Abstract
We have cloned and characterized a novel cellular gene that is promoted by an intracisternal A-particle (IAP) LTR and expressed in the mouse placenta (mouse IAP promoted placental gene, MIPP). A 1067bp cDNA clone containing an IAP LTR U5 region duplicated in its 5' terminus and an ORF coding for a potential 202 amino acids protein was isolated from an 8.5 day old mouse embryo cDNA library. Sequence analysis of the 5' region of a genomic clone revealed the presence of a solo IAP LTR with the same U5 duplication, and primer extension analysis confirmed that transcription of the MIPP gene is under the control of the IAP LTR. Expression of the MIPP gene parallels that of IAP genes in normal mouse tissues with abundant transcripts present in the placenta and also in the myeloma MOPC-315. The MIPP-encoded protein is composed of four 48-amino acid repeat units and shares homology with a vaccinia virus gene product. MIPP-related sequences were also detected in higher eukaryotic genomes including human.
Full text
PDF

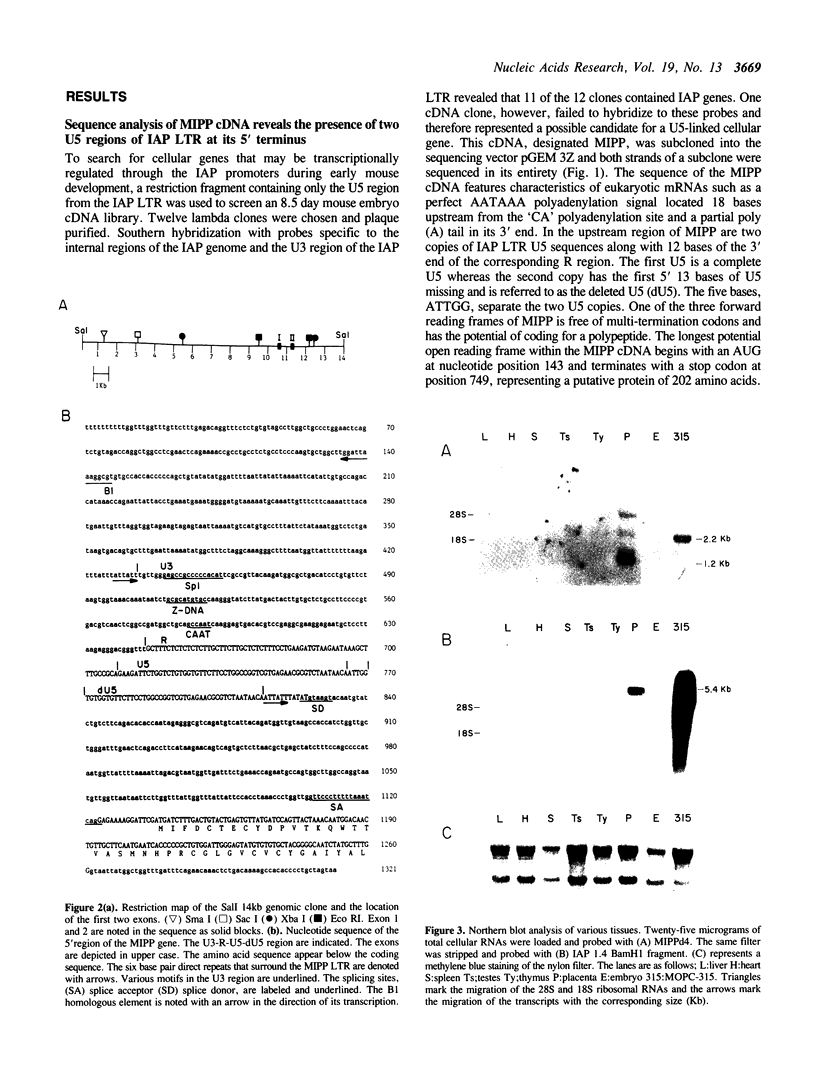


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augenlicht L. H., Kobrin D., Pavlovec A., Royston M. E. Elevated expression of an endogenous retroviral long terminal repeat in a mouse colon tumor. J Biol Chem. 1984 Feb 10;259(3):1842–1847. [PubMed] [Google Scholar]
- Banville D., Boie Y. Retroviral long terminal repeat is the promoter of the gene encoding the tumor-associated calcium-binding protein oncomodulin in the rat. J Mol Biol. 1989 Jun 5;207(3):481–490. doi: 10.1016/0022-2836(89)90458-0. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- Canaani E., Dreazen O., Klar A., Rechavi G., Ram D., Cohen J. B., Givol D. Activation of the c-mos oncogene in a mouse plasmacytoma by insertion of an endogenous intracisternal A-particle genome. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7118–7122. doi: 10.1073/pnas.80.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Huang R. C., Aaronson S. A. Genetic relatedness between intracisternal A particles and other major oncovirus genera. Virus Res. 1985 Jul;3(1):1–11. doi: 10.1016/0168-1702(85)90036-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Brown A. R., Gourlie B. B., Huang R. C. Nucleotide sequences of murine intracisternal A-particle gene LTRs have extensive variability within the R region. Nucleic Acids Res. 1985 Jan 11;13(1):289–302. doi: 10.1093/nar/13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Huang R. C. Functional analysis of the long terminal repeats of intracisternal A-particle genes: sequences within the U3 region determine both the efficiency and direction of promoter activity. Mol Cell Biol. 1988 Mar;8(3):1093–1102. doi: 10.1128/mcb.8.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. B., Unger T., Rechavi G., Canaani E., Givol D. Rearrangement of the oncogene c-mos in mouse myeloma NSI and hybridomas. Nature. 1983 Dec 22;306(5945):797–799. doi: 10.1038/306797a0. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Ono M., Huang R. C. Terminally redundant sequences in cellular intracisternal A-particle genes. J Virol. 1981 May;38(2):680–687. doi: 10.1128/jvi.38.2.680-687.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaffar I., Dianoux L., Leibovich S., Kaplan L., Emanoil-Ravier R., Peries J. Detection of IAP related transcripts in normal and transformed rat cells. Biochem Biophys Res Commun. 1990 May 31;169(1):222–231. doi: 10.1016/0006-291x(90)91457-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Furter C. S., Heizmann C. W., Berchtold M. W. Isolation and analysis of a rat genomic clone containing a long terminal repeat with high similarity to the oncomodulin mRNA leader sequence. J Biol Chem. 1989 Nov 5;264(31):18276–18279. [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Grossman Z., Mietz J. A., Kuff E. L. Nearly identical members of the heterogeneous IAP gene family are expressed in thymus of different mouse strains. Nucleic Acids Res. 1987 May 11;15(9):3823–3834. doi: 10.1093/nar/15.9.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Murialdo H., Gibson D. M., Hozumi N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7425–7429. doi: 10.1073/pnas.79.23.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F., Condamine H. Tumor viruses and early mouse embryos. Biochim Biophys Acta. 1982 Apr 29;651(2-3):105–141. doi: 10.1016/0304-419X(82)90009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Analysis of a large cluster of nonessential genes deleted from a vaccinia virus terminal transposition mutant. Virology. 1988 Dec;167(2):524–537. [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Fewell J. W. Intracisternal A-particle gene expression in normal mouse thymus tissue: gene products and strain-related variability. Mol Cell Biol. 1985 Mar;5(3):474–483. doi: 10.1128/mcb.5.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Smith L. A., Lueders K. K. Intracisternal A-particle genes in Mus musculus: a conserved family of retrovirus-like elements. Mol Cell Biol. 1981 Mar;1(3):216–227. doi: 10.1128/mcb.1.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man Y. M., Delius H., Leader D. P. Molecular analysis of elements inserted into mouse gamma-actin processed pseudogenes. Nucleic Acids Res. 1987 Apr 24;15(8):3291–3304. doi: 10.1093/nar/15.8.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A., Christy R. J., Huang R. C. Murine A type retroviruses promote high levels of gene expression in embryonal carcinoma cells. Development. 1988 Jan;102(1):23–30. doi: 10.1242/dev.102.1.23. [DOI] [PubMed] [Google Scholar]
- Morgan R. A., Huang R. C. Correlation of undermethylation of intracisternal A-particle genes with expression in murine plasmacytomas but not in NIH/3T3 embryo fibroblasts. Cancer Res. 1984 Nov;44(11):5234–5241. [PubMed] [Google Scholar]
- Moshier J. A., Deutch A. H., Huang R. C. Structure and in vitro transcription of a mouse B1 cluster containing a unique B1 dimer. Gene. 1987;58(1):19–27. doi: 10.1016/0378-1119(87)90025-4. [DOI] [PubMed] [Google Scholar]
- Ono M., Cole M. D., White A. T., Huang R. C. Sequence organization of cloned intracisternal A particle genes. Cell. 1980 Sep;21(2):465–473. doi: 10.1016/0092-8674(80)90483-3. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Segal S., Lueders K. K., Kuff E. L. RNA associated with murine intracisternal type A particles codes for the main particle protein. J Virol. 1978 Jul;27(1):118–126. doi: 10.1128/jvi.27.1.118-126.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Brown M. S., Goldstein J. L. Different combinations of cysteine-rich repeats mediate binding of low density lipoprotein receptor to two different proteins. J Biol Chem. 1989 Dec 25;264(36):21682–21688. [PubMed] [Google Scholar]
- Taylor M. E., Conary J. T., Lennartz M. R., Stahl P. D., Drickamer K. Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J Biol Chem. 1990 Jul 25;265(21):12156–12162. [PubMed] [Google Scholar]
- Tong P. Y., Gregory W., Kornfeld S. Ligand interactions of the cation-independent mannose 6-phosphate receptor. The stoichiometry of mannose 6-phosphate binding. J Biol Chem. 1989 May 15;264(14):7962–7969. [PubMed] [Google Scholar]
- Wujcik K. M., Morgan R. A., Huang R. C. Transcription of intracisternal A-particle genes in mouse myeloma and Ltk- cells. J Virol. 1984 Oct;52(1):29–36. doi: 10.1128/jvi.52.1.29-36.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]