Abstract
Background:
Recurrent Herpes Labialis (RHL) is one of most common infective vesiculoulcerative lesions. According to some studies administration of topical and/or systemic zinc compositions has been effective in treatment and prevention. This article aims to comparison of zinc level in healthy subjects and RHL patients in acute and convalescent phases.
Materials and Methods:
This was a retrospective case – control study, carried on 80 individuals (40 normal and 40 RHL patients) mean age=34.5 and 34.4, respectively. Saliva samples were taken in patients in acute phase once and after healing of lesions in convalescent phase (averagely 21 days later) and in normal individuals. Salivary zinc level concentration was measured by flame atomic absorption spectrophotometer by dry digestion method. The results were statistically analyzed with SPSS software by t-test (α=0.05).
Results:
Results showed that salivary zinc level in case group in acute and convalescent phases were 160.8 ngr/mland 205.7 ngr/ml respectivly and significant differences between them were existed (P <0.05). Also significant differences were existed between zinc concentration in healthy subjects and patient groups (in both phases) (P=.001 and .002 for acute and convalescent phases respectively).
Conclusion:
According to the results, zinc level is significantly lower in acute phase than in convalescent phase and significantly lower in both phases compared to healthy individuals,so determination of serum zinc level and prescribing zinc complement in low serum status has both treatmental and preventive effects in RHL patients.
Keywords: Acute phase, convalescent phase, recurrent herpes labialis, zinc level
INTRODUCTION
Infection with herpes simplex virus (a DNA virus) is the most prevalent infective disease of human after viral respiratory infections.[1,2]
There are two major types of virus (HSV1 and HSV2). Classically, HSV1 causes a majority of oral and pharyngeal infection, meningo-encephalitis and dermatitis above the waist while HSV2 is implicated in most genital infections.[1] After the initial infection, which is often an acute stomatitis, the causal microorganism, herpes simplex virus (HSV) type 1, remains dormant in the trigeminal ganglion and may be reactivated by stress, fever, upper respiratory tract infections, ultraviolet light, trauma and immune incompetence, but the condition is variable and unpredictable in most patients.[3] Recurrent herpes infection of the mouth [Recurrent herpes labialis (RHL)] occurs in patients who have experienced a previous herpes simplex infection.[1]
Many patients with RHL experience several episodes of recurrence during a year so the disease is an annoyance for the majority of patients and it can be disabling for patients with severe frequent lesions for example immunocompromised individuals.[1] These factors necessitate treatment of the lesion as they can cause interference with eating and speaking. Acyclovir, the principal antiherpes drug, has been shown to be effective in treatment of HSV infections, but may have toxicity or side effects and can lead to viral resistance.[3]
Consequently, a safer and more effective method to prevent, eliminate or substantially reduce recurrences should be considered. Recently the effectiveness and preventive role of zinc components in many animals and human abnormalities and diseases has been considered.[3,4] Zinc has several roles in metabolism. There are more than 100 zinc metalloenzymes, including a large number of nicotinamide adenine dinucleotide (NADH) dehidrogenases, RNA and DNA polymerases, and DNA transcription factors as well as alkaline phosphatase, superoxide dismutase and carbonic anhydrase.[3] Zinc is involved in nucleic acid and protein metabolism and in processes of cell differentiation and replication.[4] Zinc deficiency can have several effects but clinical assessment of mild zinc deficiency is difficult because many of the signs and symptoms are non-specific. Acrodermatitis enteropathica is a more severe childhood form of zinc deficiency, which manifests with periorificial (oral, anal, genital) and acral dermatitis, diarrhea, behavioral and mental changes, neurological disturbances and secondary bacterial and fungal infections, though it has not been reported to predispose to herpetic lesions.[3] biochemical signs associated with zinc deficiency include decreased levels of plasma zinc level (<70 μg/dl), decreased serum level of alkaline phosphatase, alcohol dehidrogenase in the retina (which accounts for night blindness), and decreased plasma testosterone as well as impaired T-lymphocyte function,decreased collagen synthesis (resulting in poor wound healing), and decreased RNA polymerase activity in several tissues.[3] The zinc has capacity for the safe control of viruses by cell-mediated immunity stimulation. The T-cell lymphocyte response is the basis of cellular-mediated immunity (CMI). The CMI is important in protection against virus, fungal and protozoan infections, as well as against malignant and autoimmune disease.[3] Zinc appears to increase number of helper or effector T-cells, or precursors of antibody forming cells or increased suppressor cell activity. Zinc ions stimulate lymphocyte DNA synthesis within a few days and approximately 10-40% of lymphocytes are transformed into lymphoblasts. Additionally zinc-8 hydroxyquinoline unsaturated complexes are stimulatory to lymphocyte mitosis in animals and at least two mechanisms appear to exist for zinc to stimulate lymphocytes in animal models.[3] Many studies suggest that systemic and/or topical zinc administration appeared to reduce both the number of episodes and the time of recovery of herpes labials.[3,5–9]
According to the relative ease with which saliva can be collected compared with the relative invasive method of getting blood samples and noting that saliva is a potential indicator of concentration of many blood components, and along with the relationship between salivary zinc concentrations and dietary intakes reported in the literature[10–12] while there is not still any documented research that has measured the zinc level in patients with RHL and comparison of it with healthy subjects. The aim of this study was to measure salivary zinc concentration in acute and convalescent phases of RHL in patients with history of at least three episodes per year in comparison with healthy individuals.
MATERIALS AND METHODS
This case–control retrospective study was done between three groups. The first group (study group) consisted of 40 individuals (22 female and 18 male, mean age=34.4, std. deviation=14.52) who had clinically documented herpes labialis at the examination time of ≤48 hours duration and history of at least 3 episodes of recurrence each year without any history of immunological disease. The excluding criteria swas:having any history of disease that could affect immune function of the body and having history of RHL less than three times a year.
The second group areof the same individuals in group 1 but 21 days later, at the convalescent phase of disease.
The third group (control group) consisted of 40 healthy subjects (24 female and 16 male, mean age=34.5, std.deviation=13.85) with no history of any systemic and immunological disease with immune competency, that are match in sex and age and nutritional habits (for example times of eating milk and fish meals in week) with study group. All subjects were informed of the purpose and informed consent was obtained from all the individuals and local ethical committee approval that was in accordance with the last update of Helsinki declaration was obtained.
Saliva collection
10 cc of whole unstimulated saliva was collected in sterile test tubes from each person between 9.00 and 12.00 am. All subjects were requested to avoid eating and drinking 2 hours before sampling. Saliva was collected using spitting method (saliva ejection for 5 minutes was collected), then each tube was frozen at –18°C until sending to biochemistry laboratory. Another salivary collection was done only in patient group 21 days later in convalescent phase after which lesions had completely healed. There after 120 saliva samples were sent to biochemistry lab at one time to avoid the problems of several setting up of the unit. Samples were analyzed using the dry digestion method by flame atomic absorption spectrophotometer according to following step :
Transporting the saliva samples to porcelain circulates and dry in 105°C for 24 hours.
Heating the samples in 450 cc in electrical furnace 14 hours.
Dissolving the burnings in 0.5 ml of nitric acid (7 molar) and diluted up to 10 ml with pure water.
Measurement of zinc level by direct aspiration into spectrophotometer using the hollow multielemental cathodic lamp.
Reliability of analyze was tested with comparison with cow-liver standard test of international institute of standard and technology. Finally results were analyzed in each 3 groups by SPSS statistically software using paired t-test (P<0.05).
RESULTS
Study subjects consisted of 18 men and 22 women (mean age=34.4 years, std. deviation=14.52) and healthy subjects consisted of 16 men and 24 women (mean age 34.5 years with std. deviation=13.85)
There were 120 salivary samples that 40 were from healthy persons and 40 from patients at acute phase and 40 from same patients in convalescent phase. Acute phase is the time that patient has lesions with symptoms such itching, burning, crusting and …
Convalescent phase is 21 days after beginning of lesion after lesions completely healed but viral shedding in saliva may be continued.
Duration of lesions in study group was at least 4 up to 15 days and mean was 9.2 days (std. deviation=2.84).
Number of recurrence each year was at least 3 up to 10 and most prevalence was three times of recurrence and mean was 4.8 (std. deviation=1.65)
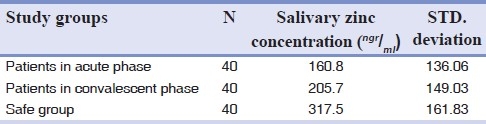
Results of salivary zinc measurements in three groups are shown in [Table 1]. According to Table 1 there is significant difference between salivary zinc levels in acute and convalescent phases of disease in patient group (P<0.05) and between both of phases and healthy group too (P<0.05).
Table 1.
Mean salivary zinc concentration (ngr/ml) in patients in acute and convalescent phases and safe group
Analyzes of the relation between salivary zinc levels in acute and convalescent phases and other questioned parameters (time of recurrence per year, duration of disease, age and sex, revealed no significant differences between zinc level and any of this parameters. (P>0.05 in all comparisons).
DISCUSSION
Herpes labialis is one of the most prevalent and clinically obvious viral disease and recurrence is a common characteristic and many patients experience frequent, large, painful or disfiguring lesions so the disease is bothering for patients and interfere with their social activity and can produce many psychological problems.[1]
Studies have suggested several mechanisms for reactivation of latent HSV, including low serum IgA,[13] decreased cell-mediated immunity[14] and depression of antibody-dependent cell cytotoxicity (ADCC).[15] Although antiviral agents, including Acyclovir, may be effective against recurrent herpes labialis, they may have many side effects and can promote viral resistance.[1,3] On the other hand many studies had emphasized the effects of zinc components in treatment of RHL.[3,5–9]
According to many investigations, zinc is associated with taste and smelling power and wound healing. It is essential to the integrity of the immune system as it plays a role in stabilization of cell membranes.[3,4] the zinc has potential in safe control of viruses by cell mediated immunity stimulation,[3] thus its deficiency may associated with many abnormalities. The purpose of this study was determination and comparison of salivary zinc level in patients with RHL in both acute and convalescent phase of disease and between the two phases of disease and healthy subjects.
According to the results of this study there is a significant differences between the mean value of salivary zinc level in acute phase of disease (160.8) and convalescent phase (205.7) (P=0.001)) [Table 1]. Significant increase of zinc level during convalescent phase of RHL may reveal the reparative role of zinc in mucosal lesions. Some biochemical signs associated with zinc deficiency include decreased collagen synthesis (resulting in poor wound healing) and decreased RNA polymerase activity in several tissues.[3]
Also this finding can confirm previous researches finding about the effects of topical and systemic compositions of zinc in shortening the duration of disease and fastening the healing process of lesions. There was significant differences between mean concentration of salivary zinc levels in patients in both phases (160.8 and 205.7, respectively) and healthy individuals (317.5), demonstrated that zinc level in healthy subjects is about two times more than its level in acute phase and 1.5 times more than its level in convalescent phase.
These results emphasized the important role of zinc as a preventive and reparative agent in mucocutaneous lesions. Thus in cases of zinc deficiency, administration of zinc may have significant therapeutic and prophylactic effects in many diseases such as RAS,[16–23] RHL,[3,5–9] periodontal diseases as indicated in many studies.[3–9,23]
CONCLUSIONS
The results of this study suggest that zinc deficiency is a potential risk factor for recurrent herpes labialis. So prescribing zinc complement if zinc level is low in patients with RHL has both theraputic and preventive effects and is recommended.
ACKNOWLEDGMENT
The authors are grateful for the financial support of Isfahan University of Medical Sciences, School of Dentistry and Torabinejad Research Center. The authors are also thankful to biochemistry laboratory of Isfahan faculty of pharmacy.
Footnotes
Source of Support: This report is based on a thesis which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, in partial fulfillment of the requirements for the MSc degree in Oral Medicine (#388539). This study was financially supported and approved by Isfahan University of Medical Sciences, Isfahan, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Greenberg M, Glick M, Ship . Burket's oral medicine. Hamilton: BC Decker Inc; 2008. pp. 42–9. [Google Scholar]
- 2.Neville B, Daam D, Allen C, Bouquo T. J Oral and Maxillofacial Pathology. 3rd ed. St. Louis: Saunders Elsevier; 2009. pp. 240–8. [Google Scholar]
- 3.Femiano F, Gombos F, Scully C. Recurrent herpes labialis: A pilot study of the efficacy of zine therapy. Oral Pathol Med. 2005;34:423–5. doi: 10.1111/j.1600-0714.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 4.Ayodele JT, Bayero AS. Lead and zinc concentrations in hair and nail of some Kano inhabitants. Afr J Environ Sci Technol. 2009;3:164–70. [Google Scholar]
- 5.Brody I. Topical treatment of recurrent herpes simplex and post-herpetic erythema multi forme with low concentrations of zincsulphate solution. J Dermatol. 1981;104:191–4. doi: 10.1111/j.1365-2133.1981.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 6.Osptelten W, Neven AK, Eekhof J. Treatment and prevention of herpes labialis. Can Fam Physician. 2008;54:1683–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Kneist W, Hempel B, Borell S. Clinical double-blind trial of topical zinc sulfate for herpes labialis recidivans. Arzneimittelforschurg. 1995;45:624–6. [PubMed] [Google Scholar]
- 8.Godfrey HR. A randomized clinical trial on the treatment of oral herpes with topical zinc oxide/glycine. Alter Ther Health Med. 2001;7:49–56. [PubMed] [Google Scholar]
- 9.Singh BB, Udani J, Vinjamury SP, Der-Martirosian C, Gandhi S, Khorsan R. Safety and effectiveness of on L.lysine, zinc, and Herbal-based production in the treatment of facial and circumoral herpes. Altern Med Rev. 2005;10:123–7. [PubMed] [Google Scholar]
- 10.Freeland-Graves JH, Hendrickson PJ, Ebangit ML, Snowden JY. Salivary zinc as an index of zinc status in women fed a low zinc diet. Am J Clin Nutr. 1981;34:312–21. doi: 10.1093/ajcn/34.3.312. [DOI] [PubMed] [Google Scholar]
- 11.Greger JL, Sickles VS. Saliva zinc levels: Potential indicators of zinc status. Am J Clin Nutr. 1979;32:1859–66. doi: 10.1093/ajcn/32.9.1859. [DOI] [PubMed] [Google Scholar]
- 12.Henkin RI, Muellel CW, Wolf RO. Estimation of zinc concentration of parotid saliva by flameless atomic absorption spectrophotometery in normal subjects and in patients with idiopathic hypogeusia. J Lab Clin Med. 1975;8:175–80. [PubMed] [Google Scholar]
- 13.Greenberg MS, Brightman VJ. Serum immunoglobulins in patients with recurrent intraoral herpes simplex infections. J Dent Res. 1971;50:781. doi: 10.1177/00220345710500034101. [DOI] [PubMed] [Google Scholar]
- 14.Heineman HS, Greenberg MS. Cell protective effect of human saliva specific for herpex simplex virus. Arch Oral Biol. 1980;25:257–61. doi: 10.1016/0003-9969(80)90031-x. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg MS, Friedman H, Cohen SG. A comparative study of herpes simplex infections in renal transplant and leukemic patients. J Infect Dis. 1987;156:280. doi: 10.1093/infdis/156.2.280. [DOI] [PubMed] [Google Scholar]
- 16.Orbak R, Cicek Y, Tezel A, Doygru Y. Effect of zinc treatment in patients with recurrent aphthous stomatitis. Dent Mater J. 2003;22:21–9. doi: 10.4012/dmj.22.21. [DOI] [PubMed] [Google Scholar]
- 17.Pang JF. Relation between treatment with traditional Chinese medicine for recurrent aphthous ulcer and human zinc and copper. (260-1).Zhongguo Zhong Xi Yi Jie He Za Zhi. 1992;12:280–2. [PubMed] [Google Scholar]
- 18.Endre L. Recurrent aphthous ulceration with zinc deficiency and cellular immune deficiency. Oral Surg Oral Med Oral Pathol. 1991;72:559–61. doi: 10.1016/0030-4220(91)90493-v. [DOI] [PubMed] [Google Scholar]
- 19.Wang SW. Clinical study on the application of zinc remedy in the treatment of recurrent aphthous ulceration. Zhonghua Kou Qiang Yi Xue Za Zhi. 1990;25:108–10. [PubMed] [Google Scholar]
- 20.Wang SW, Li HK, He JS, Yin TA. The trace element zinc and aphthosis. The determination of plasma zinc and the treatment of aphthosis with zinc. Rev Stomatol Chir Maxillofac. 1986;87:339–43. [PubMed] [Google Scholar]
- 21.Wray D. A double-blind trial of systemic zinc sulfate in recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol. 1982;53:469–72. doi: 10.1016/0030-4220(82)90459-5. [DOI] [PubMed] [Google Scholar]
- 22.Merchant HW, Gangarosa LP, Glassman AB, Sobel RE. Zinc sulfate supplementation for treatment of recurring oral ulcers. South Med J. 1977;70:559–61. doi: 10.1097/00007611-197705000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Orbak R, Kara C, Ozbek E, Tezel A, Demir T. Effects of zinc deficiency on oral and periodontal diseases in rats. J Periodontal Res. 2007;42:138–43. doi: 10.1111/j.1600-0765.2006.00939.x. [DOI] [PubMed] [Google Scholar]


