Abstract
Animal experimentation is carried out in consultation with the veterinary wing but it is essential that be familiar with experimental protocols of animal model to be able to design an approriate study. This is more so in place where the veterinary facilities are not easily available.Span Rabbits are commonly used as subjects for screening implant material. They have gained favour for their numerous advantages even though they should be ideally used prior to testing in a larger animal model. Though experimentation on rabbits seems to be easy there are many pitfalls. Our endeavor in this article is to integrate all the data about maintaining rabbits as a model and to critically analyze it on the basis of our experimentation.
Keywords: Animal model, experimental model, rabbit model
INTRODUCTION
The first step in any study is to review the literature on the model on which to perform the experiment. When research is performed on the appropriate animals, the information obtained should approximate what can be expected in human beings,[1] i.e., animal that is phylogenetically similar to human beings. The emotional attitude of some researchers, mainly in cases involving domestic animals (e.g., dogs and cats) can be a problem in long experiments where the researcher may become emotionally attached to the animal. Dogs are the most widely used animal model in periodontal research, especially in the periodontal tissue regeneration studies and as periodontal disease models. Despite the structural similarities in organic composition between human bone and canine, implant-associated changes evident in a canine model may not be as apparent in the human situation where there is a lower rate of remodeling.[2,3] Monkeys were at a second level and rats, rabbits were at the third level.[4] International standards established regarding the species suitable for testing implantation of materials in bone state that dogs, sheep, goat, pigs, or rabbits are suitable. Arriving at an animal model which suits all fields of application is a current goal in research and that is almost impossible. But the wide range of animal species gives choice to the investigators for a most appropriate animal for their study design.
If there is more than one alternative, one should select the least phylogenetically developed animals available in sufficient quantity.[5] Figure 1 represents the phylogenetic tree for vertebrates.[6]
Figure 1.
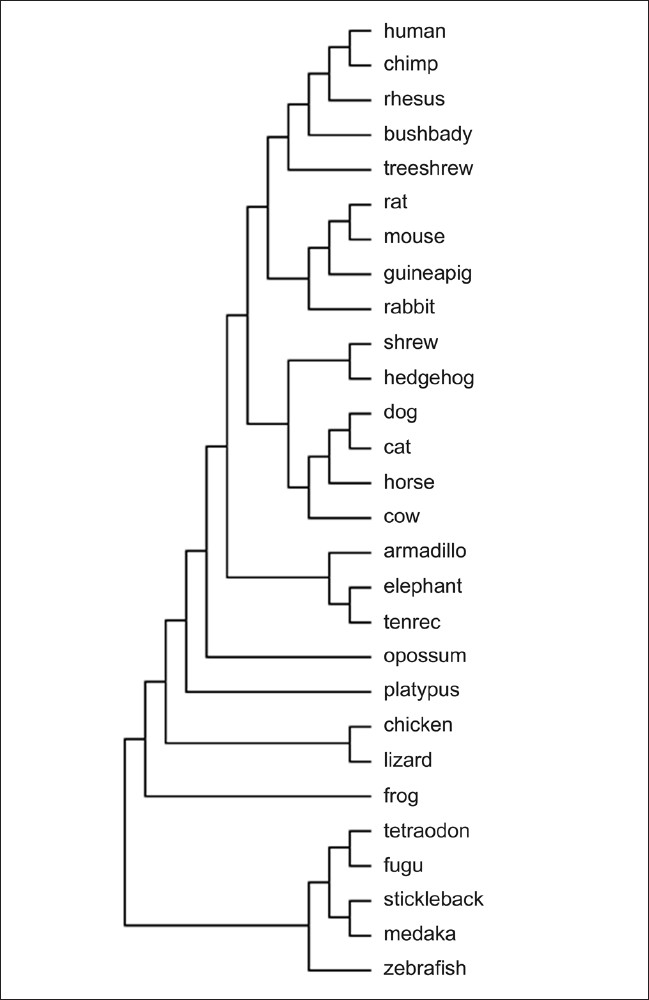
Phylogenetic tree for vertebrates
The animals used as research models are divided into the following two groups:
-
Small animal category requiring local animal ethical committee approval:
- Rats
- Mice
- Guinea pigs
- Rabbit (largest animal in this group)
-
Large animal category requiring central animal ethical committee approval in addition to the local ethical committee are:
- Dogs
- Goats
- Primates
Rabbits are small mammals in the family of Leporidae of the order Lagomorpha[7] found in several parts of the world. Their habitats include meadows, wood, forest, and grass lands. Although the macrostructure and microstructure of rabbit bone are dissimilar to human bone,[8] rabbits are commonly used for screening implant material prior to testing in a larger animal model.[9]
Amongst various strains, New Zealand white strains of rabbits are commonly being used for research activities. These strains are less aggressive in nature and have less health problems as compared with other breeds.
MICROSTRUCTURE OF RABBIT BONE
Histologically, rabbit long bones have a very different microstructure from human beings.[9–10] In comparison with the secondary bone structure of mature human bone, rabbits have a primary vascular longitudinal tissue structure, comprising vascular canals of osteons running parallel with the long axis of the bone, surrounding the medullary canal as well as the periosteal surface. The bone between these layers is comprised of dense haversian bone.[11] The maximum mean osteon diameter described was 223.79+47.69 μm, with a mean minimum diameter of 50.79+9.71 μm.
BONE COMPOSITION
Although there is minimal literature on the differences between human and rabbit bone composition and density, some similarities are reported in the bone mineral density and subsequently the fracture toughness of mid-diaphyseal bone between rabbits and human beings.[9]
BONE REMODELLING
In comparison with other species, such as primates and some rodents, the rabbit has faster skeletal change and bone turnover (significant intracortical, Haversian remodelling).[12–14] This may make it difficult to extrapolate results from studies performed in rabbits onto the likely human clinical response. However, rabbits are commonly used for screening implant materials prior to testing in a larger animal model.
ADVANTAGES OF RABBIT AS A MODEL ARE AS FOLLOWS
This animal is very docile and non-aggressive and hence easy to handle and observe.
Widely bred and very economical compared with the expense of larger animals.
Have short vital cycles (gestation, lactation, and puberty)
It comes under the small animal category, hence comes under the purview of local ethical committee. Larger animals require an additional clearance from the central ethical committee clearance which is a time-consuming process with stringent rules.
The mandatory rearing facilities for ethical clearance for surgical procedures on larger animals are very elaborate and expensive, generally only within the capacity of central animal research facilities.
Experimentation in rabbits poses a general set of problems, some of which are common to many animals.
Lack of well-equipped animal houses.
Lack of expert handlers of the animal.
Lack of easy availability of post- and intraoperative medicines, particularly for rabbits.
Scarcity of literature on the care of experimental utilization of rabbits.
The specific disadvantages as regards to implantation of dental implants are as follows:
Being a small animal, the size of the mandible does not permit insertion of dental implants and hence loading of the implants is not possible after implantation elsewhere in the body.
The rabbit is an animal of prey which has to be fast and swift in nature. Thereby, they have a small light and fragile bony structure.
The mid-femur diameter is only about 0.5 cm for a rabbit weighing 3 kg, making it inadequate for the larger size implants generally associated with human beings.
The rabbit is a vulnerable animal prone to post-antibiotic diarrhea, gastrointestinal stasis or ileus, and post-implantation fractures due to a fragile weight-bearing femur.
Veterinary surgeons specializing in rabbits are not many.
MAINTENANCE OF RABBITS IN THE ANIMAL HOUSE
Newly arrived rabbits in the animal house should be quarantined for a minimum of two weeks and examined for the most common diseases. Rabbits are easily infested with scabies, which is highly contagious and capable of damaging the airways and predisposing to pneumonia.[15] Quarantine also serves as a period of adaptation to the surroundings and the daily routine in the animal quarters. Adult rabbits should be kept in individual mesh cages (0.90 × 0.60 × 0.45 m) hung at a height of 0.8 cm from the ground so that excrement can fall out into collecting trays.[16] 12 to 14 hours of light are necessary for the colony's circadian biorhythms and animals should be routinely observed for food consumption and fecal characteristics.[17,18]
DIET
The diet of an adult rabbit consists of fresh hay, water, and fresh vegetables. Water should be changed every day and should be available round the clock. Dehydration in rabbit is a very common serious condition that needs immediate attention. Signs of dehydration can be detected by the skin on their body. The skin on the back of the rabbit's neck is raised upward until it is stretched enough that is comfortable to the rabbit. If it snaps back fast, the rabbit is healthy. The slower it takes for the skin to return, the more is the dehydration. In such a case, one can hydrate them by giving RGatorade—mix the Gatorade half and half with water and feed them with a dropper.[19]
HANDLING
Daily human contact reduces stress during handling. Gloves should always be worn when handling rabbits. These animals can become nervous and inflict severe bites or scratches from their powerful hind legs, if not properly handled and restrained. Rabbits should never be handled by the ears because of a high probability of causing cervical luxation and death.[17,20] They should be held by grasping a large fold of loose skin over the shoulders with one hand and either supporting or grasping the rear feet with the other hand. Failing to support or hold onto the rear feet may result in the animal kicking and trying to escape, which can cause severe spinal injury or a broken back.
IMPORTANT CONSIDERATIONS FOR THE USE OF RABBITS FOR EXPERIMENTAL RESEARCH
Choice of implant size
The implant size and length should be as small as possible. The recommended norm is of 2 mm diameter and 6 mm length as there is size limitation of rabbit bone.[21] Smaller size of the implant also reduces the sequencing of drills and the drilling time which is advantageous as ketamine is a short-acting anesthetic with an induction time of half an hour during which both the legs have to be opened up and implants have to be placed.
Rabbit size
Contemporary literature survey has given little importance to size of rabbit in experiments with implant surgery. But our experience shows that size does play a crucial role. Choosing a healthy large animal more than 3 to 3.5 kg leaves one with an experimental subject with a good capacity to withstand surgical trauma, preventing most of the serious postoperative problems and leading to a better survival rate. Gender is generally not a problem, but some investigators in literature have preferred female sex as it is biologically the stronger sex.
Position of implantation
We found the ideal location for implantation to be the proximal femoral condyle. It has both cortical and cancellous bone. Due to presence of cancellous bone in addition to cortical bone, it provides a cushioning effect and prevents the cortical bone from splintering which is commonly observed in other parts of the femur and tibia. Also, the bone at this place gives adequate space for the implantation and therefore a wider and longer implant can be used. A 6 mm diameter and 8 mm long implant can be placed with ease by this approach in comparison with the recommended norm of 2 mm diameter and 6 mm length.
Implantation can be by the medial approach or the lateral approach in the proximal femoral condyle. A lateral approach puts the following structures at risk of damage:[22]
Fibular collateral ligament
Peroneal nerve
Popliteus tendon
The nerve is reflected along with the fascia with this approach.
The medial approach puts the following structures at risk:
The infrapatellar branch of saphenous nerve.
The distal tibial head is the next best location. Placing more than one implant in one limb predisposes the already fragile rabbit bone to fracture and must be attempted with caution only if the implant size is small, i.e., 2 to 3 mm in diameter and 6 mm or less in length. We contemplate that a larger rabbit size and a small implant can only be the way to use more than one implant in the same limb safely.
Anesthesia
Preoperative fasting is not necessary because vomiting during induction of anesthesia has not been reported in this species. Rabbits are subject to dehydration, so it should be ascertained that they always have plenty of water. These animals can be provided with water until approximately 60 minutes prior to starting the anesthesia. Anesthetic agents are selected based on the health of the animal and the duration and invasiveness of the procedure.
Ketamine (50-60 mg kg Inter Venous) is the most preferred drug and should be administered slowly through the lateral auricular vein [Figure 2] after the animal is immobilized in a special chamber. Providing inadequate anesthesia and analgesia will lead to the rabbit regaining consciousness during surgery and flapping its legs. With ketamine alone, our first experimental rabbit kept regaining consciousness and flapping its legs wildly and kicking off the instruments from the surgical table to the ground. Most of the surgery had to be performed with the rabbit being physically constrained. Toward the end of the surgery, i.e., after implantation, the rabbit flapped its leg and broke the femur into two pieces. Subsequently, in the next rabbit, lignocaine was also used, which gave satisfactory results.
Figure 2.
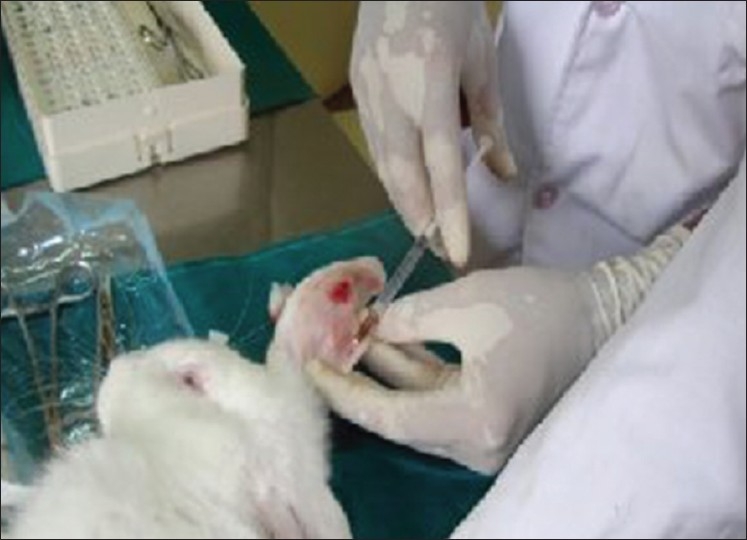
Administration of Ketamine through lateral auricular vein
But the best results were only after administration of xylazine (0.6 ml Inter Muscular) which kept the animal sedated for a longer time with minimal pain. In several laboratory species (i.e., rodents, rabbits, ruminants, ferrets), xylazine has proven to be a safe anesthetic adjunct when coadministered with ketamine to induce short periods of surgical anesthesia. When combined with ketamine, muscle relaxation and visceral analgesia are improved, and emergence from anesthesia is smoother.[23] Xylazine has a wide margin of safety. Moreover, increasing the dose does not generally increase the degree of sedation but rather the duration of effect.
Shaving and antisepsis
The region to be operated should always be shaved before any procedures. This can be done with a safety blade razor or electric shaver. All shaved hairs should be cleaned away to avoid contamination.
Surgical procedure
Lidocaine with adrenaline is administered locally. An incision is made using No. 22 blade. The incision is kept on the ventral side of the joint [Figure 3]. Once the skin is incised, the fascia is cut with a pair of scissors until the bone is reached. Care should be taken to cut the fascia only and not the muscle. The periosteum is reflected with a periosteal elevator. First, a pilot drill is used and then the implant site is enlarged to the final diameter. Intermittent drilling with a low speed rotary and profuse saline irrigation is necessary while drilling. The implant is then placed and screwed into the bone [Figure 4]. The fascia is sutured with catgut and the skin is sutured with silk. The silk sutures are kept long to prevent the rabbit from chewing the sutures off completely and opening the surgical site. Some investigators advise intracutaneous silk sutures which do not extend above the surface of the skin. Cutaneous sutures are painful if the anesthesia has started to wear off. And this can result in rabbit kicking the legs vigorously. The prevention and control of pain is the key to the practice of anesthesia. The perioperative analgesic protocol has an impact on the well-being of the animal that often extends far beyond the immediate anesthetic period.[24]
Figure 3.
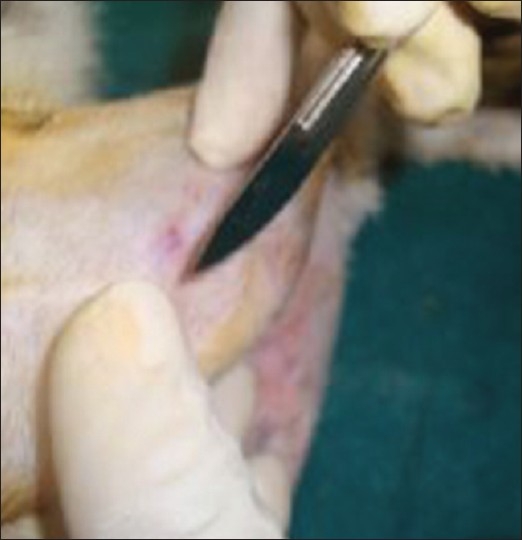
Incision being given on the ventral side of the joint
Figure 4.
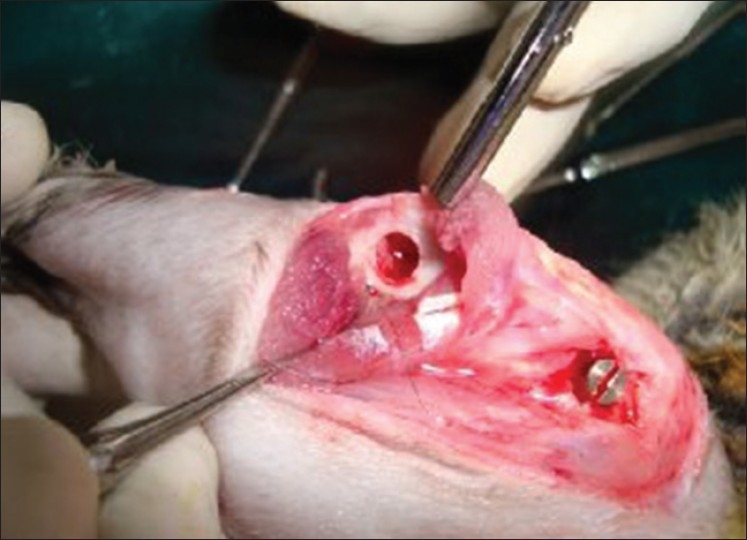
Implant placed at the femoral condyle
Postoperative management
Moderate to severe postoperative pain can represent a serious stress to the rabbit and can result in some potentially dangerous consequences. In the past, when we did not understand the full significance of pain management in rabbits, they would often survive a surgical procedure, only to die within the next 36 hours. The old cliché “the operation was a success, but the patient died” would apply to those situations. Some of those cases would most likely have survived if postsurgical pain had been managed, thereby reducing the stress on the rabbit.
How can we recognize pain in rabbits?
When working with rabbits, the practitioner must infer the presence of pain by observing changes from normal behavior.[22] A normal rabbit is bright, alert, active, inquisitive, has a smooth coat and good body condition. Pain may be evident as a limp or a change in gait, withdrawal or protection of an injured part, awkward or abnormal postures, licking, rubbing or scratching at an area, or indicated by decreased food and water intake. It is important to know that rabbits evolved as a prey species, an animal that normally needs to hide any handicap in order to escape predation. Hence, Signs of pain may be subtle, such as an increase in respiration, reluctance to move, sudden aggression, persistently squinting the eyes, a loss of interest in the surroundings, or an inability to rest or sleep normally.
Animals should be carefully evaluated during the first 24 postoperative hours, checking for signs of pain, because even if they do not vocalize them, remaining quiet and immobile in the back of their cages may be a sign of distress. Excessive pain can prolong recovery time from illness or injury. It can cause a rabbit to stop eating, loading to eventual shutting down of the gastrointestinal tract and death. Rabbits in excessive pain also can go into shock and die within 24 to 48 hours, despite the fact that the illness or injury itself may not have been life-threatening. Hence, analgesics should consistently be used for all major surgeries. The most common analgesics currently used in rabbits include butorphanol, buprenorphine, and nonsteroidal anti-inflammatory drugs, such as aspirin, carprofen, diclofenac, flunixin, ibuprofen, indomethacin, ketoprofen, meloxicam, acetaminophen, and piroxicam.
As an immediate postoperative management, one dose of Bupivacaine (A long-acting local anesthetic agent) can be given around the wound as the first dose. Later Ibugesic Plus syrup can be given twice or thrice daily for pain management. To administer the syrup, take the required amount in the dropper and hold the dropper near the rabbit's mouth. Most of the rabbits will suck the syrup out of the dropper. For the ones which do not, insert your fingers behind the incisors and the jaw of the rabbit, so that its mouth is forced open. The syrup is forced into the rabbit's mouth using a dropper until the rabbit swallows the sweet syrup by reflex. The intramuscular and subcutaneous route for the pain killer and antibiotics should be avoided for a small animal like rabbit as the trauma of the injections will add substantially to the shock of surgery and the rabbit may go into gastrointestinal ileus. The antibiotic of choice is enrofloxacin which is given orally with the help of a dropper, for 5 to 7 days. Amoxicillin should not be given as it leads to fatal diarrhea.
RABBIT BEHAVIOR POST-ANESTHESIA/SURGERY
There are specific signs to be aware of in rabbits after an anesthetic/surgical procedure.[25] They are as follows:
Quiet behavior
Your rabbit may want to sleep and stay quietly in one area. The lack of activity can be caused by residual anesthetic in the body, pain, or medication. Some analgesics have sedative properties and may add to the lethargy. Check with your veterinarian about what you should expect with any drugs you are giving to your rabbit. If your pet is extremely lethargic, crying out, or appears unable to move normally and sits hunched in one spot, you should contact your veterinarian immediately.
Poor appetite
The rabbit may not eat or drink at all for the first 24 hours after the surgery. Analgesics may improve this situation, but not completely eliminate it. You can syringe feed your pet with thin slurry blenderized fresh vegetables mixed with fruit juice one to two times during this initial 24-hour period. A food supplement called Fibreplex is available which helps maintain good gut motility and provides probiotics and prebiotics to encourage normal digestive function, which is vital for their recovery. If your rabbit still refuses to eat after 24 hours, you should contact your veterinarian immediately.
Abnormal or absence of stools
Anesthetic and analgesic agents can alter the motility of the gastrointestinal tract. In addition, the rabbit may have eaten poorly just prior to surgery; therefore, there may be an absence of stools for a period up to three days after anesthesia/surgery. Watery diarrhea should not be present and is cause for immediate concern.
Sutures
The rabbits have a tendency to bite off their sutures with their sharp teeth. This can lead to gaping of the wound. This can be overcome with a help of RHealex spray which forms a polymer layer over the wound. It should never be applied when the rabbit is conscious as the burning sensation from the spray can make the rabbit run wild leading to fracture of the already weakened legs.
Death
The most likely cause of death reported in rabbit model is gastrointestinal stasis or ileus. This is caused due to reduction in peristalsis which is caused due to factors like:
Fractures
Loss of mate
New environment (can lower their immunity making them more prone to illness)
Stress[26]
In gastrointestinal stasis due to stoppage of peristalsis, the toxins produced by Clostridium accumulate and may cause death in 12 hours. General sign of this is, the rabbit stops eating and dies eventually. This can be avoided by giving prokinetics (Cisapride 0.2 mg/kg orally, Simethicone, Metoclopramide).
As a caregiver, you can do a number of things to minimize your rabbit's discomfort, such as careful handling of the sick rabbit, prompt communication with your veterinarian, gentle nursing care to improve your rabbit's comfort, access to food and water, and a palatable diet to keep the rabbit eating. It is important to prevent changes in gastrointestinal motility, especially when the rabbit is already stressed by disease. The faster the rabbit starts eating, the faster the recovery.
Euthanasia
Euthanasia can be accomplished easily, painlessly, and without prior stress.[2] The technique preferred by veterinarians involves sedation followed by injection of a barbiturate. Sodium pentobarbital is the drug of choice because it acts fast and effectively.[16]
Our experience
One of the main complications of implant surgery in rabbits which we observed was the fracture of the femur. This can happen while drilling or postoperatively. The hallucinating effect while coming out of the short-acting anesthesia can make them jump around violently and can result in usually fracture of the femur. Despite a Spica cast (used for femur fractures and to hold the hip or thigh muscles and tendons in place after surgery to allow healing) that was placed, the rabbit managed to wriggle out of it. Death of the rabbit is also a common finding following fracture, which was confirmed by the postmortem analysis stating the cause as fat embolism. In our observation, the rabbits did not maintain a postoperative bandage or cast and removed it by biting. The rabbits with intra- and postoperative fracture should be kept under observation and their mobility should be limited by placing boxes in their cages. Taking these measures can help the rabbit overcome the trauma and can result in better healing. We were able to carry out surgical procedures successfully in rabbits following the guidelines described above in which we studied osseointegration. All have been completed without any significant untoward results for either the animals or the research staff and with positive results toward evaluating hypotheses and explaining mechanisms.
DISCUSSION
Animal research in addition to clinical research on human beings has contributed greatly to the understanding of the various physiological and pathological processes affecting human beings.[27] The use of animals in experimental research must be based on scientific, ethical, and legal principles. If the researcher keeps these three aspects in balance in his activities, he will succeed without compromising his work or his reputation.[28] Our development of appropriate research protocols reviewed by our institutional research ethics committee involving surgical procedures on white New Zealand rabbits has allowed us to carry out projects with good quality, control, and safety for both the researchers and the animals.
CONCLUSION
Animal models are being used for experimental studies in various branches of medical and dental sciences, because certain of the research areas obviously cannot be done on human beings for practical and ethical reasons. And, rabbit being an easily available and less aggressive animal is a promising model if the guidelines described above are followed.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Liebschner MA. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials. 2004;25:1697–714. doi: 10.1016/s0142-9612(03)00515-5. [DOI] [PubMed] [Google Scholar]
- 2.Bloebaum RD, Merrell M, Gustke K, Simmons M. Retrieval analysis of a hydroxyapatite-coated hip prosthesis. Clin Orthop Relat Res. 1991;267:97–102. [PubMed] [Google Scholar]
- 3.Bloebaum RD, Ota DT, Skedros JG, Mantas JP. Comparison of human and canine external femoral morphologies in the context of total hip replacement. J Biomed Mater Res. 1993;27:1149–59. doi: 10.1002/jbm.820270905. [DOI] [PubMed] [Google Scholar]
- 4.Dannan A, Alkattan F. Animal models in periodontal research. A mini-Review of the literature. [Last cited on 2010 Sept 01];The internet journal of veterinary medicine [Serial Online] 2008 5(1) Available from: http://www.ispub.com/journal/the_internet/volume_5_number_1_2.html . [Google Scholar]
- 5.Fagundes DJ, Taha MO. Animal disease model: Choice´s criteria and current animals specimens. Acta Cir Bras. 2004;19:59–65. [Google Scholar]
- 6.Miller W, Rosenbloom K, Hardison RC, Hou M, Taylor J, Raney B, et al. 28-way vertebrate alignment and conservation track in UCSC Genome Browser. Genome Res. 2007;17:1797–808. doi: 10.1101/gr.6761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: A review. Eur Cell Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 8.Okermann L. United States: Blackwell Scientific Publications; 2nd ed. United States: Blackwell Scientific Publications; 1994. Diseases of domestic rabbits; pp. 4–8. [Google Scholar]
- 9.Wang X, Mabrey JD, Agrawal CM. An interspecies comparison of bone fracture properties. Biomed Mater Eng. 1998;8:1–9. [PubMed] [Google Scholar]
- 10.Martiniakova M, Omelka R, Chrenek P, Ryban L, Parkanyi V, Grosskopf B, et al. Changes of femoral bone tissue microstructure in transgenic rabbits. Folia Biol (Praha) 2005;51:140–4. [PubMed] [Google Scholar]
- 11.Struillou X, Boutigny H, Soueidan A, Layrolle P. Experimental animal models in periodontology: A review. Open Dent J. 2010;4:37–47. doi: 10.2174/1874210601004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castañeda S, Largo R, Calvo E, Rodríguez-Salvanés F, Marcos ME, Díaz-Curiel M, et al. Bone mineral measurements of subchondral and trabecular bone in healthy and osteoporotic rabbits. Skeletal Radiol. 2006;35:34–41. doi: 10.1007/s00256-005-0022-z. [DOI] [PubMed] [Google Scholar]
- 13.Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: Current status and comparison with other animal models. Bone. 1995;16:277S–84S. doi: 10.1016/8756-3282(95)00026-a. [DOI] [PubMed] [Google Scholar]
- 14.Gilsanz V, Roe TF, Gibbens DT, Schulz EE, Carlson ME, Gonzalez O, et al. Effect of sex steroids on peak bone density of growing rabbits. Am J Physiol. 1988;255:E416–21. doi: 10.1152/ajpendo.1988.255.4.E416. [DOI] [PubMed] [Google Scholar]
- 15.Schanaider A, Silva PC. Use of animals in experimental surgery. Acta Cir Bras. 2004;19:441–7. [Google Scholar]
- 16.Calasans-Maia MD, Rossi AM, Dias EP, Santos SR, Ascoli F, Granjeiro JM. Stimulatory effect on osseous repair of zinc-substituted hydroxyapatite.Histological study in rabbit tibia. Key Eng Mater. 2008;361-363:1269–72. [Google Scholar]
- 17.Podberscek AL, Blackshaw JK, Beattie AW. The effects of repeated handling by familiar and unfamiliar people on rabbits in individual cages and group pens. Appl Anim Behav Sci. 1991;28:365–73. [Google Scholar]
- 18.Susan E, Wilson -Sanders Biology and diseases of laboratory Rabbits. Research animal models. [Last cited on 2010 Sept 01]. Available at: http://www.uac.arizona.edu/VSC443/rabdisease/rabdiseases.htm .
- 19.Carew-Helium B. How to tell if a rabbit is dehydrated. [Last accessed on 2011 Sept 12]. Available from: http://www.helium.com .
- 20.Olfert ED. Canadian Council on Animal Care. Guide to the care and use of experimental animals. Ottawa: Conseil Canadien de Protection de Animaux (CCPA); 1993. Rabbits. [Google Scholar]
- 21.Schossler JE. Choice, Restraint and Handling of Experimental Animals. Acta Cir Bras. 1993;8:166–8. [Google Scholar]
- 22.Hellyer PW, Robertson AS, Fails AD. Pain and its management. In: Tranquilli WJ, Thurmon JC, Trimm KA, editors. Veterinary anesthesia and analgesia. 4th ed. United States: Blackwell; 2007. pp. 31–60. [Google Scholar]
- 23.Fujimoto J. Laboratory animal anesthesia. In: Greene SA, editor. Veterinary anesthesia and pain management secrets. Philadelphia: Hanley and Belfus; 2002. pp. 299–306. [Google Scholar]
- 24.Jordan C, Mizabeige E. New York: Thieme; 2000. Atlas of orthopaedic surgical exposures; p. 144. [Google Scholar]
- 25.Susan Brown. Taking the fear out of rabbit anesthesia and surgery. [Last accessed on 2011 Sept 12]. Available from: http://www.veterinarypartner.com .
- 26.Reusch B. Rabbit gastroenterology. Vet Clin N Am Exot Anim Pract. 2005;8:351–75. doi: 10.1016/j.cvex.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira LM, Hochman B, Barbosa MV. Modelos experimentais em pesquisa. Acta Cir Bras. 2005;20:28–34. doi: 10.1590/s0102-86502005000800008. [DOI] [PubMed] [Google Scholar]
- 28.Petroianu A. Ethical Aspects of Animal Research. Acta Cir Bras. 1996;11:157–64. [Google Scholar]


