Abstract
Background:
Introducing new endodontic cements should await comprehensive investigations and new formulations have to be tested in vivo before applying in human beings. So, the purpose of this study was to compare the biocompatibility of new endodontic cements, calcium aluminate α-aluminate cement (CAAC), calcium aluminate α-aluminate plus cement (CAAC plus), and a mixture of wollastonite and CAAC cement (WOLCA) and mineral trioxide aggregate (MTA), in subcutaneous connective tissue of rats.
Materials and Methods:
Twenty-seven Wistar rats were divided into three groups of 7, 14, and 30 experimental days. Sterile polyethylene tubes were filled with MTA, CAAC, CAAC Plus, and WOLCA cement and implanted subcutaneously. Empty tubes were implanted as negative control. After the experimental periods, animals were sacrificed by anesthetic overdosing. The occurrence of inflammatory responses was scored according to the previously established scores. Data were statistically analyzed using Friedman, Wilcoxon, Kruskal-Wallis, and Mann-Whitney tests. The level of significance was 5% (P<0.05).
Results:
There was a statistically significant difference between experimental and negative control sites in each group (P<0.05). CAAC Plus showed the highest mean scores of inflammation, compared with MTA, CAAC, and WOLCA cement sits at the end of all periods (P<0.05). There were no statistically significant differences between inflammatory scores of each site in different experimental groups, except CAAC plus sites, in which inflammation increased significantly with time (P<0.05).
Conclusion:
According to the results of the current study, biocompatibility of CAAC and WOLCA cement were comparable with that of MTA, but CAAC Plus induced an inflammatory response higher than MTA, therefore is not biocompatible.
Keywords: Biocompatibility, mineral trioxide aggregate, new endodontic cement, sodium hexametaphosphate
INTRODUCTION
The goal of endodontics is to prevent or treat apical periodontitis[1] and most of the endodontic failures occur as the result of leakage of irritants through improperly sealed root end fillings into periradicular tissues.[2–5] An ideal orthograde or retrograde root canal filling material should seal the pathways of communication between root canal system and its surrounding tissues.[6] The materials used in root canal therapy, particularly root end filling, are frequently in direct contact with soft and hard periodontal tissues; therefore, a root filling material is necessary to be highly biocompatible and nontoxic.[7] In 1990s, mineral trioxide aggregate (MTA), a new root-ending endodontic material, was developed at the university of Loma Linda.[8] MTA is now used extensively in endodontics for pulp capping, pulpotomy, repair of root perforations, root end filling, root canal filling, and apical barrier formation in teeth with necrotic pulps and open apices.[9,10]
Despite all the benefits listed for MTA, it has also some disadvantages. The main drawbacks of MTA include the potential for tooth discoloration, presence of some toxic elements in the material composition, high cost, long setting time, difficult handling, and difficulty in its removal after setting.[11,12]
Efforts have been made to overcome these shortcomings. Introducing new substitutes for MTA should await comprehensive investigations, and new formulations have to be tested in vitro as well as in vivo before applying in human beings.[13]
Recently, investigators of Torabinejad Dental Research Center at Isfahan University of Medical Sciences (IUMS), Isfahan, Iran, have formulated new cements to be used in endodontics. These materials include calcium aluminate α-aluminate cement (CAAC), calcium aluminate α-aluminate plus cement (CAAC Plus), and a mixture of 1 to 1 wollastonite and CAAC cement (WOLCA). CAAC contains calcium aluminates (60-70% CA, 10-15% CA2, and 0-5% C12A7) and alpha aluminate (α-AL2O3 5-15%). CAAC plus is a mixture of CAAC and 5% by weight Sodium Hexametaphosphate (Na-HMP) to improve physical properties of CAAC.
Wollastonite is a naturally occurring calcium silicate (CaSio3) with a theoretical composition of 48.3% CaO and 51.7% SiO2.[14]
Although there is an extensive knowledge on the biocompatibility of MTA,[15–17] the biological properties of these new cements (CAAC, CAAC Plus, and WOLCA cement) have not been evaluated. Thus, the purpose of this study was to compare the biocompatibility of these cements with each other and MTA in subcutaneous connective tissue of rats.
MATERIALS AND METHODS
The protocol of this study was approved ethically by research council of the IUMS. In this experimental animal study, 27 healthy male Wistar rats weighing 250 to 300 g were used. Animals were divided into three groups of nine with respect to experimental duration. Rats were anesthetized with an intramuscular injection of Ketamine (60 mg/kg, Alfasan, Woerden-Holland), Acepromazine (2.5 mg/kg, Alfasan, Woerden-Holland), and Atropine (0.04 mg/kg, Alfasan, Woerden-Holland). The dorsal skin was shaved and disinfected with povidone-iodine solution (10%) (Daroupakhsh, Tehran, Iran). Five 15 mm long incisions were made through the skin using a no. 15 scalpel blade and pockets were prepared in one direction by undermining the incisions longitudinally by blunt dissection for 20 mm.
Sterile polyethylene tubes (1.5 mm of inner diameter and 7 mm in length) were filled with MTA (Dentsply Tulsa Dental, Tulsa, OK) CAAC, CAAC Plus, and WOLCA cements which have been prepared according to manufacturer's instructions, under aseptic conditions; each tube were implanted in each subcutaneous pocket of rats. Empty tubes were implanted as negative control. Wounds were sutured for 7 days. At the end of experimental periods of 7, 14, and 30 days, animals of the respective group were sacrificed by anesthetic overdosing. After histological processing, tissue samples were serially sectioned longitudinally to a thickness of 4 μm and stained with hematoxylin and eosin. Sections were evaluated under a light microscope (OLYMPUS CH30 RF200, Olympus Optical Co., Ltd. Japan) equipped with a digital camera (Sony ExwaveHAD, Tokyo, Japan) using 10× and 40× objective lenses by two independent examiners in a blind manner. The occurrence of inflammatory response were scored according to previously established scores[16] 0 (no reaction) for absence of inflammatory cells; 1+ (mild reaction) for presence of mild chronic inflammatory infiltrate, or few eosinophilic or giant cells; 2+ (moderate reaction) for presence of moderate chronic inflammatory infiltrate, or some eosinophilic or giant cells, or 3 + (severe reaction) for presence of an intense chronic inflammatory infiltrate, large number of eosinophilic or giant cells.
Differences between the inflammatory responses of sites were statistically analyzed using Friedman test while Wilcoxon test was used to compare individual pairs of groups. Differences between the three sets of data were statistically analyzed by Kruskal-Wallis and Mann-Whitney tests. The level of significance was set at α=0.05.
RESULTS
Table 1 presents the mean values of histological scores in different groups. Statistically significant differences were found between sites on 7th, 14th, and 30th days following implantation (P=0.018, P<0.001, and P<0.001, respectively). There were also statistically significant differences between experimental and negative control sites on all three experimental periods following implantation [Table 2].
Table 1.
Mean scores and standard deviation of inflammatory response after 7, 14, and 30 days following implantation
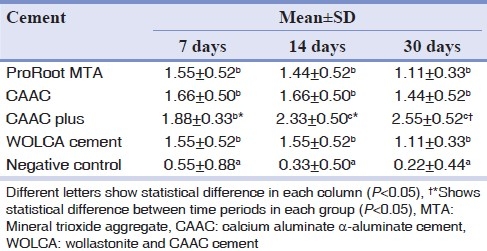
Table 2.
P values for comparisons of test groups with negative control group (Wilcoxon test)
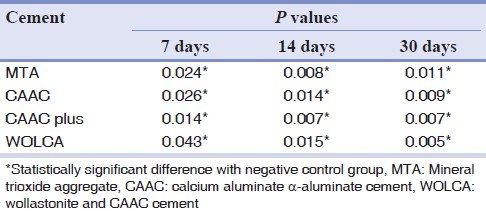
The CAAC plus group showed the highest mean scores of inflammation and was significantly different from MTA, CAAC, and WOLCA cement sites on 14th (P=0.038, P=0.034, and P=0.038, respectively) and 30th days (P=0.006, P=0.008, and P=0.009, respectively) following implantation. There were no statistically significant differences between mean inflammatory scores of other experimental sites (P>0.05).
There were no statistically significant differences between mean inflammatory scores of matched sites in different experimental periods, except CAAC plus group, in which inflammation increased with time (P=0.024).
Photomicrographs of different inflammatory reactions are presented in Figures 1 to 4.
Figure 1.
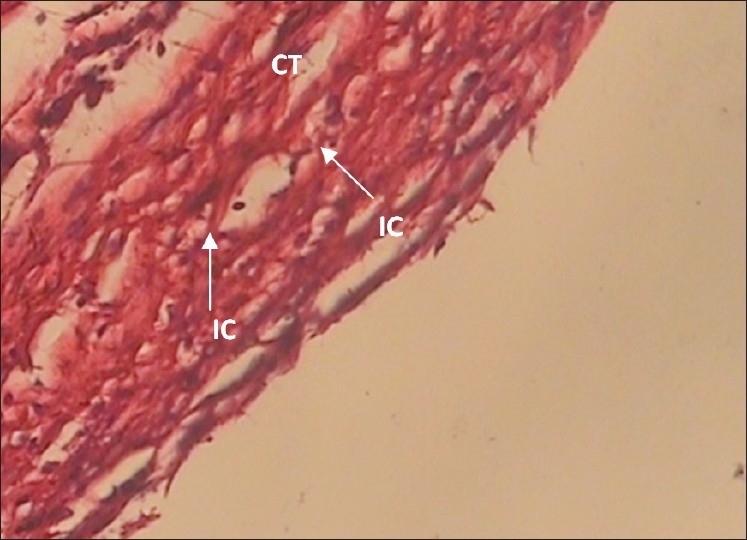
The 30-day MTA specimen with grade + inflammation (×400 Mag) Mild inflammatory cells (IC) are infiltrated in surrounding connective tissue (CT)
Figure 4.
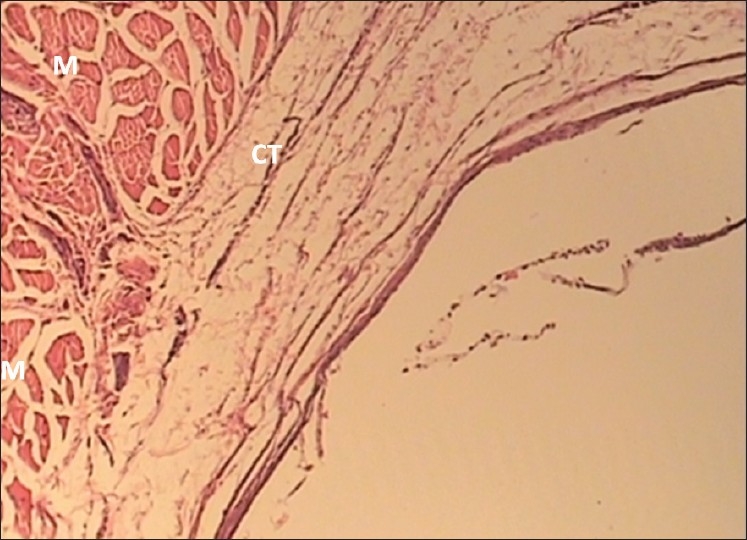
The 30-day Negative control specimen with grade 0 inflammation (×100 Mag) Inflammatory cells around the connective tissues (CT) and muscles (M) are absent
Figure 2.
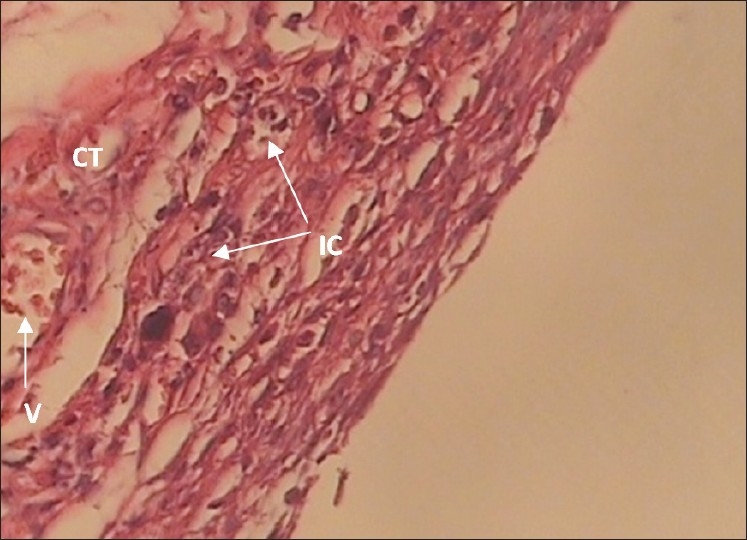
The 30-day WOLCA specimen with grade ++ inflammation (×400 Mag) Moderate infiltration of inflammatory cells (IC) can be seen in connective tissue (CT) and around the Capillaries (V)
Figure 3.
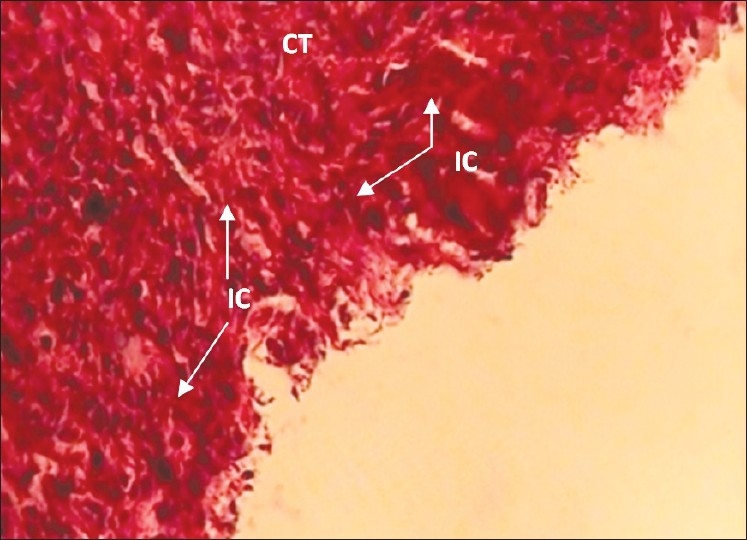
The 30-day CAAC Plus specimen with grade +++ inflammation (×400 Mag) Severe inflammatory cells (IC) are infiltrated in connective tissue
DISCUSSION
The term biocompatibility is often described as the ability of a material to perform with an appropriate host response in a specific application.[18] Because of continuous introduction of new dental materials, evaluation of their biologic potential is necessary.[19] According to ISO-6876 and 10993-5 standards,[20] tissue implantation of different materials in the body of laboratory animals has been proposed. Although data from laboratory animals could not be extended to human beings, it is considered as a valuable method to evaluate their biological properties.[21,22] Rat subcutaneous implantation studies are acceptable experimental models for this assessment[23] and the inert nature of polyethylene tubes makes them suitable for implantation studies, so implantation of polyethylene or silicon tubes filled with endodontic materials into subcutaneous connective tissue of rats simulates in situ conditions of the materials.[24]
Numerous studies have used MTA to seal the natural, pathological, and iatrogenic communications between root canal system and periapical tissues.[16,25] Biocompatibility of MTA has been reported in many in vitro and in vivo studies.[5,10,15–16,26]
Also, biocompatibility of new materials should be evaluated to ensure that a new material does not cause irritation, unwanted reactions, or tissue necrosis compared with control groups. For this reason, histological investigations evaluate the inflammatory response adjacent to the materials.[26]
The present study evaluated histological inflammatory response adjacent to test materials. The severity of inflammation against CAAC Plus cement was higher than other test materials at all periods and the inflammatory response against this material increased with time. At 7 days after implantation of materials, no statistically significant difference was observed between experimental groups, and at 14 and 30 days, severity of inflammation against CAAC Plus cement was significantly more than other groups.
In this study, empty polyethylene tubes as negative control group revealed no inflammation to mild inflammatory response, which is similar to previous studies.[10,27] Initial inflammatory response to empty tubes is probably the result of surgical process of tube implantation.[27,28]
At 7 days, the MTA group displayed a mild-to-moderate inflammatory response which was reduced to a mild reaction after 30 days. This has been reported before by several studies showing the biocompatibility of MTA.[10,17,26,29,30]
CAAC and WOLCA cement groups also revealed a similar response to MTA which was not significantly different.
According to these results, CAAC is a biocompatible material which is consistent to previous in vitro studies on biocompatibility of similar calcium aluminate cements.[31,32] WOLCA cement is a mixture of CAAC and Wollastonite in a ratio of 1 : 1. Wollastonite is a naturally occurring calcium silicate, the composition of which is similar to MTA. Previously, the biocompatibility of MTA and other calcium silicate cements has been proved.[5,15,16,26] It seems that the new material made from mixing two biocompatible cements (CAAC and calcium silicate) is still biocompatible.
The effect of aqueous surface chemistry is very important for small particles. Typically, this occurs in slurries. Weak inter-particle bonds in slurry of flocculated particles make the slurry more viscous than slurry of dispersed particles. In other words, if slurry contains highly dispersed particles, it will have a low viscosity. Since the internal structure of dispersed slurry approaches that of liquid and dispersed particles could be packed more firmly than flocculated particles, it leads to better handling. If particles in slurry are not sufficiently dispersed, the particle charge can be increased by adding a polyelectrolyte[33] such as Na-HMP.
CAAC Plus contains 5% by weight Na-HMP as a dispersant to get these advantages, but the severity of tissue inflammation against CAAC Plus increased by the time. It seems that adding this additive to CAAC reduced its biocompatibility.
Hesaraki et al. evaluated the effects of adding Na-HMP on basic properties of calcium phosphate cement and mentioned that although Na-HMP made this cement more stable and improved its injectability properties, it weakened other basic properties of this cement-like compressive strength and increased its setting time.[34]
According to these findings, it seems that severe inflammatory response to CAAC Plus cement can be due to its prolonged setting time. This is consistent with other investigations which have shown that delay in curing reaction of a substance causes an acute inflammatory response.[35,36]
Previously, the ability of Na-HMP to change the electrical charge of the materials has been demonstrated[34] which alters the proteins and cells absorbed to the material's surface.[37] This issue can also explain the difference between the biocompatibility of this cement and CAAC.
CONCLUSION
According to the results of the current study, biocompatibility of CAAC and WOLCA cements were comparable with that of MTA, but CAAC Plus showed higher inflammatory response than MTA and is not biocompatible. CAAC and WOLCA cements can be two alternatives for MTA. However, more studies are deemed necessary to evaluate the biocompatibility of these cements.
ACKNOWLEDGMENTS
The authors would like to thank Torabinejad Dental Research Center, Isfahan University of Medical Sciences for their kind assistance. This paper was based on a thesis submitted to the School of Dentistry, Isfahan University of Medical Sciences, in partial fulfillment of the requirements for MSc degree in Endodontics. This study was supported by Isfahan University of Medical Sciences Grant #389329. The authors also thank Dr. Navid Askari for his support in preparation of the manuscript.
Footnotes
Source of Support: This paper was based on a thesis submitted to the School of Dentistry, Isfahan University of Medical Sciences, in partial fulfillment of the requirements for MSc degree in Endodontics. The study was approved by the Medical Ethics and Research Office at the Isfahan University of Medical Sciences and financially supported by this University.
Conflict of Interest: None declared.
REFERENCES
- 1.Hargreaves K, Cohen S. COHEN'S pathways of the pulp. 10th ed. St Louis: Mosby Elsevier; 2011. [Google Scholar]
- 2.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review-Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro DA, Duarte MA, Matsumoto MA, Marques ME, Salvadori DM. Biocompatibility in vitro tests of mineral trioxide aggregate and regular and white Portland cements. J Endod. 2005;31:605–7. doi: 10.1097/01.don.0000153842.06657.e2. [DOI] [PubMed] [Google Scholar]
- 4.Torabinejad M, Pitt Ford TR. Root end filling materials: A review. Endod Dent Traumatol. 1996;12:161–78. doi: 10.1111/j.1600-9657.1996.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes K, Santos E, Reda S, Motta LS. Biocompatibility analysis of MTA, Portland cement and modified Portland cement on cultured fibroblast cells and subcutaneous tissue. Conscientiae Saúde. 2010;9:11–6. [Google Scholar]
- 6.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: A review of the constituents and biological properties of the material. Int Endod J. 2006;39:747–54. doi: 10.1111/j.1365-2591.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- 8.Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent Mater. 2008;24:149–64. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 9.American Association of Endodontists. New Materials/Technologies position paper. 2004 [Google Scholar]
- 10.Yavari H, Shahi SH, Rahimi S, Shakouhi SL. Connective tissue reaction to white and gray MTA mixed with distilled water or chlorhexidine in rats. Iranian Endodontic Journal. 2009;4:25–30. [PMC free article] [PubMed] [Google Scholar]
- 11.Kratchman SI. Perforation repair and one-step apexification procedures. Dent Clin North Am. 2004;48:291–307. doi: 10.1016/j.cden.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:809–15. doi: 10.1016/j.tripleo.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Maxim LD, McConnell EE. A review of the toxicology and epidemiology of wollastonite. Inhal Toxicol. 2005;17:451–66. doi: 10.1080/08958370591002030. [DOI] [PubMed] [Google Scholar]
- 15.de Morais CA, Bernardineli N, Garcia RB, Duarte MA, Guerisoli DM. Evaluation of tissue response to MTA and Portland cement with iodoform. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:417–21. doi: 10.1016/j.tripleo.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Martinez Lalis R, Esain ML, Kokubu GA, Willis J, Chaves C, Grana DR. Rat subcutaneous tissue response to modified Portland cement, a new mineral trioxide aggregate. Braz Dent J. 2009;20:112–7. doi: 10.1590/s0103-64402009000200004. [DOI] [PubMed] [Google Scholar]
- 17.Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endod. 1995;21:603–8. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- 18.Sunnegårdh-Grönberg K. Calcium Aluminate Cement as Dental Restorative. Mechanical Properties and Clinical Durability. Umeå, Sweden: Umeå University Odontological Dissertations; 2004. [Google Scholar]
- 19.Lawrence WH, Malik M, Autian J. Development of a toxicity evaluation program for dental materials and products. II. Screening for systemic toxicity. J Biomed Mater Res. 1974;8:11–34. doi: 10.1002/jbm.820080104. [DOI] [PubMed] [Google Scholar]
- 20.Camps J, About I. Cytotoxicity testing of endodontic sealers: A new method. J Endod. 2003;29:583–6. doi: 10.1097/00004770-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Recommended standard practices for biological evaluation of dental materials. Federation Dentaire International, Commission of Dental Materials, Instruments, Equipment and Therapeutics. Int Dent J. 1980;30:140–88. [PubMed] [Google Scholar]
- 22.Geneva. 1994. Organization IS. ISO 10993-6(F)-07-15. Biological evaluation of medical devices. Part 6. Tests for local effects after implantation. CP56. CH1211. [Google Scholar]
- 23.Moretton TR, Brown CE, Jr, Legan JJ, Kafrawy AH. Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. J Biomed Mater Res. 2000;52:528–33. doi: 10.1002/1097-4636(20001205)52:3<528::aid-jbm11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Mutoh N, Tani-Ishii N. A biocompatible model for evaluation of the responses of rat periapical tissue to a new zinc oxide-eugenol sealer. Dent Mater J. 2011;30:176–82. doi: 10.4012/dmj.2010-095. [DOI] [PubMed] [Google Scholar]
- 25.Lamb EL, Loushine RJ, Weller RN, Kimbrough WF, Pashley DH. Effect of root resection on the apical sealing ability of mineral trioxide aggregate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:732–5. doi: 10.1067/moe.2003.98. [DOI] [PubMed] [Google Scholar]
- 26.McNamara RP, Henry MA, Schindler WG, Hargreaves KM. Biocompatibility of accelerated mineral trioxide aggregate in a rat model. J Endod. 2010;36:1851–5. doi: 10.1016/j.joen.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Yaltirik M, Ozbas H, Bilgic B, Issever H. Reactions of connective tissue to mineral trioxide aggregate and amalgam. J Endod. 2004;30:95–9. doi: 10.1097/00004770-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Zmener O, Guglielmotti MB, Cabrini RL. Tissue response to an experimental calcium hydroxide-based endodontic sealer: A quantitative study in subcutaneous connective tissue of the rat. Endod Dent Traumatol. 1990;6:66–72. doi: 10.1111/j.1600-9657.1990.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Yanez Sanchez A, Leco-Berrocal MI, Martinez-Gonzalez JM. Metaanalysis of filler materials in periapical surgery. Med Oral Patol Oral Cir Bucal. 2008;13:E180–5. [PubMed] [Google Scholar]
- 30.Holland R, de Souza V, Nery MJ, Faraco Junior IM, Bernabe PF, Otoboni Filho JA, et al. Reaction of rat connective tissue to implanted dentin tube filled with mineral trioxide aggregate, Portland cement or calcium hydroxide. Braz Dent J. 2001;12:3–8. [PubMed] [Google Scholar]
- 31.Kimura M, Sorata Y, Yanome S, Hideshima O, Yokomoto M, Tekikou S, et al. Study of root canal cements comprising calcium aluminate.First report on cytotoxicity. Shoni Shikagaku Zasshi. 1991;29:44–54. [PubMed] [Google Scholar]
- 32.Franz A, Konradsson K, König F, van Dijken JW, Schedle A. Cytotoxicity of a calcium aluminate cement in comparison with other dental cements and resin-based materials. Acta Odontol Scand. 2006;64:1–8. doi: 10.1080/00016350500279568. [DOI] [PubMed] [Google Scholar]
- 33.Palmes J. Effect of the properties of coal surface and flocculant type on the flocculation of fine particles. Vancouver, Canada: British Colombia; 1996. [Google Scholar]
- 34.Hesaraki S, Zamanian A, Moztarzadeh F. Effect of adding sodium hexametaphosphate liquefier on basic properties of calcium phosphate cements. J Biomed Mater Res A. 2009;88:314–21. doi: 10.1002/jbm.a.31836. [DOI] [PubMed] [Google Scholar]
- 35.Ueyama Y, Ishikawa K, Mano T, Koyama T, Nagatsuka H, Matsumura T, et al. Initial tissue response to anti-washout apatite cement in the rat palatal region: Comparison with conventional apatite cement. J Biomed Mater Res. 2001;55:652–60. doi: 10.1002/1097-4636(20010615)55:4<652::aid-jbm1060>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto Y, Ishikawa K, Takechi M, Toh T, Yuasa T, Nagayama M, et al. Histological and compositional evaluations of three types of calcium phosphate cements when implanted in subcutaneous tissue immediately after mixing. J Biomed Mater Res. 1999;48:36–42. doi: 10.1002/(sici)1097-4636(1999)48:1<36::aid-jbm8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Thevenot P, Hu W, Tang L. Surface chemistry influences implant biocompatibility. Curr Top Med Chem. 2008;8:270–80. doi: 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]


