Abstract
Background:
The purpose of this study was to determine the salivary levels of alkaline phosphatase (ALP) and acid phosphatase (ACP) activities in patients with periodontal disease and to evaluate the use of these enzymes as biochemical markers for periodontal tissue damage.
Materials and Methods:
In this prospective analytical study, we examined the activities of salivary ALP and ACP in patients with periodontal disease, before and after periodontal treatment. The experimental groups consisted of 20 gingivitis patients and 20 periodontitis patients and the control group had healthy subjects (20 samples). The stimulated saliva of the patient was collected in a sterile test tube and analyzed using Hitachi's Diagnostic Automatic Analyser. Periodontal disease was determined based on clinical parameters such as gingival index, probing depth and clinical attachment loss. Patients with periodontal disease were under conventional periodontal treatment. The statistical analysis applied was Student's t-test. Probabilities less than 0.05 (P < 0.05) were considered significant.
Results:
The obtained results showed statistically significant increased activities of ALP and ACP in saliva from patients with periodontal disease in relation to control group. A significant reduction in the enzyme levels was seen after conventional periodontal therapy.
Conclusions:
Based on these results, salivary ALP and ACP can be considered to be the biomarkers for evaluating periodontal tissue damage.
Keywords: Acid phosphatise, alkaline phosphatise, periodontal disease, saliva
INTRODUCTION
Analyses of various gingival crevicular fluid (GCF) biochemical markers have been proposed as a means to predict clinical attachment loss (CAL). Among the important GCF components are the various enzymes. A response of an organism to the periodontal infection includes production of several enzyme families which are released from stromal, epithelial, inflammatory or bacterial cells. The enzymes of tissue degradation are aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), alkaline phosphatase (ALP) and acid phosphatase (ACP).[1–3] There are enough studies available in the literature, correlating the levels of these enzymes in GCF with the severity of periodontal disease.[4,5] However, there are inherent problems in collecting GCF in a routine office dental setting. The sampling technique is not easy as a long time is required for sample collection and it only reflects gingival inflammation at each specific site sampled. Thus, GCF is not suitable for mass screening.[6] Recent studies have shown that these enzymes can be quantified easily in saliva sample.[3,7,8] The use of saliva to measure these biomarkers (enzymes) offers several advantages over GCF. As collection of saliva requires no specialized equipment or techniques, it is faster and more convenient for the patient and the practitioner to collect. In addition, whole saliva represents a pooled sample with contributions from all periodontal sites, and analysis of biomarkers in saliva may provide an overall assessment of disease status as opposed to site-specific GCF analysis.[9]
Research objectives in this study were the following:
Measurement of activities of ALP and ACP in saliva of healthy persons and comparison with the activities in patients with periodontal disease.
Comparison of the activities of ALP and ACP in saliva of the patients with periodontal disease before and after treatment.
MATERIALS AND METHODS
Experimental design included 20 patients with gingivitis; 20 patients with periodontitis, and 20 healthy adult volunteers. The categorization of the patients was done based on the following evaluation parameters: gingival index (gingival index (GI); Loe and Sillness), probing depth (PD) and CAL. Patients having at least four teeth with ≥5 mm CAL and moderate bone loss on orthopantomogram (OPG) were categorized in periodontitis group. Patients with a history of smoking, alcohol consumers, those who had taken antibiotics in past 6 months or who had undergone periodontal treatment in the past 6 months were excluded from the study. Pregnant and lactating mothers were also excluded.
As the initial examination, each subject completed a detailed medical questionnaire and received a complete periodontal examination, which included: GI (Loe and Sillness), PD and CAL. Patients with periodontal disease were under conventional periodontal treatment consisting of oral hygiene instructions, scaling and root planing. Saliva sample was collected at baseline and 3 weeks post treatment. Patient was asked to rinse with 15 ml of water (to wash out exfoliated cells) and then chew paraffin wax for 5 minutes. The stimulated saliva (10 ml) of the patient was collected in a sterile test tube. The saliva sample was stored in refrigerator (2°C–8°C) until it is transported in an ice bag to the lab where the activities of the salivary enzymes, ALP and ACP, were determined spectrophotometrically with the help of an autoanalyser. The saliva samples were centrifuged (Remi C-24, India) at 11180 g for 10 minutes. The enzyme activities in saliva were determined spectrometrically by the international federation of clinical chemistry (IFCC) method on the Hitachi Automatic Analyser using ALP reagent and ACP reagent as substrates. Group 1 (healthy subjects) was provided no treatment. Group 2 (gingivitis patients) and Group 3 (periodontitis patients) received oral prophylaxis which included scaling and root planing. The statistical analyses applied were the following: mean value, standard deviation, standard error, Student's t-test. Probabilities less than 0.05 (P<0.05) were considered statistically significant.
RESULTS
The obtained results showed that the activities of enzymes in saliva of the patients with periodontal disease were significantly higher in relation to the control group. The established differences showed a high level of statistical significance (P < 0.001) [Tables 1a and 1b]. After conventional periodontal treatment, the activities of salivary enzymes along with various evaluation parameters decreased significantly [Table 2, Figures 1–3].
Table 1a.
Differences between ALP and ACP activity (IU/L±SD) in saliva of healthy and patients with periodontal disease (gingivitis patients and periodontitis patients)
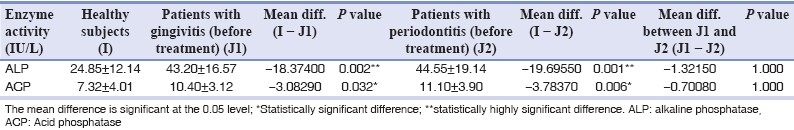
Table 1b.
Mean difference of baseline and post-treatment salivary enzyme levels
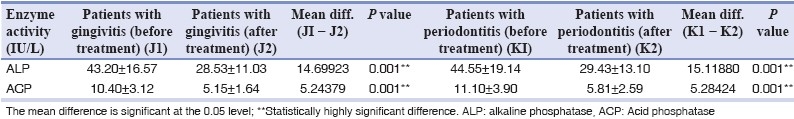
Table 2.
Mean values of various parameters at baseline and 3 weeks after periodontal therapy in all the groups

Figure 1.
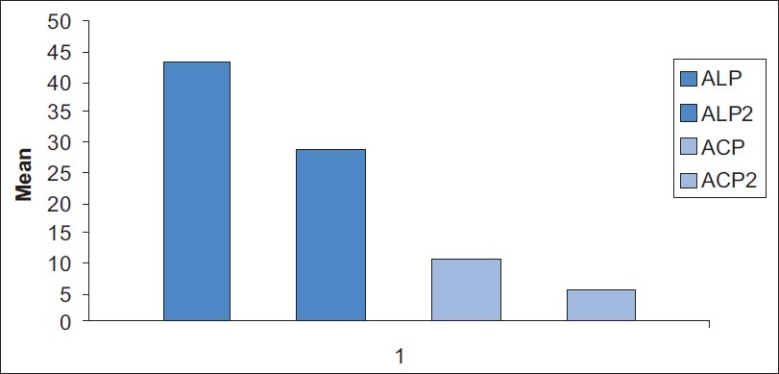
Mean comparison of pre treatment and post treatment salivary enzymes in gingivitis patients
Figure 3.
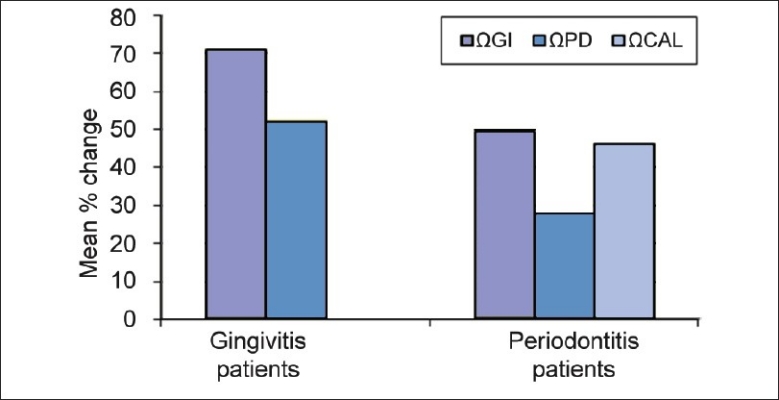
Mean percentage reduction in evaluation parameters after periodontal therapy (ΩGI, ΩPD, ΩCAL = Mean percentage change in parameters after periodontal therapy)
Figure 2.
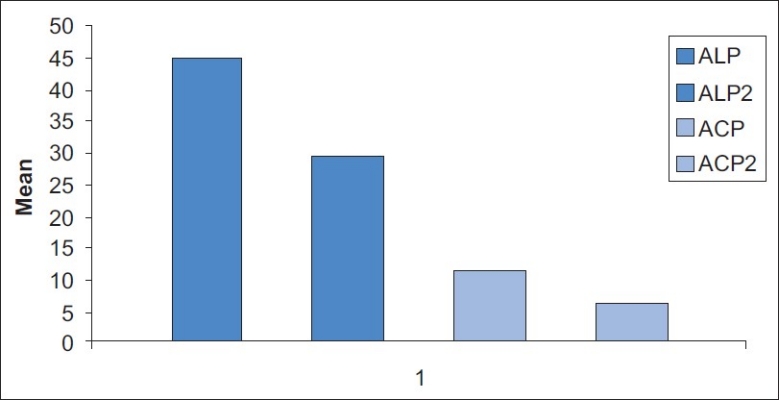
Mean comparison of pre treatment and post treatment salivary enzymes in periodontitis patients
DISCUSSION
CAL readings by the periodontal probe and radiographic evaluations of alveolar bone loss measure damage from past episodes of destruction and require a 2–3 mms threshold change before a site can be identified as having experienced a significant anatomic event. Advances in oral and periodontal disease diagnostic research are, therefore, moving toward methods whereby periodontal risk can be identified and quantified by objective measures such as use of biomarkers.[10] Numerous biomarkers in GCF have been proposed as diagnostic tests for periodontal disease.
These enzymes are indicators of higher levels of cellular damage and their increased activities in GCF are a consequence of their increased release from the damaged cells of the soft tissues of the periodontium and are a reflection of metabolic changes in inflamed gingiva. There are enough studies available in the literature, correlating the levels of these enzymes in GCF with the severity of periodontal disease.[4,5]
ALP and ACP are intracellular enzymes present in most of the tissues and organs, particularly in bones. Their increased activities might be a consequence of destructive processes in alveolar bone in advanced stages of development of periodontal disease.[3] Some studies have shown a remarkably increased activity of ALP in the acute phase of periodontal disease, and after periodontal therapy, the activities of these enzymes restored to the values found in healthy persons.[11] Here again, these studies referred to the GCF.[12,13]
Previous studies mainly investigated the activities of these enzymes in the GCF, which is in a much closer contact with periodontal tissues and, due to this, it surely reflects the occurrences in them much better. However, the problem with the GCF is that the technique of collecting is rather complicated and that in a routine procedure, which possibly might be established, it would be hardly feasible in practice.
The activities of these enzymes can also be proved in saliva, as these enzymes are found even in blood of healthy persons. When a periodontal tissue becomes diseased or its cells become damaged due to edema or destruction of a cellular membrane, i.e. of a cell as a whole, these intracellular enzymes are increasingly released into the GCF and saliva where their activity can be measured. Contrary to the GCF, there is plenty of saliva, the procedure of its sampling is much easier and more bearable for the patient. Being a simple and non-invasive method of collection, salivary diagnostic tests appear to hold promise for the future.[14,15]
This study shows that the increased activity of certain tissue enzymes in periodontal disease can be proved in saliva as a reflection of pathological changes in the cells of periodontal tissues. The present study indicates that salivary ACP and ALP levels are significantly increased in patients with periodontal disease and the salivary enzyme levels decrease in concomitance with the periodontal treatment.
The increased activity of ACP, and especially that of ALP, indicates that the pathological destructive process had affected the alveolar bone, which means that periodontal disease had significantly advanced.
ALP and ACP are among the enzymes most commonly associated with bone metabolism.[16] ALP is enriched in the membranes of mineralizing tissue cells (e.g. osteoblasts) and is also present in polymorphonuclear leukocytes (PMN) granules. ALP is produced by some oral bacteria, including gram-negative microorganisms found in subgingival plaque. In our study, we could see a highly significant rise in salivary ALP levels in gingivitis (P=0.002) and periodontitis patients (P=0.001) as compared to the healthy controls [Table 1a], thereby indicating the pathological changes occurring in the underlying periodontal tissues.
The potential value of ALP as a biomarker of periodontal disease was identified by Ishikawa and Cimasoni.[17] They showed a significant correlation (r=0.49; P<0.05) between ALP concentration in GCF and pocket depth. More recently, a study with longitudinal design demonstrated a 20-fold increase of ALP activity at sites with 2 mm or more of attachment loss.[18] As per the reported results of this study, though the levels of salivary ALP were higher in periodontitis patients as compared to the gingivitis patients, the difference was not statistically significant (P=1.000) [Table 1a]. The difference between the post-treatment and the pre-treatment values of salivary ALP was statistically significant (P=0.001) [Table 1b], which is consistent with the findings reported by Numabe et al.,[14] wherein a decrease of ALP activity in saliva was observed after scaling and root planing. As per Zambon et al.,[19] ALP activity which was reported to be 30.0±8.9 decreased to a level of 24.0±8.0, 2 weeks post treatment.
ACP is among the enzymes associated with bone metabolism. It is present in neutrophils and considered a lysosomal marker. Desquamated epithelial cells, macrophages and several bacteria, including Actinobacillus, Capnocytophaga and Veillonella, also produce this enzyme. In our study, statistically significant increase was seen in the activity of salivary ACP in patients with gingivitis and chronic periodontitis when compared to healthy subjects [Table 1a]. Our findings are related to the previous findings of Todorovic et al.,[4] who, in 2006, reported a statistically significant increase in ACP activity in patients of chronic periodontitis when compared to healthy subjects. Although the levels of salivary ACP in this study were higher in periodontitis patients as compared to the gingivitis patients, the difference was not statistically significant. The activity of salivary ACP was found to significantly decrease post treatment (P=0.001) [Table 1b]. Similar results were reported by Todorovic et al.,[4] where a significant decrease in activity of ACP was seen after periodontal therapy. Results of salivary ACP levels in this study do not correlate with the findings of Zambon et al.,[19] who reported no change in the levels of ACP activity when pre-treatment levels (40.0±0) were compared to 2 weeks post-treatment levels (40.0±0).
CONCLUSION
Salivary ALP and ACP levels reflect inflammation and destruction of periodontal tissues, suggesting their use as clinically useful biomarkers. Their raised levels in diseased individuals are probably a consequence of pathological processes in periodontal tissues where from these intracellular enzymes are increasingly released into the secretion which surrounds them (saliva) and the decrease in the activities of these enzymes after periodontal treatment is probably a result of periodontal tissues’ repair.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Armitage GC. Periodontal diseases: Diagnosis. Ann Periodontol. 1996;1:137–215. doi: 10.1902/annals.1996.1.1.37. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis.A review. J Clin Periodontol. 2000;27:453–65. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- 3.Yoshie H, Tai H, Kobayashi T, Oda-Gou E, Nomura Y, Numabe Y, et al. Salivary enzyme levels after scaling and interleukin 1 genotypes in Japanese patients with chronic periodontitis. J Periodontol. 2007;78:498–503. doi: 10.1902/jop.2007.060216. [DOI] [PubMed] [Google Scholar]
- 4.Todorovic T, Dozic I, Vicente-Barrero M, Ljuskovic B, Pejovic J, Marjanovic M, et al. Salivary enzymes and periodontal disease. Med Oral Patol Oral Cir Bucal. 2006;11:E115–9. [PubMed] [Google Scholar]
- 5.Cesco Rde T, Ito IY, de Albuquerque RF., Jr Levels of asparatate Aminotransferase (AST) in saliva of patients with different periodontal conditions. J Clin Periodontol. 2003;30:752–5. doi: 10.1034/j.1600-051x.2003.00373.x. [DOI] [PubMed] [Google Scholar]
- 6.Nomura Y, Tamaki Y, Tanaka T, Arakawa H, Tsurumoto A, Kirimura K, et al. Screening of periodontitis with salivary enzyme tests. J Oral Sci. 2006;48:177–83. doi: 10.2334/josnusd.48.177. [DOI] [PubMed] [Google Scholar]
- 7.Persson GR, Page RC. Diagnostic characteristics of crevicular fluid asparatate aminotransferase levels associated with periodontal disease activity. J Clin Periodontol. 1992;19:43–8. doi: 10.1111/j.1600-051x.1992.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 8.Totan A, Greabu M, Totan C, Spinu T. Salivary asparatate aminotransferase, alanine aminotransferase, and alkaline phosphatase: Possible markers in periodontal disease. Clin Chem Lab Med. 2006;44:612–5. doi: 10.1515/CCLM.2006.096. [DOI] [PubMed] [Google Scholar]
- 9.Ng PY, Donley M, Hausmann E, Hutson AD, Rossomando EF, Scannapieco FA. Candidate salivary biomarkers associated with alveolar bone loss: Cross sectional and in-vitro studies. FEMS Immunol Med Microbiol. 2007;49:252–60. doi: 10.1111/j.1574-695X.2006.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perinetti G, Paolantonio M, Femminella B, Serra E, Spoto G. Gingival crevicular fluid alkaline phosphatase activity reflects periodontal healing recurrent inflammation phases in chronic periodontitis patients. J Periodontol. 2008;79:1200–7. doi: 10.1902/jop.2008.070519. [DOI] [PubMed] [Google Scholar]
- 11.Yan F. Alkaline phosphatase level in gingival crevical fluid of peri-odontitis before and after periodontal treatment. Chinese Journal of Stomatology. 1995;30:255–66. [PubMed] [Google Scholar]
- 12.Chapple IL, Socransky SS, Dibart S, Glenwright IL, Mathews JB. Chemiluminescent assay to alkaline phosphatases in human gingival crevical fluid: Investigations with on experimental gingivitis model and studies on the source of the enzyme within crevical fluid. J Clin Periodontol. 1996;23:587–94. doi: 10.1111/j.1600-051x.1996.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima K, Roechrich N, Cimasoni G. Osteocalcin, prostaglandin E2 and alkaline phosphatases in gingival crevical fluid: Their relation to periodontal status. J Clin Periodontol. 1994;21:327–33. doi: 10.1111/j.1600-051x.1994.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 14.Numabe Y, Hisano A, Kamoi K, Yoshie H, Ito K, Kurihara H. Analy-sis of saliva for periodontal diagnosis and monitoring. Periodontology. 2004;40:115–9. [Google Scholar]
- 15.Ozmeric N. Advances in periodontal disease markers. Clin Chim Acta. 2004;343:1–16. doi: 10.1016/j.cccn.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 16.van Lente F. Alkaline and Acid phosphatase determinations in bone disease. Orthop Clin North Am. 1979;10:437–50. [PubMed] [Google Scholar]
- 17.Ishikawa I, Cimasoni G. Alkaline phosphatase in human gingival fluid and its relation to periodontitis. Arch Oral Biol. 1970;15:1401–4. doi: 10.1016/0003-9969(70)90032-4. [DOI] [PubMed] [Google Scholar]
- 18.Binder TA, Goodson JM, Socransky SS. Gingival fluid levels of acid and alkaline phosphatase. J Periodontal Res. 1987;22:14–9. doi: 10.1111/j.1600-0765.1987.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 19.Zambon JJ, Nakamura M, Slots J. Effect of periodontal therapy on salivary enzymatic activity. J Periodontal Res. 1985;20:652–9. doi: 10.1111/j.1600-0765.1985.tb00850.x. [DOI] [PubMed] [Google Scholar]


