Abstract
Objective:
Recombinant human erythropoietin (rhEPO) therapy under circumstances of moderate chronic renal failure (CRF), with yet lower kidney and heart lesion, may have a protective cardiac effect beyond the correction of anemia, whose mechanism deserves better elucidation, namely by clarifying the impact on gene expression profile of markers of apoptosis, inflammation, proliferation, angiogenesis, and lesion/stress in the heart.
Materials and Methods:
Four groups of rats were studied over a period of 15 weeks (n=7 each): control—without surgery and without drug treatment; rhEPO—treated with 50 IU/kg/week of rhEPO—beta; CRF—submitted to partial nephrectomy (3/4); CRF + rhEPO—CRF with rhEPO treatment after the 3rd week of surgery. The heart was collected in order to evaluate the gene expression, by real-time qPCR, of markers of apoptotic machinery, inflammation/immunology, proliferation/angiogenesis, and lesion/stress.
Results:
The main findings obtained were (a) CRF rats have demonstrated overexpression of EPO-R in the heart without changes on EPO expression, together with overexpression of Bax/Bcl2 ratio, PCNA, and IL-2; (b) rhEPO therapy on the heart of the rats with CRF induced by partial 3/4 nephrectomy promoted nonhematopoietic protection, demonstrated by the apoptosis prevention, viewed by the Bax/Bcl2 balance, by the promotion of proliferation, due to PCNA increment, and by the immunomodulatory action, expressed by a trend to prevent the IL-2 increment.
Conclusion:
In this model of moderate CRF, rhEPO treatment showed important cardiac nonhematopoietic effects, expressed mainly by the antiapoptotic and the proproliferative action, suggesting that early rhEPO therapy in moderate stages of CRF might have further therapeutic benefits.
KEY WORDS: Apoptosis, cardiovascular protection, gene expression, moderate chronic renal failure, proliferation/angiogenesis
The appearance and development of chronic kidney disease (CKD) is often associated with a state of low levels of erythropoietin (EPO) production, due to loss of kidney peritubular cells, responsible for its formation, with consequent reduction in erythropoiesis, resulting in anemia.[1] The reduction of tissue oxygenation then causes an increase of cardiac function, and subsequent left ventricular hypertrophy, in order to reply to the oxygen demands by the peripheral tissues, which accounts for subsequent development of heart failure—a triad of dysfunctions known as cardiorenal anemia syndrome.[1–3] The relationship between the anemia secondary to kidney disease and the heart failure is already well described and accounts for the high morbidity and mortality rates found in CKD patients.[4,5]
The use of recombinant human erythropoietin (rhEPO) has emerged in the early 1990s as the most appropriate method to control anemia in patients with CRF, and was responsible for a better and longer life in those patients, both from amelioration of anemia and beneficial cardiovascular impact.[4–6] Therefore, EPO has been recognized as a key player in a broad variety of processes in cardiovascular pathophysiology, including apoptosis, cell proliferation, ischemia, and the nitric oxide (NO) pathway, which reinforces their putative use in nonhematological conditions.[7] However, for a significant percentage of patients, rhEPO loses efficacy, becoming resistant, recommending dose increment with further deterioration of heart function, most probably due to the expected hyperviscosity and thromboembolisms.[7–10] Therefore, although enhanced EPO synthesis is viewed as an appropriate compensatory mechanism in the cardiorenal syndrome, excessive EPO synthesis in the advanced stages of both the chronic renal failure (CRF) and congestive heart failure appears to be predictive of higher mortality.[10] Thus, early rhEPO use, in stages of yet moderate renal failure and initial cardiac deterioration, might have additional benefits. However, this impact remains poorly investigated.
Considering that the use of human tissues is obviously limited by ethical reasons, our group has previously extensively characterized a model of moderate CRF,[11] in which rhEPO treatment has promoted erythrocytosis and prevented tachycardia, catecholamines increment and dyslipidemia, with a rise of serum TGF-b1.[12,13] Thus, rhEPO therapy, under these circumstances, has promoted a beneficial effect on the cardiac tissue, which might be attributed to its protective actions, namely of proproliferative and antiapoptotic nature, as was previously suggested in other conditions and pathologies,[3–6] but that deserves a more extensive evaluation, particularly in moderate stages of CKD, before critical impairment of the kidney and heart tissues.
EPO gene activation, mainly by hypoxic states, is linked with several important pathways, with unequivocal impact on renal and cardiac tissues function and structure. The 3× end of the EPO gene contains an element of response to situations of hypoxia (HRE), which interacts with multiple transcription factors, including hypoxia-induced factor (HIF), a regulator of the genes that are involved in adaptation to changes in oxygen levels. The HIF will act on several genes, including the vascular endothelial growing factor (VEGF) and the glucose transporter GLUT-1.[14] Although the regulation of erythropoiesis occurs mainly in the peritubular cells of the kidneys, 10% of EPO is expressed in extrarenal tissues, such as in liver, brain, spleen, and lungs.[15] Binding of EPO to its receptor (EPO-R) causes a change in intracellular calcium levels, including the formation of IP3 that is able to inhibit the occurrence of apoptosis due to activation of its effector, Akt, an apoptotic blocker involved in proliferation and cell survival.[16] The interaction between the EPO and its receptor also leads to activation of ras/MAPK signaling pathways, by activating nuclear factor κB (NF-κB), with consequent strengthening of cell proliferation.[17]
This study was designed to assess the effects of rhEPO therapy on heart gene expression profile in a moderate stage of CRF. We hypothesize that rhEPO use under these circumstances of lower tissue lesion/impairment may have a protective effect beyond the correction of anemia, whose mechanism deserves better elucidation by clarifying the impact on gene expression profile of several markers of apoptosis, inflammation, proliferation, angiogenesis, and lesion/stress.
Materials and Methods
Animals and treatments
Male Wistar rats (Charles River Lab. Inc., Barcelona, Spain), weighing ±275 g, were maintained in an air conditioned room, subjected to 12:12 h dark/light cycles and given standard rat chow (IPM-R20, Letica, Barcelona, Spain) and free access to tap water. Animal experiments were conducted according to the European Communities Council Directives on Animal Care. The rats were divided into 4 groups (7 rats each), during a 15-week protocol: control—without drugs and surgery; rhEPO(beta)—50 IU/kg/week s.c. Recormon® (Roche Pharmaceuticals), without surgery; CRF-induced by a 2-stage (3/4) nephrectomy: first, about half of the left kidney was removed and, 1 week later, the entire right kidney was removed; CRF+rhEPO-treated with rhEPO after the 3rd week of surgery. All the animals have completed the protocol. Control sham-operated rats were considered not required based on preliminary studies from us, which have demonstrated a similar pattern for hematologic and renal parameters with the nonoperated controls throughout the treatment period.
Renal and hematologic data
Blood samples were collected in rats under intraperitoneal anesthesia with a 2 mg/kg body weight of a 2:1 (v:v) 50 mg/mL ketamine (Ketalar®, Parke–Davis, Lab. Pfeizer Lda, Seixal, Portugal) solution in 2.5% chlorpromazine (Largactil®, Rhône–Poulenc Rorer, Lab. Vitória, Amadora, Portugal), by venipuncture from the jugular vein into syringes without an anticoagulant (for serum sample collection) or with EDTA for hematologic studies. Serum creatinine, urea, and uric acid concentrations were used as renal function indexes through automatic validated methods and equipments (Hitachi 717 analyzer). Red blood cell (RBC) count, hematocrit (HTC), and hemoglobin (Hb) concentration were assessed in whole blood EDTA by using an automatic Coulter Counter® (Beckman Coulter Inc., CA, USA).
Heart collection and preparation
The rats were sacrificed by cervical dislocation, under intraperitoneal anesthesia, previously described, and the heart was immediately removed, placed in ice-cold Krebs buffer and carefully cleaned of adherent fat and connective tissue, freezing therefore in RNA later™ tubes at -80°C.
Heart gene expression analysis
Total RNA isolation
The heart was isolated in autopsy and stored in RNA later™ solution (Ambion, Austin, TX, USA). Samples were removed from preservation solution and 1200 μL of RLT Lysis Buffer were added to proceed with disruption and homogenization for 2 min at 30 Hz using TissueLyser (Qiagen, Hilden, Germany). Tissue lysate were processed according to the protocol from RNeasy® Mini Kit (Qiagen, Hilden, Germany). Total RNA was eluted in 50 μL of RNase-free water (without optional treatment with DNAse). In order to quantify the amount of total RNA extracted and verify RNA integrity (RIN, RNA Integrity Number), samples were analyzed using 6000 Nano Chip® kit, in Agilent 2100 bioanalyzer (Agilent Technologies, Walbronn, Germany) and 2100 expert software, following manufacturer instructions. The yield from isolation was from 0.5 to 3 μg; RIN values were 6.0–9.0 and purity (A260/A280) was 1.8–2.0.
Reverse transcription
RNA was reverse transcribed with SuperScript™ III first-strand synthesis system for RT-PCR (Invitrogen, California, USA). One microgram of total RNA was mixed with a 2× First-Strand Reaction Mix and a SuperScript™ III Enzyme Mix (Oligo(dT) plus Random hexamers). Reactions were carried out in a thermocycler Gene Amp PCR System 9600 (Perkin Elmer, Norwalk, CT, USA), 10 min at 25°C, 50 min at 50°C and 5 min at 85°C. Reaction products were then digested with 1 μL RNase H for 20 min at 37°C and, finally, cDNA eluted to a final volume of 100 μL and stored at –20°C.
Relative quantification of gene expression
Performed using 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). A normalization step preceded the gene expression quantification, using geNorm Housekeeping Gene Selection kit for Rattus norvegicus (Primer Design, Southampton, UK) and geNorm software (Ghent University Hospital, Center for Medical Genetics, Ghent, Belgium) to select optimal housekeeping genes to this study.[18] Real-time PCR reactions used specific QuantiTect Primer Assays (Qiagen, Hilden, Germany) with optimized primers for TGF-β1 and PCNA, as proliferative markers; VEGF as an angiogenesis marker; a synthase 2 (inducible) and 3 (constitutive, endothelial) from nitric oxide, NOS2 and NOS3 as indicators of endothelial and constitutive enzyme activity; Cytochrome c as a vascular damage factor; IL-2, NF-κB, and TNF-α as inflammatory markers; and at least apoptotic indicators, such as caspase 9, caspase 3, Bax, Bcl2, Fas and Fas ligand. Endogenous controls were also used: GAPDH, ACTB, TOP1, and RPL13 together with QuantiTect SYBR Green PCR Kit Gene expression (Qiagen, Hilden, Germany) according to manufacturer's instructions. RT-qPCR reactions were carried out with 100 ng cDNA sample, primers (50–200 nM) and 1X QuantiTect SYBR Green PCR Master Mix. Nontemplate control reactions were performed for each gene, in order to assure no unspecific amplification. Reactions were performed with the following thermal profile: 10 min at 95°C plus 40 cycles of 15 s at 95°C and 1 min. at 60°C. Real-time PCR results were analyzed with SDS 2.1 software (Applied Biosystems, Foster City, CA, USA) and quantification used the 2−ΔΔCt method.[19]
Statistical analysis
For statistical analysis, we used the GraphPad Prism, Version 5.0. Results are presented as means ± standard error of means (SEM). Comparisons between groups were performed using ANOVA and the post hoc Bonferroni test. Significance was accepted at P < 0.05.
Results
Effect of rhEPO on renal function and hematologic data
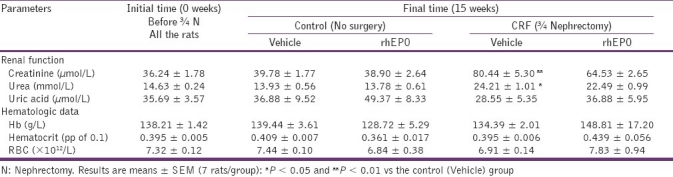
In table 1 we present the renal function markers and hematologic data for the different groups, before starting experiments and at the end of the experimental period (15 weeks: 12 weeks after partial nephrectomy). There was a statistically significant increase in serum creatinine and urea concentrations at 3 weeks after surgery (data not shown), and this increase in renal function markers remained high along the following 12 weeks of the experimental procedure. The rhEPO treatment in the CRF animals produced no significant effects in those parameters [Table 1]. Concerning to hematologic data, 3 weeks after nephrectomy, the CRF animals showed a statistically significant decrease for RBC count, HTC, and Hb (data not shown). These parameters normalized in the laboratorial evaluation performed 9 weeks after surgical intervention and remained stable until the end of the follow-up period. The rhEPO treatment in the CRF animals (CRF+rhEPO group) showed a trend to increased hematologic values (RBC, HTC, and Hb) [Table 1].
Table 1.
Effects of rhEPO treatment in renal function and hematological data in a rat model of moderate CRF
Effect of rhEPO on heart EPO and EPO-R mRNA gene expression
We found that rhEPO treatment, per se (rhEPO group), in the cardiac tissue, was able to significantly (P < 0.05) increase EPO gene expression [Figure 1a], together with a trend to higher values of EPO-R [Figure 1b], when compared with the control animals. In the CRF animals, no significant changes were encountered in EPO gene expression in this tissue [Figure 1a], but a statistically significant higher values of EPO-R genetic expression [Figure 1b] were found when compared with the control rats. rhEPO treatment in the CRF animals (CRF+rhEPO group) showed a trend to higher values of EPO gene expression in the heart, without changes on EPO-R when compared with the CRF rats without rhEPO therapy [Figure 1b].
Figure 1.
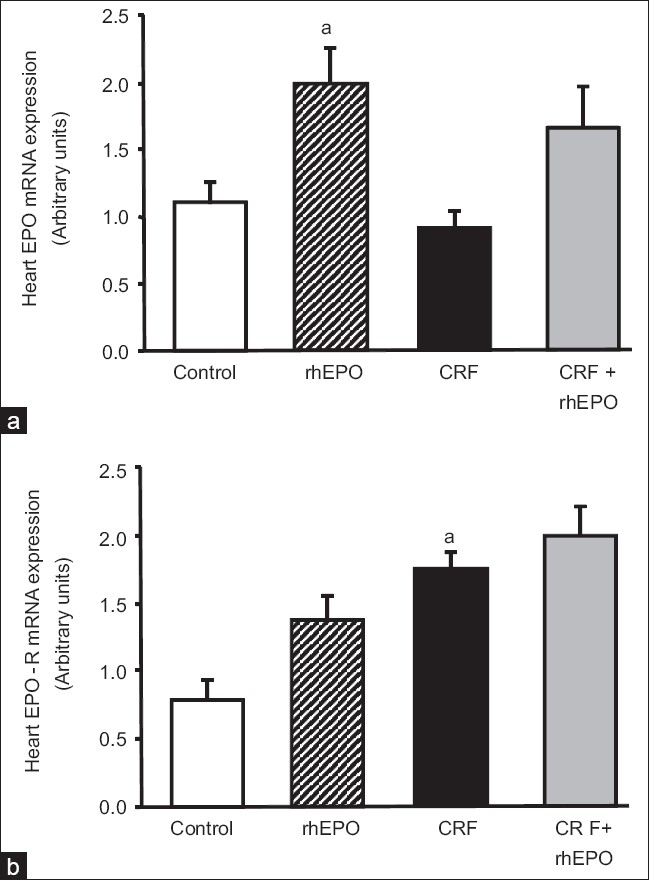
Heart mRNA erythropoietin (a) and erythropoietin receptor (b) gene expression for the groups under study, at the final time. Results are means ± SEM of arbitrary units (7 rats/group): aP < 0.05 and aaP < 0.01 vs the control group.
Effect of rhEPO on heart mRNA gene expression of apoptotic markers
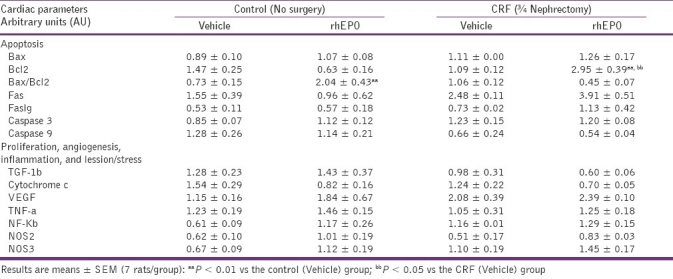
The following proteins were evaluated as markers of the apoptotic machinery: Bax, Bcl2, Fas, FasLg, and caspases 3 and 9. Concerning the rhEPO treatment, we found in the heart tissue that rhEPO was able to promote a statistically significant increment (P < 0.01) in Bax/Bcl2 ratio gene expression [Figure 2a], as a result of a trend to higher values of the proapoptotic protein Bax and lower of the antiapoptotic Bcl2 [Table 2]. The other markers of apoptosis in the heart tissue were also unchanged, with again a trend to higher values of caspase 3 and 9 gene expression [Table 2]. In the CRF animals, a statistically significant increment (P < 0.05) in Bax/Bcl2 ratio [Figure 2a] was found, together with no further changes on the other proteins, Fas, FasLg, and caspases 3 and 9 [Table 2]. Concerning the rats under rhEPO treatment with CRF (CRF+rhEPO group), there was a prevention of the proapoptotic effect promoted by the renal failure, with a statistically significant (P < 0.05) reduction of Bax/Bcl2 ratio when compared with the animals without rhEPO therapy [Figure 2a] due to a significant increment (P < 0.01) on Bcl2 gene expression [Table 2]. Once again, no further changes were found for the other parameters [Table 2].
Figure 2.
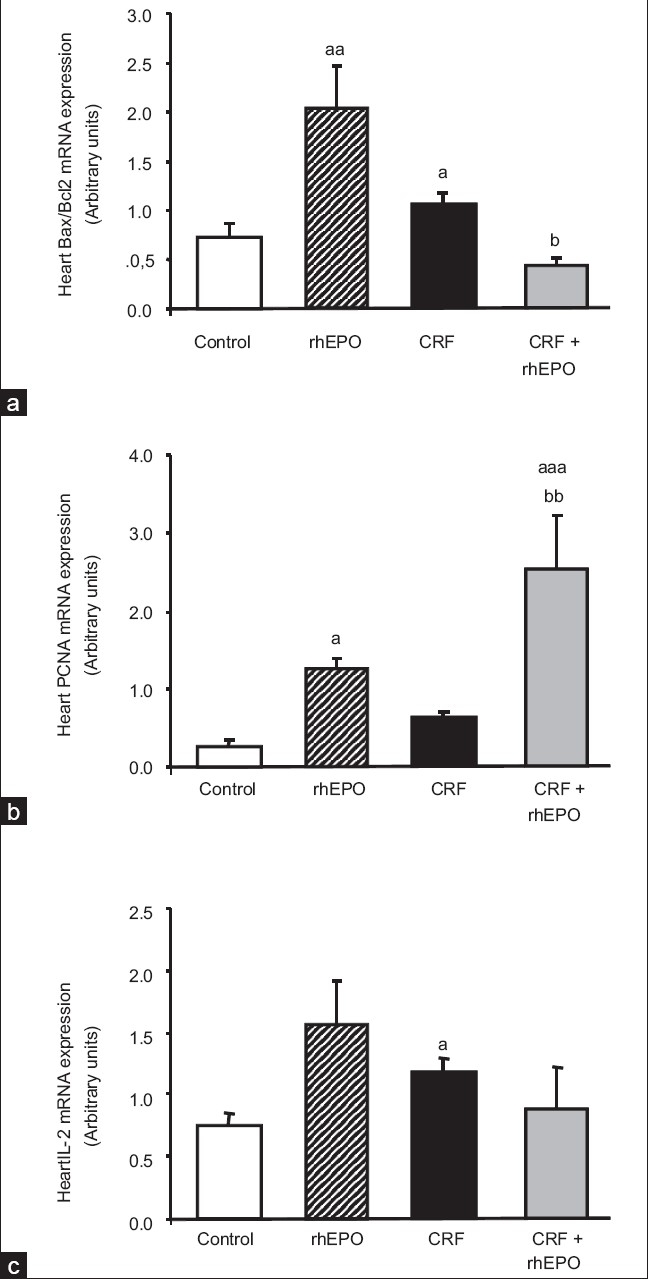
Heart mRNA Bax/Bcl2 ratio (a), PCNA (b), and IL-2 (c) gene expression for the groups under study, at the final time. Results are means ± SEM of arbitrary units (7 rats/group): aP < 0.05, aaP < 0.01, and aaaP < 0.001 vs the control group; bP < 0.05 and bbP < 0.01 vs the CRF group
Table 2.
Effects of rhEPO treatment on heart mRNA gene expression of markers of apoptosis, proliferation, angiogenesis, inflammation, and lesion/oxidative stress in a rat model of moderate CRF, at the final time (15 weeks)
Effect of rhEPO on heart mRNA gene expression of proliferative and angiogenic markers
To assess the influence of rhEPO treatment on heart proliferation and angiogenesis, we evaluated the following proteins: PCNA, TGF-b1, cytochrome c, and VEGF. Concerning the rhEPO treatment, per se (rhEPO group), we found a remarkable increment in PCNA mRNA gene expression in the heart (P < 0.05) [Figure 2b]. rhEPO treatment promoted no changes on TGF-b1, cytochrome c and VEGF gene expression, despite a trend to higher values of TGF-b1 and lower of cytochrome c [Table 2]. In the CRF animals, we found a trend to higher values of PCNA (0.63 ± 0.10 AU), when compared with the control animals (0.27 ± 0.07 AU) [Figure 2b]. Concerning the other markers, no changes were encountered for TGF-b1, cytochrome c, and VEGF gene expression levels [Table 2]. In the rats under rhEPO treatment with CRF (CRF+rhEPO group), PCNA gene expression was substantially elevated (P < 0.01) when compared to the CRF group [Figure 2b]. No other significant changes were encountered concerning the other proteins [Table 2].
Effect of rhEPO on heart mRNA gene expression of markers of inflammation and lesion/stress
We also assess the influence of rhEPO treatment on heart IL-2, TNF-a, NF-κB, and NOS2 and NOS3 gene expression. Concerning the rhEPO treatment, per se (rhEPO group), we found no significant changes for all the parameters analyzed in the heart tissue, despite a trend to higher expression in all of them [Figure 2c and Table 2]. In the CRF animals, IL-2 gene expression was higher (P < 0.05) that in the control rats [Figure 2c], without no further significant changes on all the other markers [Table 2]. In the CRF+rhEPO group, rhEPO treatment showed a trend to prevent the IL-2 gene overexpression found in the CRF animals [Figure 2c]. Apart from those changes, a trend to higher values was found in the heart NF-κB gene expression comparing to the CRF group [Table 2].
Discussion
The introduction of rhEPO therapy allowed a significant reduction of anemia-associated adverse effects, allowing for a prolonged life expectancy in end-stage renal disease stages.[4] Apart from the anemia correction, rhEPO therapy has been associated with positive beneficial effects on nonhematopoietic cells,[7–9] which have been attributed to its antiapoptotic, anti-inflammatory, and antioxidant actions, that underlie the cardio- and neuroprotection in other conditions.[20,21] Functional EPO receptors have been found in several tissues, including those of the cardiovascular system, such as the endothelial cells and cardiomyocytes.[22,23] Furthermore, EPO is a key player in a broad variety of processes in cardiovascular pathophysiology, including apoptosis, cell proliferation, and ischemia.[7] An increase in cardiac systolic function has been observed in patients with chronic heart failure treated with rhEPO. Other beneficial effects appear to be related to the proangiogenic properties on endothelial cells, which could be useful for treatment of ischemic heart disease.[24] These findings suggest that rhEPO could provide therapeutic benefits in the management of cardiovascular diseases beyond anemia correction. Nevertheless, although enhanced EPO synthesis is viewed as an appropriate compensatory mechanism in the cardiorenal syndrome, excessive EPO synthesis in the advanced stages of both the CRF and CHF appears to be predictive of higher mortality.[10] Therefore, for a significant percentage of patients, rhEPO loses efficacy, becoming resistant, recommending dose increment with further deterioration of heart function, most probably due to the expected hyperviscosity and thromboembolisms.[7–10] In this context, earlier rhEPO therapy in anemic CKD patients might have a positive cardiorenal impact, such as previously reported in ischemic injury, contributing to organ protection/regeneration.[25,26] However, the impact of early rhEPO use, in stages of yet moderate renal failure and low cardiac deterioration, remains poorly investigated.
In order to further evaluate the effect of rhEPO on the cardio-renal axis, our group has previously characterized a rat model of moderate CRF induced by partial (3/4) nephrectomy, which demonstrated a moderate but maintained degree of CRF, together with transitory anemia and iron metabolism disturbances. The remnant kidney presented a reasonable degree of functionality, mainly due to hypertrophic compensation, and there was several important cardiovascular modifications, including hypertension, tachycardia, dyslipidemia, erythropoietic disturbances, sympathetic activation, proliferation, angiogenesis, and oxidative stress, which are features seen in CKD patients.[11–13] The use of rhEPO in that model showed interesting cardiorenal effects, including prevention of tachycardia, of catecholamines increment and of dyslipidemia, together with a notorious proproliferative action on the remnant kidney and on the heart tissue.[12] Considering that EPO interaction with its receptor leads to activation of several important pathways related with apoptosis, proliferation, angiogenesis, inflammation, and lesion/stress, we hypothesize that the putative cardioprotective effect of early rhEPO use might be linked with adaptations on heart gene expression.[15–17,21]
In our study, rhEPO treatment, per se, without CRF, showed an overexpression of EPO and EPO-R gene in the heart, which was statistically evident for the EPO gene. In the group with CRF, rhEPO treatment promoted a trend to higher values of both genes in this tissue. This data suggests that rhEPO use, in this circumstance of moderate CRF, might produce a relevant extrarenal impact, namely in the cardiac tissue, as previously suggested by us using this model, as well as by others in other conditions.[6,27] Our study also showed that CRF rats presented a proapoptotic profile, viewed by the significantly increased ratio of Bax/Bcl2 expression. This pattern was accompanied by the absence of other significant changes, including on gene expression of the par Fas-Faslg and on caspases 3 and 9. rhEPO use in these animals promoted prevention of apoptosis in the heart, due to the significant prevention of Bax/Bcl2 increment induced by renal failure. This effect was previously documented for rhEPO use in other situations, in both the kidney[28–31] and in the heart, due to effect on cardiac myocytes[32–34] but was not previously evaluated on a moderate stage of CRF. A similar preventive use, attributed to the pleiotropic action of rhEPO, which includes antiapoptotic mechanisms, was reported by Bernhardt and Eckardt (2008) as a protective mechanism against development of acute kidney injury in critically ill patients at higher risk of acute nephropathy.[35]
CKD is associated with inflammatory mechanisms, viewed by the increment of acute phase protein synthesis, such as C-reactive protein, a prominent marker of inflammatory response in the general population and in CKD patients, which was reported elevated in hemodialyzed patients.[36] Chronic inflammation is also associated with atherosclerotic cardiovascular disease (CVD) and the high rate of morbidity and mortality observed in hemodialyzed patients[37] could reflect the inflammatory process in CKD patients favoring CVD events. rHEPO has been shown a pleiotropic action,[38] which includes an anti-inflammatory activity already reported on other conditions, including in cardiomyocytes with hypoxia/reoxygenation injury, in the brain, as well as in the kidney.[39,40] In our study, CRF was not associated with significant changes on the inflammatory markers, viewed by normal gene profile of both TNF-a and NF-κb in the heart, which is in agreement with our previous assays in serum markers of inflammation.[11–13] However, the IL-2 gene overexpression was prevented with rhEPO, suggesting an inhibitory effect on T-cell-induced IL-2 production. Previous studies on CKD patients have shown serum IL-2 increment when compared with controls,[41,42] an effect that was reduced by rhEPO treatment, demonstrating that rhEPO has an immunomodulatory action. Our data reinforces this notion, demonstrating that the rhEPO effect is due to downregulation of CRF induced IL-2 gene overexpression on a moderate model of CRF.
EPO is a hormone regulating the proliferation and differentiation of erythroid precursor cells, which have been demonstrating proproliferative and proangiogenic activity.[24,43] In our model of moderate CRF, heart gene expression of TGF-b1 and VEGF, which are well-recognized markers of proliferation and angiogenesis, respectively, was unchanged. This result suggests that other pathways should play a major role on the proproliferative profile encountered in this model, previously demonstrated by us through the increment in the trophism of both tissues, as well as through the increased levels of serum TGF-b1.[13] rhEPO treatment, accordingly, did not modify the expression of both genes in the CRF rats. However, rhEPO was able to exacerbate the effect of CRF on PCNA expression, thus demonstrating a proliferative action associated with DNA synthesis and repair, which are known functions of PCNA,[44,45] thus suggesting a regenerative function of rhEPO in this tissue. Similar effect was reported in a model of acute renal failure induced by cisplatin in the rat,[46] as well as in the liver after resection in the rat but was not previously documented on the heart tissue of moderate CRF models.[47]
Because EPO has been indicated as an antioxidant tissue-protective cytokine,[48] we also assessed the influence of rhEPO treatment on heart gene expression of cytochrome c, viewed as a marker of mitochondrial injury, as well as on the expression of NO synthase 2 and 3, which are the key enzymes in inducible and constitutive NO synthesis and which participate in oxidative stress under unimpaired circumstances. In our model of moderate CRF, both cytochrome C and NO synthases gene expression were unchanged, suggesting that this tissue was in an earlier stage of oxidative lesion.
In conclusion, in this model of moderate CRF, rhEPO treatment showed important cardiac nonhematopoietic effects, expressed mainly by the antiapoptotic and the proproliferative action on the heart. This data suggests that early rhEPO in moderate stages of CRF, before critical lesion of the tissues, might have further benefits.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Deicher R, Hörl WH. Anaemia as a risk factor for the progression of chronic kidney disease. Curr Opin Nephrol Hypertens. 2003;12:139–43. doi: 10.1097/00041552-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Miranda Sde P, Macedo RN, Silva Júnior GB, Daher Ede F. Cardio-renal syndrome: Pathophysiology and treatment. Rev Assoc Med Bras. 2009;55:89–94. doi: 10.1590/s0104-42302009000100022. [DOI] [PubMed] [Google Scholar]
- 3.van der Meer P, Voors AA, Lipsic E, van Gilst WH, van Veldhuisen DJ. Erythropoietin in cardiovascular disease. Eur Heart J. 2004;25:285–91. doi: 10.1016/j.ehj.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anaemia on cardiomyopathy morbidity and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28:53–61. doi: 10.1016/s0272-6386(96)90130-4. [DOI] [PubMed] [Google Scholar]
- 5.Locatelli F, Conte F, Marcelli D. The impact of hematocrit levels and erythropoietin treatment on overall and cardiovascular mortality and morbidity – the experience of the Lombardy dialysis Registry. Nephrol Dial Transplant. 1998;13:1642–4. doi: 10.1093/ndt/13.7.1642. [DOI] [PubMed] [Google Scholar]
- 6.Latini R, Brines M, Fiordaliso F. Do non-hemopoietic effects of erythropoietin play a beneficial role in heart failure? Heart Fail Rev. 2008;13:415–23. doi: 10.1007/s10741-008-9084-z. [DOI] [PubMed] [Google Scholar]
- 7.Mastromarino V, Volpe M, Musumeci MB, Autore C, Conti E. Erythropoietin and the heart: Facts and perspectives. Clin Sci (Lond) 2011;120:51–63. doi: 10.1042/CS20100305. [DOI] [PubMed] [Google Scholar]
- 8.van der Putten K, Braam B, Jie KE, Gaillard CA. Mechanisms of Disease: erythropoietin resistance in patients with both heart and kidney failure. Nat Clin Pract Nephrol. 2008;4:47–57. doi: 10.1038/ncpneph0655. [DOI] [PubMed] [Google Scholar]
- 9.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–90. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 10.Lipsic E, Schoemaker RG, van der Meer P, Voors AA, van Veldhuisen DJ, van Gilst WH. Protective effects of erythropoietin in cardiac ischemia: from bench to bedside. J Am Coll Cardiol. 2006;48:2161–7. doi: 10.1016/j.jacc.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Garrido P, Reis F, Costa E, Teixeira-Lemos E, Parada B, Alves R, et al. Characterization of a rat model of moderate chronic renal failure--focus on hematological, biochemical, and cardio-renal profiles. Ren Fail. 2009;31:833–42. doi: 10.3109/08860220903151435. [DOI] [PubMed] [Google Scholar]
- 12.Garrido P, Reis F, Costa E, Almeida A, Parada B, Teixeira-Lemos E, et al. Effect of recombinant human erythropoietin in a rat model of moderate chronic renal failure - focus on inflammation, oxidative stress and function/renoprotection. The Open Drug Discovery J. 2010;2:25–32. [Google Scholar]
- 13.Teixeira AM, Garrido P, Santos P, Alves R, Parada B, Costa E, et al. Recombinant human erythropoietin treatment protects the cardio-renal axis in a model of moderate chronic renal failure. Ren Fail. 2010;32:1073–80. doi: 10.3109/0886022X.2010.509897. [DOI] [PubMed] [Google Scholar]
- 14.Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2004;286:R977–88. doi: 10.1152/ajpregu.00577.2003. [DOI] [PubMed] [Google Scholar]
- 15.Jelkmann W. Control of erythropoietin gene expression and its use in medicine. Methods Enzymol. 2007;435:179–97. doi: 10.1016/S0076-6879(07)35010-6. [DOI] [PubMed] [Google Scholar]
- 16.Lappin T. The cellular biology of erythropoietin receptors. Oncologist. 2003;8(Suppl 1):15–8. doi: 10.1634/theoncologist.8-suppl_1-15. [DOI] [PubMed] [Google Scholar]
- 17.Weidemann A, Johnson RS. Nonrenal regulation of EPO synthesis. Kidney Int. 2009;75:682–8. doi: 10.1038/ki.2008.687. [DOI] [PubMed] [Google Scholar]
- 18.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2- ΔΔCT.Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Bogoyevitch MA. An update on the cardiac effects of erythopoietin cardioprotetion by erythropoietin and the lessons learnt from studies in neuroprotection. Cardiovasc Res. 2004;63:208–16. doi: 10.1016/j.cardiores.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Manolis AS, Tzeis S, Triantafyllou K, Michaelidis J, Pyrros I, Sakellaris N, et al. Erythropoietin in heart failure and other cardiovascular diseases: hematopoietic and pleiotropic effects. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:355–75. doi: 10.2174/156800605774370326. [DOI] [PubMed] [Google Scholar]
- 22.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–8. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, et al. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308:990–4. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- 24.Raddino R, Robba D, Caretta G, Bonadei I, Teli M, Zanini G, et al. Erythropoietin: a new perspective in cardiovascular therapy. Monaldi Arch Chest Dis. 2008;70:206–13. doi: 10.4081/monaldi.2008.414. [DOI] [PubMed] [Google Scholar]
- 25.Abdelrahman M, Sharples EJ, McDonald MC, Collin M, Patel NS, Yaqoob MM, et al. Erythropoietin attenuates the tissue injury associated with hemorrhagic shock and myocardial ischemia. Shock. 2004;22:63–9. doi: 10.1097/01.shk.00001276869.21260.9d. [DOI] [PubMed] [Google Scholar]
- 26.Ma R, Xiong N, Huang C, Tang Q, Hu B, Xiang J, et al. Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signalling pathway. Neuropharmacology. 2009;56:1027–34. doi: 10.1016/j.neuropharm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 27.van der Meer P, van Veldhuisen DJ. [New applications of erythropoietin in cardiovascular disease: from haematopoiesis to cardiac protection] Ned Tijdschr Geneeskd. 2008;152:923–7. [PubMed] [Google Scholar]
- 28.Moriyama MT, Tanaka T, Morita N, Ishii T, Chikazawa I, Suga K, et al. Renal protective effects of erythropoietin on ischemic reperfusion injury. Cell Transplant. 2010;19:713–21. doi: 10.3727/096368910X508816. [DOI] [PubMed] [Google Scholar]
- 29.Pallet N, Bouvier N, Legendre C, Beaune P, Thervet E, Choukroun G, et al. Antiapoptotic properties of recombinant human erythropoietin protects against tubular cyclosporine toxicity. Pharmacol Res. 2010;61:71–5. doi: 10.1016/j.phrs.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Toba H, Sawai N, Morishita M, Murata S, Yoshida M, Nakashima K, et al. Chronic treatment with recombinant human erythropoietin exerts renoprotective effects beyond hematopoiesis in streptozotocin-induced diabetic rat. Eur J Pharmacol. 2009;612:106–14. doi: 10.1016/j.ejphar.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Zhang J. Protective effect of erythropoietin against aristolochic acid-induced apoptosis in renal tubular epithelial cells. Eur J Pharmacol. 2008;588:135–40. doi: 10.1016/j.ejphar.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 32.Timmer SA, De Boer K, Knaapen P, Götte MJ, Van Rossum AC. The potential role of erythropoietin in chronic heart failure: From the correction of anemia to improved perfusion and reduced apoptosis? J Card Fail. 2009;15:353–61. doi: 10.1016/j.cardfail.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Fu P, Arcasoy MO. Erythropoietin protects cardiac myocytes against anthracycline-induced apoptosis. Biochem Biophys Res Commun. 2007;354:372–8. doi: 10.1016/j.bbrc.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, et al. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308:990–4. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- 35.Bernhardt WM, Eckardt KU. Physiological basis for the use of erythropoietin in critically ill patients at risk for acute kidney injury. Curr Opin Crit Care. 2008;14:621–6. doi: 10.1097/MCC.0b013e328317ee82. [DOI] [PubMed] [Google Scholar]
- 36.Schindler R, Senf R, Frei U. Influencing the inflammatory response of haemodialysis patients by cytokine elimination using large-pore membranes. Nephrol Dial Transplant. 2002;17:17–9. doi: 10.1093/ndt/17.1.17. [DOI] [PubMed] [Google Scholar]
- 37.Hung CY, Chen YA, Chou CC, Yang CS. Nutritional and inflammatory markers in the prediction of mortality in Chinese hemodialysis patients. Nephron Clin Pract. 2005;100:c20–6. doi: 10.1159/000084654. [DOI] [PubMed] [Google Scholar]
- 38.Chatterjee PK. Pleiotropic renal actions of erythropoietin. Lancet. 2005;365:1890–2. doi: 10.1016/S0140-6736(05)66622-6. [DOI] [PubMed] [Google Scholar]
- 39.Chang YK, Choi DE, Na KR, Lee SJ, Suh KS, Kim SY, et al. Erythropoietin attenuates renal injury in an experimental model of rat unilateral ureteral obstruction via anti-inflammatory and anti-apoptotic effects. J Urol. 2009;181:1434–43. doi: 10.1016/j.juro.2008.10.105. [DOI] [PubMed] [Google Scholar]
- 40.Qin C, Xiao YB, Zhong QJ, Chen L, Wang XF. Anti-inflammatory effect of erythropoietin pretreatment on cardiomyocytes with hypoxia/reoxygenation injury and the possible mechanism. Chin J Traumatol. 2008;11:352–8. doi: 10.1016/s1008-1275(08)60071-1. [DOI] [PubMed] [Google Scholar]
- 41.Costa E, Lima M, Alves JM, Rocha S, Rocha-Pereira P, Castro E, et al. Inflammation, T-cell phenotype, and inflammatory cytokines in chronic kidney disease patients under hemodialysis and its relationship to resistance to recombinant human erythropoietin therapy. J Clin Immunol. 2008;28:268–75. doi: 10.1007/s10875-007-9168-x. [DOI] [PubMed] [Google Scholar]
- 42.Debska-Slizien A, Rutkowski B, Manitius J, Zdrojewski Z, Szołkiewicz M, Bułło B, et al. Influence of erythropoietin on immunological system of patients with chronic renal failure. Pol Merkur Lekarski. 2003;15:326–7. [PubMed] [Google Scholar]
- 43.Efthimiadou A, Pagonopoulou O, Lambropoulou M, Papadopoulos N, Nikolettos NK. Erythropoietin enhances angiogenesis in an experimental cyclosporine A-induced nephrotoxicity model in the rat. Clin Exp Pharmacol Physiol. 2007;34:866–9. doi: 10.1111/j.1440-1681.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- 44.Shivji KK, Kenny MK, Wood RD. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992;69:367–74. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- 45.Essers J, Theil AF, Baldeyron C, van Cappellen WA, Houtsmuller AB, Kanaar R, et al. Nuclear dynamics of PCNA in DNA replication and repair. Mol Cell Biol. 2005;25:9350–9. doi: 10.1128/MCB.25.21.9350-9359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagnis C, Beaufils H, Jacquiaud C, Adabra Y, Jouanneau C, Le Nahour G, et al. Erythropoietin enhances recovery after cisplatin-induced acute renal failure in the rat. Nephrol Dial Transplant. 2001;16:932–8. doi: 10.1093/ndt/16.5.932. [DOI] [PubMed] [Google Scholar]
- 47.Schmeding M, Boas-Knoop S, Lippert S, Ruehl M, Somasundaram R, Dagdelen T, et al. Erythropoietin promotes hepatic regeneration after extended liver resection in rats. J Gastroenterol Hepatol. 2008;23:1125–31. doi: 10.1111/j.1440-1746.2007.05265.x. [DOI] [PubMed] [Google Scholar]
- 48.Johnson DW, Forman C, Vesey DA. Novel renoprotective actions of erythropoietin: new uses for an old hormone. Nephrology (Carlton) 2006;11:306–12. doi: 10.1111/j.1440-1797.2006.00585.x. [DOI] [PubMed] [Google Scholar]


