Abstract
Purpose:
Aqueous and hydroalcoholic extracts of Nigella sativa (400 mg/kg, orally) for 7 days were administered and evaluated for their neuroprotective effects on middle cerebral artery occluded (MCAO) rats.
Materials and Methods:
Cerebral ischemia was induced by middle cerebral artery occlusion for 2 h followed by reperfusion for 22 h. After 24 h of ischemia, grip strength, locomotor activity tests were performed in the surgically operated animals. After behavioral tests, animals were immediately sacrificed. Infarct volumes followed by the estimation of markers of oxidative stress in the brains were measured.
Results:
Locomotor activity and grip strength of animals were improved in both aqueous and hydroalcoholic extracts pretreated rats. Infarct volume was also reduced in both extracts pretreated rats as compared with MCAO rats. An elevation of thiobarbituric acid reactive substance (TBARS) and a reduction in glutathione and antioxidant enzymes, viz., superoxide dismutase (SOD) and catalase levels were observed following MCAO. Pretreatment of Nigella sativa extracts showed the reduction in TBARS, elevation in glutathione, SOD and catalase levels as compared with MCAO rats.
Conclusion:
The present study observed the neuroprotective effects of both the extracts of Nigella sativa in cerebral ischemia. The neuroprotective effects could be due to its antioxidant, free radical scavenging, and anti-inflammatory properties.
KEY WORDS: Infarct volume, MCAO, Nigella sativa extracts, SOD, TBARS
The world wide pattern of disease is shifting from infectious diseases to noncommunicable diseases, especially with heart diseases and stroke is now being the chief causes of death globally.[1] There is a desperate need for more research into stroke. The exact pathophysiology of the disease is not known clearly; various mechanisms such as excessive release of excitatory neurotransmitters, generation of free radicals, intracellular accumulation of calcium, inhibition of protein synthesis, etc. have been implicated for the induction of ischemia.[2] Several new drugs are being evaluated in clinical trial such as glutamate receptor antagonists, sodium and calcium channel blockers, free radical scavengers, and anti-inflammatory agents. These drugs not only improve the blood flow to the dying neurons, but also have beneficial effect on the biochemical mechanisms involved in the development of stroke and thus will prove to be more useful in the treatment of cerebral ischemia.[3] Although many neuroprotective drugs that were effective in animal's models of stroke yet they failed to show promising effects in the clinical trials.[1]
Nigella sativa L. (Ranunculaceae) is an annual herbaceous flowering plant that originated in the Mediterranean region but has been cultivated in other parts of the world such as Asia, Africa, and the Arabian peninsula. The plant or its constituents have been reported to demonstrate many pharmacological activities such as analgesic,[4] anti-inflammatory,[5] antioxidant, and anti-eicosanoid,[6] antibacterial,[7] calcium channel blocking effects[8] and a decreasing effect on intracellular calcium in the mast cells,[9] and protective action against ischemia/ reperfusion-induced gastric mucosal lesions.[10]
The major active constituent present in Nigella sativa is thymoquinone, has a strong antioxidant property.[11] Nigella sativa or its active constituents are also reported to posses some of the pharmacological properties such as it protect carbon tetrachloride induced hepatotoxicity,[12] nephropathy produced by cisplatin,[13] autoimmune as well as allergic encephalomyelitis.[14,15]
The present study was undertaken to evaluate the neuroprotective effects of aqueous and hydro alcoholic extracts of Nigella sativa seeds in rats using middle cerebral artery occlusion (MCAO) model of focal cerebral ischemia. MCAO followed by reperfusion is a model of focal ischemia in rats, which resembles to that of stroke in human and is widely used for evaluating the therapeutic intervention.
Materials and Methods
Plant material
The dried seeds of Nigella sativa were procured from the Khari Bawli, Delhi. The seeds were identified, authenticated; extracts were standardized by Department of Pharmacognosy and Phytochemistry, Jamia Hamdard, New Delhi, before the commencement of the study.
Preparation of extracts
Two types of extracts were prepared, viz., aqueous and hydroalcoholic. [Aqueous extract used distilled water, hydro alcoholic-equal volume of water and alcohol]. Nigella sativa seeds were crushed to coarse powder and then 100 g of the prepared powder was mixed with 500 ml of respective solvent in the soxhlet apparatus by heating at their boiling point and the solutions were filtered and evaporated under reduced pressure to get hydroalcoholic extract.[16] In literature, it is described that the active constituents present in the Nigella sativa are soluble in aqueous and hydro alcoholic solvent. Its active constituent is thymoquinone, hence, the present study used two extracts.
Animals
The study was carried out under controlled conditions using adult Wistar albino rats of 250-300 g, procured from Central Animal House Facility of Hamdard University, New Delhi, selected and acclimatized accordingly. All animals were housed in cages kept at a temperature of 23-30°C with a natural light-dark cycle. They had free access to standard pellet diet (Amrut Laboratory rat and mice feed, NavMaharastra Chakan Oil Mills Ltd, Pune) and tap water. The experimental protocol was approved by an Institutional Animal Ethics Committee CPCSEA Jamia Hamdard New Delhi (Project no. 575). Ethical norms were strictly followed during all experimental procedures.
Drugs and administration
Animals were divided into six groups, each group consisting of six rats each receiving different treatments orally for seven days. GP I - Normal control, GP II - Sham operated, GP III - MCAO only, GP IV - Aspirin + MCAO, GP V - Aqueous extract + MCAO, GP VI - Hydroalcoholic + MCAO. All the different extracts were made accordingly and administered at a dose of 400 mg/kg orally daily for 7 days; care was taken to ensure the same proportion of each extract to be dosed to the each animal for the comparisons. The last dose was administered 3 h before inducing cerebral ischemia in rats. Normal control group and sham operated animals received distilled water. One hundred milligrams of aspirin was suspended in 5 ml of the 0.5% CMC suspension and 0.5 ml of this suspension was administered orally at 100 mg/kg for the same days as above.
Ischemia was induced for 2 h followed by reperfusion for 22 h. After 24 h of ischemia, behavioral parameters were assessed and the animals were then immediately sacrificed for infarct volume and oxidative stress parameters in brains.
Induction of cerebral ischemia
Rats were anesthetized with chloral hydrate (dissolved in distilled water) at a dose of 400 mg/kg i.p. A middle incision was made and the right common carotid artery, external carotid artery, and internal carotid artery was exposed under an operative magnifying glass. A 4.0 monofilament Nylon thread (40-3033 Pk 10, Doccol Corporation Pennsylvania Ave, Red lands, CA, USA) with its tip rounded by heating quickly near a flame was advanced from the external carotid artery into the lumen of the internal carotid artery until resistance was felt, which ensures the occlusion of the origin of the middle cerebral artery.[17] The nylon filament was allowed to remain in place for 2 h. After 2 h the filament was retracted so as to allow the reperfusion of ischemic region. Sham-operated rats had the same surgical procedures except that the occluding monofilament was not inserted. Adequate precautions were taken to prevent the infection.
After 24 h, the animals were studied for locomotor activity, grip strength test. Immediately after behavioral tests the animals were sacrificed, their brain removed, infarct volume was measured, homogenate was prepared from the brain slices and biochemical estimations were carried out.
Behavioral tests
Locomotor activity (closed field activity monitoring)
Spontaneous locomotor activity was assessed using a digital photoactometer. Each animal was observed for a period of 10 min in a square closed arena equipped with infrared light sensitive photocells. The apparatus was housed in a darkened light and sound attenuated ventilated testing room.[18] During activity testing, only one animal was tested at a time.
Grip strength test
Grip strength meter was used for recording the grip strength of the animal. The animal's front paws were placed on the grid of grip strength meter and was moved down until its front paws grasping the grid was released. The force achieved by animal was than displayed on the screen and was recorded as kilogram unit.[19]
Estimation of oxidative stress markers
Following the behavioral testing, the animals were decapitated and the brains were quickly removed and necrotic part of the brains were taken for estimation, weighed and homogenized in ice cold KCI phosphate buffer (0.1 M pH 7.4) 10 times (w/v) and centrifuged at 2000 rpm for 5 min at 4°C. The supernatant containing crude membrane were used for the estimation of TBARS and GSH. The remaining supernatant was again centrifuged at 10,000 rpm at 4°C for 20 min. The postmitochondrial supernatant was used for the study of antioxidant enzyme activities and protein estimation. Catalase and super oxide dismutase activities were determined immediately after sample preparation. Protein concentrations were determined according to Lowry et al,[20] using purified bovine serum albumin as standard.
Measurement of lipid peroxidation
Thiobarbituric acid reactive substance (TBARS), a measure of lipid peroxidation was measured as described by Ohkawa et al.[21] Briefly, 1 ml of suspension medium was taken from the 10% tissue homogenate. 0.5 ml of 30% TCA was added to it, followed by 0.5 ml of 0.8% TBA reagent. The tubes were covered with aluminum foil and kept in shaking water bath for 30 min at 80°C. After 30 mins, tubes were taken out and kept in ice-cold water for 30 min. These were centrifuged at 3000 rpm for 15 min.
The absorbance of the supernatant was read at 540 nm at room temperature against appropriate blank. Blank consist of 1 ml distilled water, 0.5 ml of 30% TCA, and 0.5 ml of 0.8% TBA. TBARS values were expressed as n moles MDA/mg protein.
Measurement of reduced glutathione
Glutathione was measured according to the method of Ellman.[22] The equal quantity of homogenate (w/v) and 10% trichloroacetic acid were mixed and centrifuged to separate the proteins. To 0.01 ml of this supernatant, 2 ml of phosphate buffer (pH 7.4), 0.5 ml 5,5’-dithiobisnitro benzoic acid (DTNB) and 0.4 ml of double distilled water was added. The mixture was vortexed and the absorbance was read at 412 nm within 15 min. Measurement of reduced glutathione (GSH) values were expressed as μ moles GSH mg protein.
Measurement of catalase
Catalase activity was measured by the method of Claiborn.[23] A total of 0.1 ml of supernatant was added to cuvette containing 1.9 ml of 50 mM phosphate buffer (pH 7). The reaction was started by the addition of 1 ml freshly prepared 30 mM H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically at 240 nm. Catalase values were expressed as n moles H2O2 consumed/min/mg protein.
Measurement of superoxide dismutase
Superoxide dismutase activity was measured by the method of Kagiyama et al.[24] The supernatant was assayed for Measurement of superoxide dismutase (SOD) activity by following the inhibition of pyrogallol auto-oxidation. 100 μl of cytosolic supernatant was added to Tris-HCI buffer (pH 8.5). The final volume of 3 ml was adjusted with the same buffer. At least 25 μl of pyrogallol was added and changes in absorbance at 420 nm was recorded at 1 min interval for 3 min. The increase in absorbance at 420 nm after the addition of pyrogallol was inhibited by the presence of SOD.
Infarct volume estimation
Rats were sacrificed and their brains were quickly removed and sectioned coronally into six slices each with a 2 mm thickness. The six brain slices were immersed in 2% triphenyltetrazolium chloride (TTC) for 30 min at 37°C and then fixed with formalin. Infracted areas were identified as regions lacking the brick red staining of normal brain tissue. It was expressed as mm3.
Statistical analysis
All the data were expressed as the mean ± SEM. For a statistical analysis of the data, group means were compared by one-way analysis of variance (ANOVA) followed by Dunnett's ‘t’ test. P value < 0.05 was considered significant. It is carried out with graph pad in Stat 3 software.
Results
Effect of aqueous and hydroalcoholic extracts of Nigella sativa on locomotor activity in MCAO rats
Spontaneous locomotor activity was observed over a period of 10 min for each rat in each group. In the MCA occluded rats significant reduction in locomotor count was observed (P < 0.00l). There was significant improvement in locomotor counts observed in both extracts as compared to the MCAO rats (P < 0.001) [Table 1].
Table 1.
Effect of aqueous and hydroalcoholic extracts of Nigella sativa on locomotor activity and grip strength in middle cerebral artery occluded rats
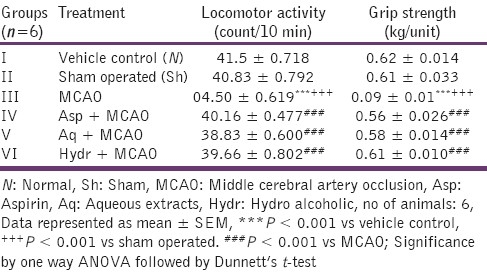
Effect of aqueous and hydroalcoholic extracts of Nigella sativa on grip strength in MCAO rats
MCAO group showed a significant decrease in grip strength as compared to the normal rats (P < 0.001). Pretreatment of both extracts showed improvement in grip strength when compared with MCAO rats (P < 0.001) [Table 1].
Effect of aqueous and hydroalcoholic extracts of Nigella sativa on tissue TBARS in MCAO rats
The TBARS levels measured after 24 h of middle cerebral artery occlusion were found to be significantly increased in the MCAO rats than in the normal rats. Hydroalcoholic extract produced significantly reduction in TBARS levels when compared to that of MCAO group [Table 2].
Table 2.
Effect of aqueous and hydroalcoholic extracts of Nigella sativa on TBARS, GSH, SOD and catalase in middle cerebral artery occluded rats
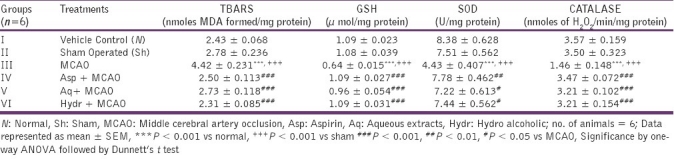
Effect of aqueous and hydroalcoholic extracts of Nigella sativa on tissue glutathione in MCAO rats
The brain glutathione levels were estimated in all the groups. Levels of reduced glutathione in MCAO rats were significantly reduced as compared to the sham operated rats. In all extracts pretreated rats, the glutathione levels were significantly elevated when compared with MCAO group (P < 0.001) [Table 2].
Effect of aqueous and hydroalcoholic extracts of Nigella sativa on tissue SOD in MCAO rats
The levels of SOD after 24 h in MCA occluded group were significantly reduced as compared to the normal rats. In both aqueous and hydro alcoholic pretreated group, the levels of SOD were significantly increased (7.22 ± 0.613 and 7.44 ± 0.562 units/mg protein) as compared to the MCAO group (4.43 ± 0.407 units/mg protein) [Table 2].
Effect of aqueous and hydroalcoholic extracts of Nigella sativa on tissue catalase in MCAO rats
The levels of catalase were reduced in MCA occluded group (P < 0.001) as compared to the normal rats. All extracts pretreated rats showed elevation in the levels as compared to the MCAO group (P < 0.001) [Table 2].
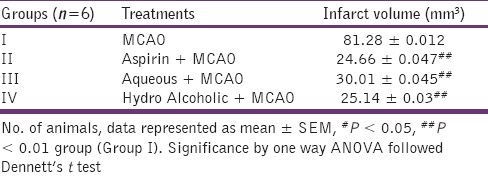
Effect of aqueous and hydroalcoholic extracts of of Nigella sativa on infarct volume in MCAO rats
TTC dye staining of brains of all extracts pretreated animals showed significant improvement in the infarct volume Aspirin, aqueous and hydroalcoholic extracts pretreated animals showed a highly significant reduction in infarct volume as compared with MCAO rats (P < 0.001) [Table 3].
Table 3.
Effects of aqueous and hydroalcoholic extracts of Nigella sativa on infarct volume in middle cerebral artery occlusion occluded rats
Discussion
Thrombolytics, NMDA receptor antagonists, calcium channel blockers and antioxidants etc have been used or being evaluated, an ideal drug for the treatment of stroke is still needed.[25] Preventive measures are used to reduce the severity of the disease, but no effective curative medication is available till today.
Herbal preparations are gaining popularity nowadays because of their beneficial effects in various conditions without any adverse effects. The present study was planned to evaluate the role of aqueous and hydroalcoholic extracts of Nigella sativa for the treatment and preventions of stroke and its consequences in animal model of focal cerebral ischemia.
In the present study, we observed that N. sativa extracts significantly decreased TBARS as compared to the MCAO rats [Table 2]. This was similar to earlier reports which showed that MCA occlusion followed by reperfusion increased the TBARS formation.[1,2,26,27] Regarding the effect of black seed on lipid peroxidation and defense system, it has been found that pretreatment with N. sativa extracts decreased blood MDA levels and increased the antioxidant defense system activity. It prevents oxidative injury in various MCAO models and may prevent membrane lipid peroxidation.[28] Thymoquinone (TQ) is the main active constituent of Nigella sativa is effective in protecting rats against transient forebrain ischemia-induced damage in the rat hippocampus.[28] TQ is also works as a scavenger of various reactive oxygen species, including super oxide anion and hydroxyl radicals.[29,30]
Pretreatment of aqueous and hydro alcoholic extracts showed a highly significant reduction in TBARS levels indicating that the active constituents responsible for having antioxidant and free radical scavenging properties may be more soluble in aqueous and hydro alcoholic solvent.[29,31]
Glutathione (GSH) is the master and indigenous antioxidant enzymes which prevent/overcome the generation of reactive oxygen species (free radicals) and oxidative damage. We observed a significant decrease in GSH levels (P < 0.001) in MCA occluded rats as compared to pretreated groups. Similar observations were also reported elsewhere in which the oxidative stress decreased GSH levels.[1,2,26] Pretreatment of N. sativa extracts resulted in significant elevation of GSH levels as compared to MCA occluded rats thus confirming its antioxidant and free radical scavenging properties.[29–31]
GSH, which is considered the most prevalent and important intracellular nonprotein thiol, has a crucial role as a reactive oxygen species scavenger. In the current work, GSH content was significantly reduced by ischemic insult. This could be explained by the consumption of GSH due to scavenging of the rapidly generating reactive oxygen species in ischemia. The current work also showed that there was a significant decline in activity of the endogenous antioxidant enzymes. Pretreatment with Nigella sativa extracts for 7 days followed by MCA occlusion indicate reversals in the reduction of GSH level as compared with MCA occluded rats and the potency of the reversal may be dependent on the availability of the chemicals constituents present in each extracts. Elevation of GSH levels could be due to the antioxidant effects which have been observed in hydro alcoholic and aqueous extracts.
In normal brain tissues, the production of reactive oxygen species (ROS) and peroxynitrite anion is balanced by endogenous enzymatic and non-enzymatic anti-oxidative defenses. However, after cerebral ischemia and particularly reperfusion this free radical production is dramatically elevated disrupting the endogenous antioxidant systems.[32,33]
A significant reduction in motor performance tests were also observed, particularly in grip strength and locomotors counts. These results were similar to the previously reported observations and results.[1,2,26] The possible mechanism for this deficit could be due to neuronal damage in the territory of the MCA.[25] The ischemic insult observed could be due to the alterations induce both biochemical (TBARS, GSH, SOD, and Catalase) and morphologically in rats brain. However, in our study pretreatment with hydroalcoholic and aqueous extracts showed improvement in the motor performance tests (P < 0.001). These finding suggested that the improvement in the motor performance test could be due to reduction in the ischemic lesion in the territory area.[25]
Our results indicated that 2 h of cerebral ischemia followed by reperfusion induced selective neuronal damage infarction as evident by triphenyltetrazolium chloride (TTC) dye staining. Nigella sativa extracts significantly reduced the infarct volume of the rat's brain tissue as compared to MCA occluded rats. Pretreatment with Nigella sativa extracts offered significant improvements in hydro alcoholic and aqueous extracts (P < 0.001).
Conclusion
In the present study, both hydro alcoholic and aqueous extracts of Nigella sativa reduced TBARS levels, elevated GSH, SOD and catalase levels. Decrease in infarct volume was also observed. The significant improvements by aqueous and hydro alcoholic pretreated animals could be due to the high polarity of the solvent (aqueous and hydro alcoholic). The other added advantage for the hydro alcoholic extracts is its antiseptic property. Our results are preliminary, further studies are required to confirm the anti ischemic effect of Nigella sativa by using both extracts as pretreatment and post treatment as multiple drug administration. Thus, we may conclude that the neuroprotective effects exhibited by both extracts of N. sativa against ischemia in rat are more or less similar to the observations produced by aspirin by virtue of their antioxidant and free radical scavenges properties. Further estimation of other antioxidant enzymes with simultaneous recording of mean arterial blood pressure; ECG, pulse rate, etc., are also required to confirm the beneficial role of N. sativa for improving the blood flow and reducing the neurological deficits in cerebral ischemia.
Acknowledgments
Financial support under RPS scheme from AICTE New Delhi is greatly acknowledged.
Footnotes
Source of Support: RPS scheme from AICTE New Delhi
Conflict of Interest: None declared.
References
- 1.Pratap R, Pillai KK, Khanam R, Islam F, Ahmad SJ, Akhtar M. Protective effect of irbesartan an angiotensin II receptor antagonist alone and in combination with aspirin on middle cerebral artery occlusion model of focal cerebral ischemia in rats. Hum Exp Toxicol. 2011;30:354–62. doi: 10.1177/0960327110371257. [DOI] [PubMed] [Google Scholar]
- 2.Sinha K, Degaonkar N, Jagannathan NR, Gupta YK. Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur J Pharmacol. 2001;428:185–92. doi: 10.1016/s0014-2999(01)01253-5. [DOI] [PubMed] [Google Scholar]
- 3.Adams H, Adams R, Del Zoppo G, Goldstein LB. Guidelines for the early management of patients with ischemic stroke: Guidelines update.A scientific statement from the stroke council of the American Heart Association/American Stroke Association. Stroke. 2005;36:916–23. doi: 10.1161/01.STR.0000163257.66207.2d. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Fattah M, Matsumoto K, Watanabe H. Anti-nociceptive effects of Nigella sativa oil and its major components in mice. Eur J Pharmacol. 2000;400:89–97. doi: 10.1016/s0014-2999(00)00340-x. [DOI] [PubMed] [Google Scholar]
- 5.Mutabagani A, El-Mahdy SA. Study of the anti-inflammatory activity of Nigella sativa L. and thymoquinone in rats. Saudi Pharm J. 1997;5:110–3. [Google Scholar]
- 6.Turkdogan MK, Agaoglu Z, Yener Z, Sekeroglu R, Akkan HA, Avci ME. The role of antioxidant vitamins (C and E), selenium and Nigella sativa in the prevention of liver fibrosis and cirrhosis in rabbits: New hopes. Dtsch Tierarztl Wochenschr. 2001;108:71–3. [PubMed] [Google Scholar]
- 7.Morsi NM. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol Pol. 2000;49:63–74. [PubMed] [Google Scholar]
- 8.Gilani AH, Aziz N, Khurram IM, Chaudhary KS, Iqbal A. Bronchodilator, spasmolytic and calcium antagonist activities of Nigella sativa seeds (Kalonji): A traditional herbal product with multiple medicinal uses. J Pak Med Assoc. 2001;51:115–20. [PubMed] [Google Scholar]
- 9.Chakravarty N. Inhibition of histamine release from mast cells by nigellone. Ann Allergy. 1993;70:237–42. [PubMed] [Google Scholar]
- 10.El-Abhar HS, Abdallah DM, Saleh S. Gastroprotective activity of Nigella sativa oil and its constituent, thymoquinone, against gastric mucosal injury induced by ischemia/reperfusion in rats. J Ethnopharmacol. 2003;84:251–8. doi: 10.1016/s0378-8741(02)00324-0. [DOI] [PubMed] [Google Scholar]
- 11.Houghton PJ, Zarka R, de las Herass B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–6. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 12.Nagi MN, Alam K, Badary OA, al-Shabanah OA, al-Sawaf HA, al-Bekairi AM. Thymoquinone protects against carbon tetrachloride hepatotoxicity in mice via an antioxidant mechanism. Biochem Mol Biol Int. 1999;47:153–9. doi: 10.1080/15216549900201153. [DOI] [PubMed] [Google Scholar]
- 13.Badary OA, Al-Shabanah OA, Nagi MN, Al-Rikabi AC, Elmazar MM. Inhibition of benzo (a) pyrene-induced forestomach carcinogenesis in mice by thymoquinone. Eur J Cancer Prev. 1999;8:435–40. doi: 10.1097/00008469-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed A, Afridi DM, Garani O, Tucci M. Thymoquinone inhibits the activation of NF-kappa B in the brain and spinal cord of experimental autoimmune encephalomyelitis. Biomed Sci Instrum. 2005;41:388–93. [PubMed] [Google Scholar]
- 15.Mohamed A, Shoker A, Bendjelloul F, Mare A, Alzrigh M, Benghuzzi H, Desin T. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; an oxidative stress inhibitor. Biomed Sci Instrum. 2003;39:440–5. [PubMed] [Google Scholar]
- 16.Muhd TS, Ralid AK, Indul I. Antimicrobial activity of black seed against multi drug resistant of coagulase negative staphylococci. Pak J Med Res. 2002;41:77–83. [Google Scholar]
- 17.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Lannert H, Hcfyer S. lntracerebroventricular administration of streptozotocin causes long term diminution in learning and memory abilities and intracerebral energy metabolism in adult rats. Stroke. 1998;28:2060–6. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- 19.Ali A, Ahmed FJ, Pillai KK, Vohora D. Evidence of antiepileptic potential of amiloride with neuropharamacological benefits in rodents models of epilepsy and behaviour. Epilepsy Behav. 2004;5:322–8. doi: 10.1016/j.yebeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosenburg NJ, Farr AL, Randale RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 21.Okhawa H, Ohishi N, Yagi K. Assay of lipid peroxides in animals tissue by thiobarbituraic acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL. Tissue sulphhydryl groups. Arc Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Claiborne A. Green world R.A Handbook of method of oxygen free radical research. Boca Raton, Florida: CRC Press; 1985. pp. 283–5. [Google Scholar]
- 24.Kagiyama T, Kagiyama S, Phillips MI. Expression of angiotensin type 1 and 2 receptors in brain after transient middle cerebral artery occlusion in rats. Regul Pept. 2003;110:241–7. doi: 10.1016/s0167-0115(02)00223-9. [DOI] [PubMed] [Google Scholar]
- 25.Gupta R. Trends in hypertension epidemiology in India. J Hum Hypertens. 2004;18:73–8. doi: 10.1038/sj.jhh.1001633. [DOI] [PubMed] [Google Scholar]
- 26.Akhtar M, Pillai KK, Vohra D. Effects of thioparamide on oxidative stress markers in midle crebral artey occulusoin model of focal cereal ischemia in rats. Hum Exp Tox. 2008;27:761–7. doi: 10.1177/0960327108094608. [DOI] [PubMed] [Google Scholar]
- 27.Kanter M, Coskun M, Budankamnak N. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J Gastroenterol. 2005;11:6684–8. doi: 10.3748/wjg.v11.i42.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosseinzadeha H, Parvardehb S, Aslb MN, Sadeghniab HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–7. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity - the secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- 30.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 31.Badary OA, Taha RA, Gamal El-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26:87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- 32.Pourghassem-Gargaril, Vahideh Ebrahimzadeh-Attary, Maryam Rafraf, Abolfazl Gorbani. Effect of dietary supplementation with Nigella sativa L.on serum lipid profile, lipid peroxidation and antioxidant defense system in hyperlipidemic rabbits. J Med Plants Res. 2009;3:815–21. [Google Scholar]
- 33.Margaill T, Plotkine M, Lerouet D. Antioxidant strength in the treatment of stroke. Free Radic Biol Med. 2005;39:429–43. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]


