subclasses of terpenoids are biosynthesized from the basic five-carbon unit, IPP, and from the initial prenyl (allylic) diphosphate, dimethylallyl diphosphate, which is formed by isomerization of IPP. In reactions catalyzed by prenyltransferases, monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20) are derived from the corresponding intermediates by sequential head-to-tail addition of C5 units. Triterpenes (C30) are formed from two C15 (farnesyl) units joined head-to-head, and tetraterpenes (C40) are formed from two C20 (geranylgeranyl) units joined head-to-head
-
i
Production of flavonoids in yeast
-
ii
Production of terpenoids in yeast
- Production of monoterpenoids in yeast
- Production of sesquiterpenes in yeast
- Production of carotenoids in yeast
-
iii
Production of plant-origin alkaloids in yeast
Production of Flavonoids in Yeast
Flavonoids are produced in yeast by expressing phenylpropanoid pathway. Many flavonoid compounds are successfully produced in yeast by cloning genes from different plant species and microorganisms. Flavanone has been successfully produced in yeast by expressing phenyl ammonia lyase (PAL), cinnamate-4-hydroxylase (C4H), 4-coumarate-CoA (4CL), and chalcone synthase (CHS) genes.[73] Flavones have also been produced in flavanone-producing recombinant yeast by expressing flavone synthase I (FSI) and flavone synthase II (FSII) genes.
Production of terpenoids in yeast
The biosynthesis of terpenes in higher plant cells shows two entirely separate enzymatic pathways: mevalonic acid pathway (MVA) and methylerythritol 4-phosphate pathway [Figure 5]. In yeast, only MVA pathway is involved in the biosynthesis of ergosterol as the major end product.
Production of monoterpenoids in yeast
Oswald et al.[74] engineered yeasts to produce monoterpenoids by expressing linalool synthase and geraniol synthase genes, and yeast strains successfully produced those monoterpenoid alcohols by using internal geranyl pyrophosphate.
Production of sesquiterpenes in yeast
Sesquiterpenes are the most diverse class of isoprenoids; some of them are interesting and extremely important compounds in human health because of their potent anticancer, antitumor, cytotoxic, antiviral, and antibiotic properties. Amorphadiene, a sesquiterpene of the antimalarial drug artemisin, is synthesized by the cyclization of farnesyl pyrophosphate (FPP). Ro et al.[75] used Saccharomyces cerevisiae to produce high amount of artemisinic acid using an engineered mevalonate pathway, amorphadiene synthase, and a novel cytochrome P450 monooxygenase from Artemisia annua.
Production of carotenoids in yeast
Schizosaccharomyces pombe, a noncarotenogenic yeast, is not able to produce any carotenoids but it synthesizes ergosterol from FPP through the sterol biosynthetic pathway. Gunel et al.[76] cloned a gene encoding geranyl geranyl pyrophosphate synthase from bell pepper (C. annum) in S. pombe and successfully redirected carbon flow from the terpenoid pathway leading to ergosterol formation toward the production of carotenoid through the heterologous expression of carotenoid biosynthetic gene in a noncarotenogenic yeast, S. pombe.
Production of plant-origin alkaloids in yeast
Geerlings et al.[77] expressed genes coding for strictosidine synthase and strictosidine glucosidase enzymes from medicinal plant C. roseus in S. cerevisiae and successfully produced cathenamine from tryptamine and secologanin by functionally expressing those two enzymes in yeast. Along with the increased knowledge on biosynthetic pathways of many plant secondary metabolites, utilities of different yeast species should be investigated in future for the efficient microbial production of such compounds. Overcoming rate-limiting steps, reducing flux through competitive pathways, reducing catabolism, and over expression of regulatory genes are some of the strategies that can be used for increased secondary metabolites production through metabolic engineering.
Conclusion
In future, metabolic engineering and biotechnological approaches can be used as an alternative production system to overcome the limited availability of biologically active, commercially valuable, and medicinally important plant secondary metabolite compounds. Advances in biotechniques, particularly methods for culturing plant cell cultures, should provide new means for the commercial processing of even rare plants and the chemicals they provide. The advantage of this method is that it can ultimately provide a continuous, reliable source of natural products. The major advantage of the cell cultures includes synthesis of bioactive secondary metabolites, running in controlled environment, independently from climate and soil conditions. The use of in vitro plant cell culture for the production of chemicals and pharmaceuticals has made great strides building on advances in plant science. The increased use of genetic tools and an emerging picture of the structure and regulation of pathways for secondary metabolism will provide the basis for the production of commercially acceptable levels of product. Knowledge of biosynthetic pathways of desired phytochemicals in plants and in cultures is often still in its infancy, and consequently strategies needed to develop an information based on a cellular and molecular level. These results show that in vitro plant cell cultures have potential for commercial production of secondary metabolites. The introduction of newer techniques of molecular biology, so as to produce transgenic cultures and to effect the expression and regulation of biosynthetic pathways, is also likely to be a significant step toward making cell cultures more generally applicable to the commercial production of secondary metabolites. The commercial values of plant secondary metabolites have been the main impetus for the enormous research effort put into understanding and manipulating their biosynthesis using various chemical, physiological, and biotechnological pathways. In vitro propagation of medicinal plants with enriched bioactive principles and cell culture methodologies for selective metabolite production is found to be highly useful for commercial production of medicinally important compounds. The increased use of plant cell culture systems in recent years is perhaps due to an improved understanding of the secondary metabolite pathway in economically important plants. Advances in plant cell cultures could provide new means for the cost-effective, commercial production of even rare or exotic plants, their cells, and the chemicals that they will produce. Knowledge of the biosynthetic pathways of desired compounds in plants as well as of cultures is often still rudimentary, and strategies are consequently needed to develop information based on a cellular and molecular level. A key to the evaluation of strategies to improve productivity is the realization that all the problems must be seen in a holistic context. At any rate, substantial progress in improving secondary metabolite production from plant cell cultures has been made within last few years. These new technologies will serve to extend and enhance the continued usefulness of higher plants as renewable sources of chemicals, especially medicinal compounds. We hope that a continuation and intensification efforts in this field will lead to controllable and successful biotechnological production of specific, valuable, and as yet unknown plant chemicals.[102]
Acknowledgments
We are grateful to Dr. K.F.H. Nazeer Ahamed, Assistant Professor, Department of Pharmacology, Vel's College of Pharmacy, Chennai, for his assistance and encouragement. We extend our sincere thanks to Mr. Md. Zaheen Hassan Ansari, research scholar, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi, for critically reading the manuscript and providing the valuable suggestions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Canter PH, Thomas H, Ernst E. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 2005;23:180–5. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Rao SR, Ravishankar GA. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol Adv. 2002;20:101–53. doi: 10.1016/s0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 3.Rodney C, Toni M, Kutchan N, Lewis G. Natural Products. In: Buchanan B, Gruissem W, Jones R, editors. Biochemistry and molecular biology of plants. 2000. pp. 1253–348. [Google Scholar]
- 4.Vijaya SN, Udayasri PV, Aswani KY, Ravi BB, Phani KY, Vijay VM. Advancements in the production of secondary metabolites. J Nat Prod. 2010;3:112–23. [Google Scholar]
- 5.DiCosmo F, Misawa M. Plant cell and tissue culture: alternatives for metabolite production. Biotechnol Adv. 1995;13:425–53. doi: 10.1016/0734-9750(95)02005-n. [DOI] [PubMed] [Google Scholar]
- 6.Giri A, Narasu ML. Transgenic hairy roots. recent trends and applications. Biotechnol Adv. 2000;18:1–22. doi: 10.1016/s0734-9750(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 7.Hansen G, Wright MS. Recent advances in the transformation of plants. Trends Plant Sci. 1999;4:226–31. doi: 10.1016/s1360-1385(99)01412-0. [DOI] [PubMed] [Google Scholar]
- 8.Abdin MZ. Enhancing bioactive molecules in medicinal plants. In: Zhu Y, Tan B, Bay B, Liu C, editors. Natural Products-Essential resources for human. Singapore: World Scientific Publishing Co. Pvt. Ltd; 2007. pp. 45–57. [Google Scholar]
- 9.Abdin MZ, Kamaluddin A. Traditional systems of medicine. India: Publishing House Pvt. Ltd; 2006. Improving quality of medicinal herbs through physico-chemical and molecular approaches; pp. 30–9. [Google Scholar]
- 10.Sajc LD, Grubisic G, Vunjak Novakovic. Bioreactors for plant engineering: an outlook for further research. Biochem Eng J. 2000;4:89–99. [Google Scholar]
- 11.Karuppusamy S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res. 2009;3:1222–39. [Google Scholar]
- 12.Davioud E, Kan C, Hamon J, Tempe J, Husson HP. Production of indole alkaloids by in vitro root cultures from Catharanthus trichophyllus. Phytochemistry. 1989;28:2675–80. [Google Scholar]
- 13.Smetanska I. Production of secondary metabolites using plant cell cultures. Adv Biochem Eng Biotechnol. 2008;111:187–228. doi: 10.1007/10_2008_103. [DOI] [PubMed] [Google Scholar]
- 14.Gao SL, Zhu DN, Cai ZH, Jiang Y, Xu DR. Organ culture of a precious Chinese medicinal plant - Fritillaria unibracteata. Plant Cell Tiss Org Cult. 2004;59:197–201. [Google Scholar]
- 15.Namdeo AG. Plant cell elicitation for production of secondary metabolites: A review. Pharmacog Rev. 2007;1:69–79. [Google Scholar]
- 16.Wink M, Alfermann AW, Franke R, Wetterauer B, Distl M, Windhovel J, et al. Sustainable bioproduction of phytochemicals by plant in vitro cultures: anticancer agents. Plant Genetic Resour. 2008;12:113–23. [Google Scholar]
- 17.Xu H, Kim YK, Suh SY, Udin MR, Lee SY, Park SU. Deoursin production from hairy root culture of Angelica gigas. J Korea Soc Appl Biol Chem. 2008;51:349–51. [Google Scholar]
- 18.Yagi A, Shoyama Y, Nishioka I. Formation of tetrahydroanthrcence glucosides by callus tissue of Aloe saponaria. Phytochemistry. 1983;22:1483–4. [Google Scholar]
- 19.Yamanaka M, Ishibhasi K, Shimomura K, Ishimaru K. Polyacetylene glucosides in hairy root cultures of Lobelia cardinalis. Phytochemistry. 1996;41:183–5. [Google Scholar]
- 20.Kim OT, Kim MY, Hong MH, Ahn JC, Hwang B. Stimulation of asiticoside accumulation in the whole plant cultures of Centella asiatica (L.) urban by elicitors. Plant Cell Rep. 2004;23:339–44. doi: 10.1007/s00299-004-0826-7. [DOI] [PubMed] [Google Scholar]
- 21.Karppinen K, Hokkanen J, Tolonen A, Mattila S, Hohtola A. Biosynthesis of hyperforin and adhyperforin from amino acid precursors in shoot cultures of Hypericum perforatum. Phytochemistry. 2007;68:1038–45. doi: 10.1016/j.phytochem.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Kiong AL, Mahmood M, Fodzillan NM, Daud SK. Effects of precursor supplementation on the production of triterpenes by Centella asiatica callus culture. Pak J Biol Sci. 2005;8:1160–9. [Google Scholar]
- 23.Jeong GA, Park DH. Enhanced secondary metabolite biosynthesis by elicitation in transformed plant root system: effect of abiotic elicitors. Appl Biochem Biotechnol. 2006;129:436–46. doi: 10.1385/abab:130:1:436. [DOI] [PubMed] [Google Scholar]
- 24.Staniszewska I, Krolicka A, Mali E, Ojkowska E, Szafranek J. Elicitation of secondary metabolites in in vitro cultures of Ammi majus L. Enzyme Microbiol Technol. 2003;33:565–8. [Google Scholar]
- 25.Jin JH, Shin JH, Kim JH, Chung IS, Lee HJ. Effect of chitosan elicitation and media components on the production of anthraquinone colorants in madder (Rubia akane Nakai) cell culture. Biotechnol Bioprocess Eng. 1999;4:300–4. [Google Scholar]
- 26.Palazon J, Pinol MT, Cusido RM, Morales C, Bonfill M. Application of transformed root technology to the production of bioactive metabolites. Recent Res Dev Plant Phys. 1997;1:125–43. [Google Scholar]
- 27.Sevón N, Oksman-Caldentey KM. Agrobacterium rhizogenes-mediated transformation: root cultures as a source of alkaloids. Planta Med. 2002;68:859–68. doi: 10.1055/s-2002-34924. [DOI] [PubMed] [Google Scholar]
- 28.Jouanin L. Restriction map of an agropine-type Ri plasmid and its homologies with Ti plasmids. Plasmid. 1984;12:91–102. doi: 10.1016/0147-619x(84)90055-6. [DOI] [PubMed] [Google Scholar]
- 29.Zambryski P, Tempe J, Schell J. Transfer and function of T-DNA genes from Agrobacterium Ti and Ri plasmids in plants. Cell. 1989;56:193–201. doi: 10.1016/0092-8674(89)90892-1. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto T, Yun DJ, Yamada Y. Production of tropane alkaloids in genetically engineered root cultures. Pyhtochemistry. 1993;32:713–8. [Google Scholar]
- 31.Jennewein S, Rithner CD, Williams RM, Croteau RB. Taxol biosynthesis: Taxane 13 alpha-hydroxylase is a cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci U S A. 2001;98:13595–600. doi: 10.1073/pnas.251539398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang P, Zhou PP, Yu LJ. An endophytic taxol-producing fungus from Taxus media, Cladosporium cladosporioides MD2. Curr Microbiol. 2009;59:227–32. doi: 10.1007/s00284-008-9270-1. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Li M, Yang DL, Xu R, Zhang Y. Production of podophyllotaxin by root culture of Podophyllum hexandrum Royle. Electron J Biol. 2009;5:34–9. [Google Scholar]
- 34.Engels B, Dahm P, Jennewein S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab Eng. 2008;10:201–6. doi: 10.1016/j.ymben.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Komaraiah P, Ramakrishna SV, Reddanna P, Kavi Kishor PB. Enhanced production of plumbagin in immobilized cells of Plumbago rosea by elicitation and it situ adsorption. J Biotechnol. 2003;10:181–7. doi: 10.1016/s0168-1656(02)00338-3. [DOI] [PubMed] [Google Scholar]
- 36.Vanisree M, Chen YL, Shu-Fung L, Satish MN, Chien YL, HsinSheng T. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bott Bull Acad Sin. 2004;45:1–22. [Google Scholar]
- 37.Jordon MA, Wilson L. Microtuble polymerization dynamics, mitotic, and cell death by paclitaxel at low concentration. Am Chem Soc Symp Ser. 1995;583:138–53. [Google Scholar]
- 38.Cragg GM, Schepartz SA, Suffness M, Grever MR. The taxol supply crisis.New NCI policies for handling the large-scale production of novel natural product anticancer and anti-HIV agents. J Nat Prod. 1993;56:1657–68. doi: 10.1021/np50100a001. [DOI] [PubMed] [Google Scholar]
- 39.Suffness M. Taxol: Science and Applications. Boca Raton, FL: CRC Press; 1995. [Google Scholar]
- 40.Fett-Neto AG, Stewart JM, Nicholson SA, Pennington JJ, DiCosmo F. Improved taxol yield by aromatic carboxylic acid and and amino acid feeding to cell cultures of T. cuspidata. Biotechnol Bioeng. 1994;44:967–71. doi: 10.1002/bit.260440813. [DOI] [PubMed] [Google Scholar]
- 41.Ciddi V, Srinivasan V, Shuler VM. Elicitation of Taxus cell cultures for production of taxol. Biotechnol Lett. 1995;17:1343–6. [Google Scholar]
- 42.Strobel GA, Stierle A, van Kuijk JG. Factors influencing the in vitro production of radiolabelled taxol by Pacific yew, Taxus brevifolia. Plant Sci. 1992;84:65–74. [Google Scholar]
- 43.Yukimune Y, Tabata H, Higashi Y, Hara Y. Methyljasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat Biotechnol. 1996;14:1129–32. doi: 10.1038/nbt0996-1129. [DOI] [PubMed] [Google Scholar]
- 44.Tam WH, Constabel F, Kurz WG. Codeine from cell suspension cultures of Papaver somniferum. Phytochemistry. 1980;19:486–7. [Google Scholar]
- 45.Yoshikawa T, Furuya T. Morphinan alkaloid production by tissues differentiated from cultured cells of Papaver somniferum (1) Planta Med. 1985;2:110–3. [Google Scholar]
- 46.Furuya T, Ikuta A, Syono K. Alkaloids from callus cultures of Papaver somniferum. Phytochemistry. 1972;11:3041–4. [Google Scholar]
- 47.Guggenheim M. Dioxyphenylalanin, eine neue Aminosaure aus Vinca faba. Z Physiol Chem. 1913;88:276. [Google Scholar]
- 48.Daxenbichler ME, VanEtten CH, Hallinan EA, Earle FR, Barclay AS. Seeds as sources of L-DOPA. J Med Chem. 1971;14:463–5. doi: 10.1021/jm00287a030. [DOI] [PubMed] [Google Scholar]
- 49.Brain KR, Lockwood GB. Hormonal control of steroid levels in tissue cultures from Trigonella foenumgraecum. Phytochemistry. 1976;15:1651–4. [Google Scholar]
- 50.Tal B, Rokem JS, Goldberg I. Factors affecting growth and product formation in plant cells grown in continuous culture. Plant Cell Rep. 1983;2:219–22. doi: 10.1007/BF00270109. [DOI] [PubMed] [Google Scholar]
- 51.Zenk MH, el-Shagi H, Schulte U. Anthraquinone production by cell suspension cultures of Morinda citrifolia. Planta Med. 1978;(Suppl):79–101. doi: 10.1055/s-0028-1104768. [DOI] [PubMed] [Google Scholar]
- 52.Ravishankar GA, Suresh B, Giridhar P, Rao SR, Johnson TS. Biotechnological studies on capsicum for metabolite production and plant improvement. In: DE, Krishna Amit., editors. The genus Capsicum. UK: Harwood Academic Publishers; 2003. pp. 96–128. [Google Scholar]
- 53.Sharma A, Kumar V, Giridhar P, Ravishankar GA. Induction of in vitro flowering in Capsicum frutescens under the influence of silver nitrate and cobalt chloride and pollen transformation. Plant Biotechnol J. 2008;11 full text-8. [Google Scholar]
- 54.Lindsey K. Manipulation by nutrient limitation of the biosynthetic activity of immobilized cells of Capsicum frutescens Mill. ev. annum. Planta. 1995;165:126–33. doi: 10.1007/BF00392221. [DOI] [PubMed] [Google Scholar]
- 55.Holden RR, Holden MA, Yeoman MM. The effects of fungal elicitation on secondary metabolism in cell cultures of Capsicum frutescens. In: Robins RJ, Rhodes MJC, editors. Manipulating secondary metabolism in culture. Cambridge England: Cambridge University Press; 1988. pp. 67–72. [Google Scholar]
- 56.Ravishankar GA, Ramachandra Rao. Biotechnological production of phytopharmaceuticals. J Biochem Mol Biol Biophys. 2000;4:73–102. [Google Scholar]
- 57.Sanatombi K, Sharma GJ. Micropropagation of Capsicum frutescens L. using axillary shoot explants. Sci Hortic. 2007;113:96–9. [Google Scholar]
- 58.Padmanabha BV, Chandrashekar M, Ramesha BT, Hombe Gowda HC, Rajesh PG, Suhas S, et al. Patterns of accumulation of camptothecin, an anti-cancer alkaloids in Nothapodytes nimmoniana Graham, in the Western Ghats, India: Implications for identifying high-yielding sources of the alkaloid. Curr Sci. 2006;90:95–100. [Google Scholar]
- 59.Sakato K, Misawa M. Effects of chemical and physical conditions on growth of Camptotheca acuminata cell cultures. Agri Biol Chem. 1974;38:491–7. [Google Scholar]
- 60.Thengane SR, Kulkarni DK, Shrikhande VA, Joshi SP, Sonawane KB, Krishnamurthy KV. Influence of medium composition on callus induction and camptothecin(s) accumulation in Nothapodytes foetida. Plant Cell Tiss Org Cult. 2003;72:247–51. [Google Scholar]
- 61.Vanisree M, Tsay HS. Plant Cell Cultures - An Alternative and Efficient Source for the Production of Biologically Important Secondary Metabolites. International J Appl Sci Engin. 2004;2:29–48. [Google Scholar]
- 62.Breuling M, Alfermann AW, Reinhard E. Cultivation of cell cultures of Berberis wilsonae in 20l airlift bioreactors. Plant Cell Rep. 1985;4:220–3. doi: 10.1007/BF00269294. [DOI] [PubMed] [Google Scholar]
- 63.Sato F, Yamada Y. High berberine producing cultures of Coptis japonica cells. Phytochemistry. 1984;23:281–5. [Google Scholar]
- 64.Nakagawa K, Konagai A, Fukui H, Tabata M. Release and crystalization of berberine in the liquid medium of Thalictrum minus cell suspension cultures. Plant Cell Rep. 1984;3:254–7. doi: 10.1007/BF00269306. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki M, Nakagawa K, Fukui H, Tabata M. Alkaloid production in cell suspension cultures of Thalictrum flavum and T. dipterocarpum. Plant Cell Rep. 1988;7:26–9. doi: 10.1007/BF00272971. [DOI] [PubMed] [Google Scholar]
- 66.Nakagawa K, Fukui H, Tabata M. Hormonal regulation of berberine production in cell suspension cultures of Thalictrum minus. Plant Cell Rep. 1986;5:69–71. doi: 10.1007/BF00269722. [DOI] [PubMed] [Google Scholar]
- 67.Morimoto T, Hara Y, Kato Y, Hiratsuka J, Yoshioka T, Fujita Y, et al. Berberine production by cultured Coptis japonica cells in one-stage culture using medium with a high copper concentration. Agri Biol Chem. 1988;52:1835–6. [Google Scholar]
- 68.Funk C, Gugler K, Brodelius P. Increased secondary product formation in plant cell suspension cultures after treatment with a yeast carbohydrate preparation (elicitor) Phytochemistry. 1987;26:401–5. [Google Scholar]
- 69.Hara M, Kobayashi Y, Fukui H, Tabata M. Enhancement of berberine production by spermidine in Thalictrum minus cell suspension cultures. Plant Cell Rep. 1991;10:494–7. doi: 10.1007/BF00234580. [DOI] [PubMed] [Google Scholar]
- 70.Lessard P. Metabolic engineering: the concept coalesces. Nat Biotechnol. 1996;14:1654–5. doi: 10.1038/nbt1296-1654. [DOI] [PubMed] [Google Scholar]
- 71.Kinney AJ. Manipulating flux through plant metabolic pathways. Curr Opin Plant Biol. 1998;1:173–8. doi: 10.1016/s1369-5266(98)80021-6. [DOI] [PubMed] [Google Scholar]
- 72.Sato F, Hashimoto T, Hachiya A, Tamura K, Choi KB, Morishige T, et al. Metabolic engineering of plant alkaloid biosynthesis. Proc Natl Acad Sci U S A. 2001;2:367–72. doi: 10.1073/pnas.011526398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan A, Kohli A, Koffas MA. Biosynthesis of natural flavanones in Saccharomyces cerevisiae. Appl Environ Microbiol. 2005;71:5610–3. doi: 10.1128/AEM.71.9.5610-5613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oswald M, Fischer M, Dirninger N, Karst F. Monoterpenoid biosynthesis in Saccharomyces cerevisiae. FEMS Yeast Res. 2007;7:413–21. doi: 10.1111/j.1567-1364.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 75.Ro DK, Paradise EM, Ouellet M. Production of the anti-malarial drug precursor artemisinic acid in engineered yeast. Nature. 2007;440:940–4. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 76.Gunel T, Kuntz M, Arda N, Erturk S, Temizkan G. Metabolic engineering for production of geranylgeranyl pyrophosphate synthase in non-carotenogenic yeast Schizosaccharomyces pombe. Biotechnol Biotechnol Eq. 2006;20:76–82. [Google Scholar]
- 77.Geerlings A, Redondo FJ, Contin A, Memelink J, van der Heijden R, Verpoorte R. Biotransformation of tryptamine and secologanin into plant terpenoid indole alkaloids by transgenic yeast. Appl Microbiol Biotechnol. 2001;56:420–4. doi: 10.1007/s002530100663. [DOI] [PubMed] [Google Scholar]
- 78.Anderson LA, Roberts MF, Phillipson JD. Studies on Ailanthus altissima cell suspension cultures. The effect of basal media on growth and alkaloid production. Plant Cell Rep. 1987;6:239–41. doi: 10.1007/BF00268489. [DOI] [PubMed] [Google Scholar]
- 79.Anderson LA, Hay CA, Roberts MF, Phillipson JD. Studies on Ailanthus altissima cell suspension cultures. Plant Cell Rep. 1986;5:387–90. doi: 10.1007/BF00268609. [DOI] [PubMed] [Google Scholar]
- 80.Malpathak NP, David SB. Flavor formation in tissue cultures of garlic (Allium sativum L) Plant Cell Rep. 1986;5:446–7. doi: 10.1007/BF00269638. [DOI] [PubMed] [Google Scholar]
- 81.Yagi A, Shoyama Y, Nishioka I. Formation of tetrahydroanthrcence glucosides by callus tissue of Aloe saponaria. Phytochemistry. 1983;22:1483–4. [Google Scholar]
- 82.Goleniowski M, Tirppi VS. Effect of growth medium composition on psilostachyinolides and altamisine production. Plant Cell Tiss Org Cult. 1999;56:215–8. [Google Scholar]
- 83.Wang PJ, Huang CI. Production of saikosaponins by callus and redifferentiated organs of Bupleurum falcatum L. In: Fujiwara, editor. Plant Tissue Culture. Tokyo: Maruzen; 1982. pp. 71–2. [Google Scholar]
- 84.Nazif NM, Rady MR, el-Nasr MM. Stimulation of anthraquinone production in suspension cultures of Cassia acutifolia by salt stress. Fitoterapia. 2000;71:34–40. doi: 10.1016/s0367-326x(99)00101-x. [DOI] [PubMed] [Google Scholar]
- 85.Moreno PR, Heijden RV, Verpoorte R. Effect of terpenoid precursor feeding and elicitation on formation of indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Rep. 1993;12:702–5. doi: 10.1007/BF00233423. [DOI] [PubMed] [Google Scholar]
- 86.Zhao J, Zhu W, Hu Q. Enhanced catharanthine production in Catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enzyme Microb Technol. 2001;28:673–81. doi: 10.1016/s0141-0229(01)00306-4. [DOI] [PubMed] [Google Scholar]
- 87.Barthe GA, Jourdan PS, McIntosh CA, Mansell RL. Naringin and limonin production in callus culture and regenerated shoots from Citrus sp. J Plant Physiol. 1987;127:55–65. [Google Scholar]
- 88.Waller GR, Mac Vean CD, Suzuki T. High production of caffeine and related enzyme activities in callus cultures of Coffea arabica L. Plant Cell Rep. 1983;2:109–12. doi: 10.1007/BF00269330. [DOI] [PubMed] [Google Scholar]
- 89.Iwasa K, Takao N. Formation of alkaloids in Corydalis ophiocarpa callus cultures. Phytochemistry. 1982;21:611–4. [Google Scholar]
- 90.Morimoto H, Murai F. The effect of gelling agents on planuotol accumulation in callus cultures of Croton sublyratus Kurz. Plant Cell Rep. 1989;8:210–3. doi: 10.1007/BF00778534. [DOI] [PubMed] [Google Scholar]
- 91.Dornenburg H, Knorr D. Semicontinuous processes for anthraquinone production with immobilized Cruciata glabra cell cultures in a three-phase system. J Biotechnol. 1999;50:55–62. [Google Scholar]
- 92.Huang WW, Cheng CC, Yeh FT, Tsay HS. Tissue culture of Dioscorea doryophora HANCE 1. Callus organs and the measurement of diosgenin content. Chin Med Coll J. 1993;2:151–60. [Google Scholar]
- 93.O’Dowd N, McCauley PG, Richardson DH, Wilson G. Callus production, suspension culture and in vitro alkaloid yields of Ephedra. Plant Cell Tiss Org Cult. 1993;34(149):55. [Google Scholar]
- 94.Tanahashi T, Zenk MH. Isoquinoline alkaloids from cell suspension cultures of Fumaria capreolata. Plant Cell Rep. 1985;4:96–9. doi: 10.1007/BF00269216. [DOI] [PubMed] [Google Scholar]
- 95.Venkateswara R, Sankara Rao, Vaidyanathan CS. Phytochemical constituents of cultured cells of Eucalyptus tereticornis SM. Plant Cell Rep. 1986;3:231–3. doi: 10.1007/BF00269127. [DOI] [PubMed] [Google Scholar]
- 96.Carrier D, Chauret N, Mancini M, Coulombe P, Neufeld R, Weber M, et al. Detection of ginkgolide A in Ginkgo biloba cell cultures. Plant Cell Rep. 1991;10:256–9. doi: 10.1007/BF00232570. [DOI] [PubMed] [Google Scholar]
- 97.Ayabe S, Takano H, Fujita T, Hirota H, Takahshi T. Titerpenoid biosynthesis in tissue cultures of Glycyrrhiza glabra var. glandulifera. Plant Cell Rep. 1990;9:181–4. doi: 10.1007/BF00232175. [DOI] [PubMed] [Google Scholar]
- 98.Arrebola ML, Ringbom T, Verpoorte R. Anthraquinones from Isoplexis isabelliana cell suspension cultures. Phytochemistry. 1999;52:1283–6. [Google Scholar]
- 99.Uden W, Pras N, Vossebeld EM, Mol JN, Malingre TM. Production of 5-methoxypodophyllotoxin in cell suspension cultures of Linum flavum L. Plant Cell Tiss Org Cult. 1990;20:81–7. [Google Scholar]
- 100.Wichers HJ, Visser JF, Huizing HJ, Pras N. Occurrence of L-DOPA and dopamine in plants and cell cultures of Mucuna pruriens and effects of 2,4-D and NaCl on these compounds. Plant Cell Tiss Org Cult. 1993;33:259–64. [Google Scholar]
- 101.Zhong JJ, Zhu QX. Effect of initial phosphate concentration on cell growth and ginsenoside saponin production by suspended cultures of Panax notoginseng. Appl Biochem Biotechnol. 1995;55:241–6. [Google Scholar]
- 102.Baumert A, Groger D, Kuzovkina IN, Reisch J. Secondary metabolites produced by callus cultures of various Ruta species. Plant Cell Tiss Org Cult. 1992;28:159–62. [Google Scholar]


