Abstract
Background:
Nanosized ceramics may represent a promising class of bone graft substitutes due to their improved osseointegrative properties. Nanocrystalline Hydroxyapatite (NcHA) bind to bone and stimulate bone healing by stimulation of osteoblast activity. The present study aims to explore the clinical and radiographical outcome of NcHA bonegraft (Sybograf®) with collagen membrane (Periocol®), in comparison with open flap debridement (OFD), in the treatment of intrabony periodontal defects.
Materials and Methods:
A parallel-group, randomized, controlled clinical trial was designed to conduct the study. Eighteen intrabony defects in 14 systemically healthy patients aged between 25 to 65 years were randomly assigned to test and control group. The plaque index, gingival index, probing pocket depth (PPD), clinical attachment level (CAL), and gingival recession (REC) were recorded at baseline, and were reevaluated at 6 months. In addition to this, radiographic bone fill was assessed using digital software. At the test site, NcHA bone graft and collagen membrane was placed, whereas at the control site, only OFD was done. Recall appointments were made at 7 days, 30 days, and then at 3 months and 6 months.
Results:
The data were subjected to statistical analysis using the Mann-Whitney ‘U’ Test and Wilcoxon signed rank sum test. In the control group, the mean reduction of PPD was 3.22±1.09 mm (P=0.007) and CAL gain was 2.77±1.09 mm (P=0.007). In the test group, the mean PPD reduction of 4.33±0.5 mm (P=0.006) and mean gain in CAL was 3.77±0.66 mm (P=0.006) at 6 months. The mean increase in REC was 0.55±0.72 mm (P=0.025) in test, and 0.44±0.52 mm (P=0.046) in control group. The mean gain in radiographic defect fill was 2.07±0.67 mm (P=0.008) in test and 0.91±0.21 mm (P=0.007) in control group.
Conclusion:
The nanocrystalline hydroxyapatite bone graft in combination with collagen membrane demonstrated clinical advantages beyond that achieved by OFD alone.
Keywords: Bone graft, collagen membrane, nanocrystalline hydroxyapatite, periodontal regeneration
INTRODUCTION
The advent of regenerative approaches in contemporary periodontics has increased patient's treatment options and enhanced the long-term prognosis of many teeth that have advanced periodontal destruction.
Preliminary experimental studies have shown that nanosized ceramics may represent a promising class of bone graft substitutes due to their improved osseointegrative properties.[1,2] Accordingly, a synthetic nanocrystalline hydroxyapatite (NcHA) bone graft has been introduced for augmentation procedures in intrabony defects. Advantages of NcHA material are osteoconductivity, bioresorbablity, and close contact. A special feature of nanostructured materials is an extremely high number of molecules on the surface of material. When the NcHA was used as a bone graft substitute, rapid healing of critical size defects was observed in animal experiments and in human applications.[3] NcHA binds to bone and stimulate bone healing by stimulation of osteoblast activity.[4] NcHA has been used for the treatment of metaphyseal fractures in orthopedic surgery,[5] ridge augmentation,[6] and peri-implantitis lesions.[7]
Various collagen barriers of mammalian origin such as bovine[8] and porcine[9] have been used successfully for guided tissue regeneration (GTR) in human intrabony and furcation defect and gingival recession Recently, interest has been developed in non-mammalian collagen sources, primarily fish collagen. A bioresorbable collagen barrier membrane (Periocol®) of fish origin has been developed for GTR applications in human periodontal intrabony and furcation defects. Periocol® collagen membrane have been used as a sustained release chlorhexidine chip in chronic periodontitis patients and reported to resorbed after 30 days.[10]
However, collagen membrane when dampened by biological fluid results in a poor membrane resistance to collapse, allowing undesirable cell types to enter the secluded wound area.[11,12] The collapse may be prevented by means of implantation of bone grafts or bone graft substitutes into the defect to support the membrane, preserving its original position.[7] This association is called combined periodontal regenerative technique.[13] Clinical research performed in periodontal regeneration has suggested that one of the most predictable techniques in improving clinical attachment level (CAL) and bone fill is achieved when using combination of a graft material and GTR.[13–15]
During periodontal therapy, deep intraosseous defects represent a major challenge for the clinician, often requiring open flap debridement (OFD) alone or combined with bone-regenerative procedures. Various clinical and radiographic studies[9,13,16–22] demonstrated significantly more probing pocket depth (PPD) reduction and greater gain in CAL in the combined group compared with OFD group. This study was designed to evaluate the efficacy of NcHA bone replacement graft (Sybograf®) in combination with bioresorbable collagen membrane (Periocol®), compared with OFD alone.
MATERIALS AND METHODS
The present study was a parallel-group, randomized, controlled clinical trial. Sixteen systemically healthy patients (nine males and seven females), aged between 25 to 65 years, were screened after a detailed clinical and radiographic examination from the out patient Department of Periodontology, Manipal College of Dental Sciences, Mangalore. Inclusion of patients in test group (10 defects) or control group (10 defects) was determined randomly through the coin flip method.
Study inclusion criteria were as follows: Systemically healthy patients; presence of at least one or two radiographically detectable intrabony defect with PPD ≥5 mm and radiographic depth of the defect ≥3 mm; no antibiotics taken prior to six months of initial examination and did not require antibiotic premedication for any systemic condition; no periodontal surgery performed in the areas to be treated within the last 12 months; good level of oral hygiene (Plaque index (PI)<1). Subjects with previously implanted materials, natural or synthetic and physical barriers in the selected defects, smokers, pregnant and lactating females; teeth exhibiting mobility exceeding Grade II and teeth with endodontic involvement were excluded from the study.
All 16 patients with 20 intrabony defects (nine in maxilla and eleven in mandible), following an initial examination, diagnosis, and treatment plan, were subjected to Phase-I therapy, which comprises of full mouth supragingival and subgingival scaling and root planning. All patients were given detailed plaque control instructions.
A customized acrylic stents was fabricated on study casts for each patient and trimmed to the height of contour of the teeth, and one vertical groove was prepared to reproduce the probe angulation and position. All measurements were recorded to nearest millimeter (mm) with the help of a UNC-15 graduated periodontal probe. Clinical parameters (PI,[23] GI,[24] PPD, CAL, REC) were recorded at baseline and 6 months at the six aspects (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual) per tooth. Only one site representing the deepest point of the PPD was included in the study.
Using the apical margin of the customized acrylic stent as the fixed reference point (RP), the following measurements were recorded at the proximal line angle of the tooth with the associated bony defect: (a) RP to the gingival margin (GM), (b) RP to the cementoenamel junction (CEJ), (c) RP to the base of the pocket (BOP). The following clinical parameters were recorded using the acrylic stent: (a) PPD=(RP to BOP) – (RP to GM), (b) CAL=(RP to BOP) – (RP to CEJ), and (c) REC=(RP to GM) – (RP to CEJ).
An intraoral periapical radiograph was taken for each selected site using the long cone paralleling technique with mm grid scale, at base line and 6 months post-surgery [Figures 1–3]. All radiographs were digitalized using the digital camera and transferred to the computer as JPEG image to measure the linear radiographic depth (in mm) of the defect (DD) using software, Image J, which was designed by National Institute of Health (NIH) for the image analysis [Figure 1]. The percentage of bone fill was calculated using the following formula.[25]
Figure 1.
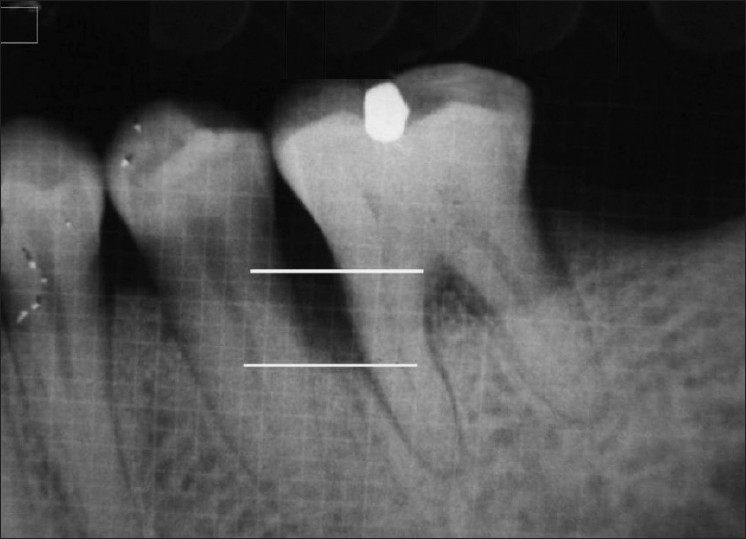
Linear radiographic interpretation with J-image computer software (parallel lines showed the base of the defect and crest of alveolar bone)
Figure 3.
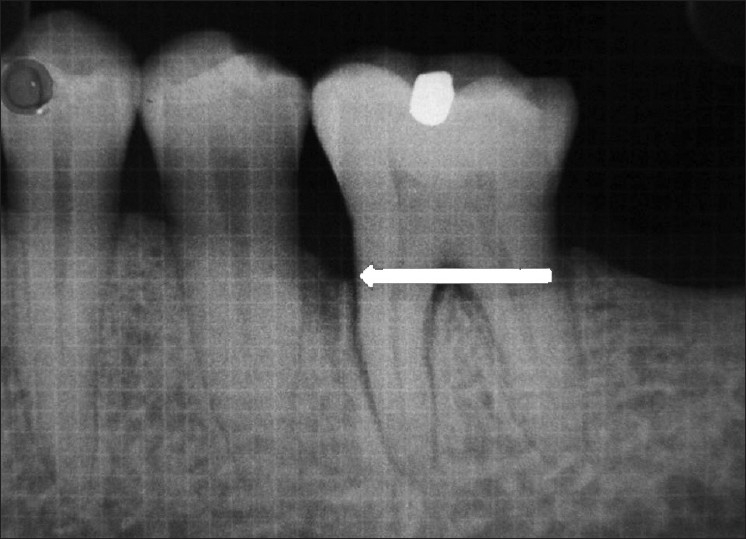
Postoperative radiograph
Figure 2.
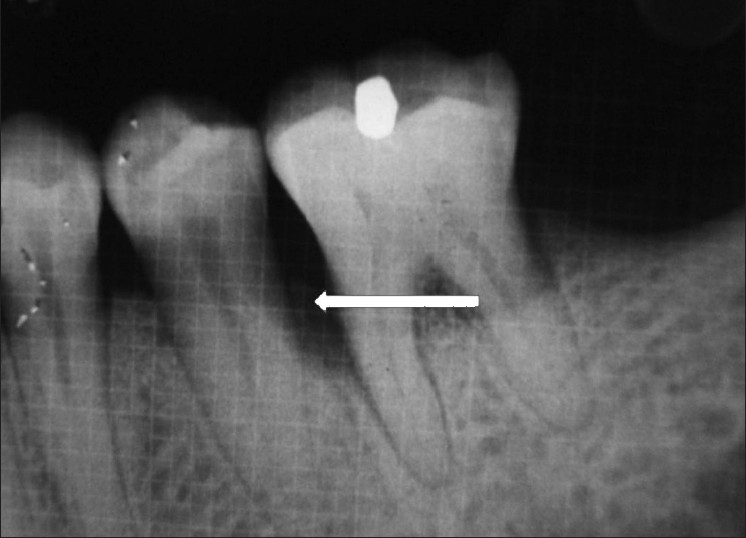
Preoperative radiograph
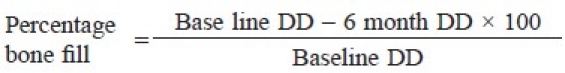
Following clinical data collection, a preoperative mouth rinse with 10 ml 0.2% Chlorhexidine Gluconate (Plakil™ India) was used by all patients. Asepsis was maintained throughout the surgical procedure. Area subjected to surgery was anesthetized by nerve block/infiltration depending on the surgical site using local anesthesia. Full thickness flap were raised buccally and lingually until 1 mm bone was exposed in control group, and 2 to 3 mm in patients of test group. The osseous defect was debrided of granulation tissue and the root surface was planed to remove plaque and calculus, using hand and ultrasonic scalers until a smooth hard consistency was found. The defect's architecture had to be confirmed by direct observation and classified based on the number of bony walls present.[26] Following surgical debridement, no root bio-modification or osseous resective procedures was attempted.
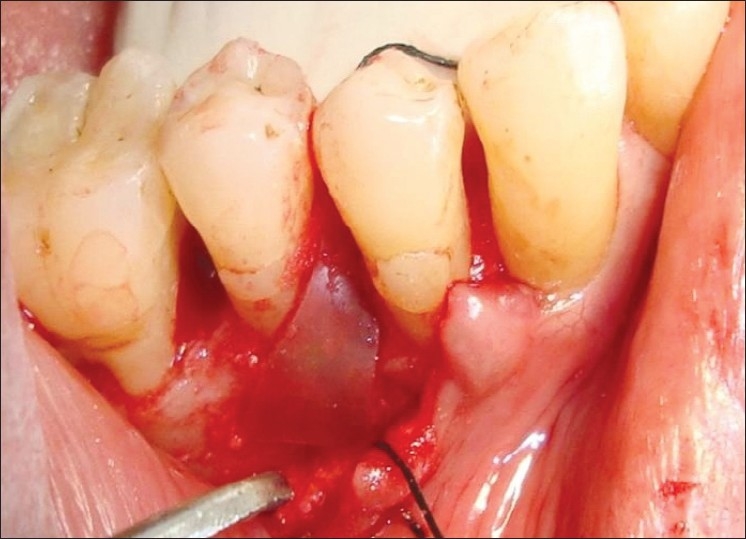
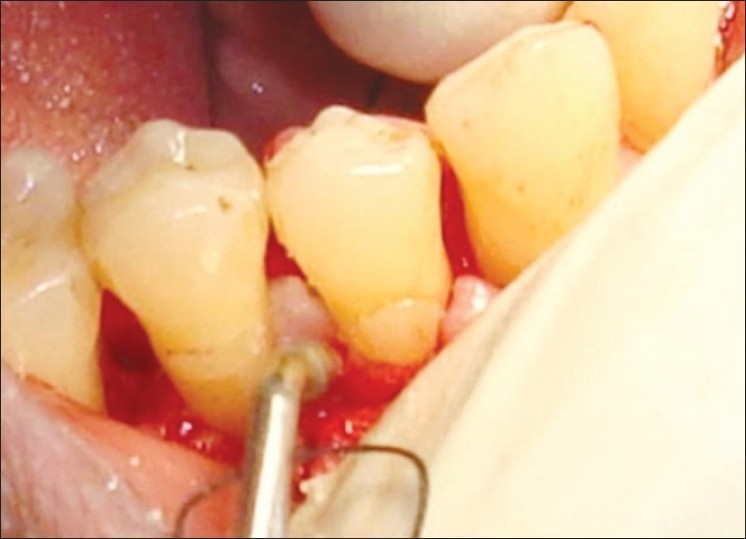
In patients selected for test group in addition to OFD, NcHA bone replacement grafts having particle size 40 to 50 nm (Sybograf®) was utilized to fill the defects to the most coronal level of the osseous walls and Type-I sterile collagen GTR membrane (Periocol®) was adapted over the bone graft extended 2 to 3 mm beyond the margin of the defect apically and mesiodistally and 1 mm below the CEJ [Figures 4–6]. Periocol® is an orange-brown-colored membrane having the size of 25 × 30 mm in a sterile double blister pack. The source of collagen in Periocol® GTR membrane is from the air bladder of fresh water fishes.
Figure 4.
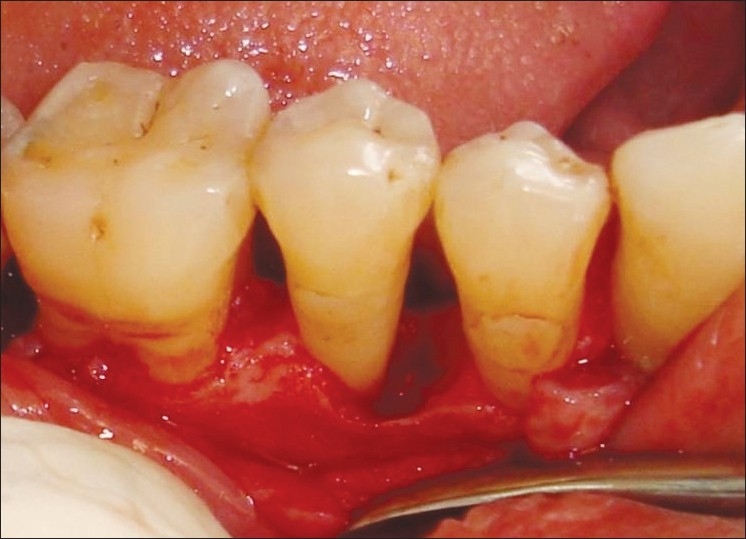
Intrabony defect after degranulation
Figure 6.
GTR membrane adapted
Figure 5.
Bone graft placed
Primary soft tissue closure was done with interrupted sutures using non-resorbable 3-0 black silk sutures. Periodontal dressing was applied at the surgical site.
Postoperatively, subjects were prescribed antibiotics (Amoxicillin 500 mg tid for 7 days) to prevent postoperative infection and analgesics (Ibuprofen 400 mg tid for 5 days) for pain control. Patients were instructed to rinse with 0.2% chlorhexidine gluconate (twice daily for 4 weeks). Following surgery, the patients were asked to refrain from tooth brushing, flossing, and interdental cleaning techniques in the treated area for 4 weeks after surgery. At 1 week, dressing, sutures, and any plaque present at the surgical site were removed. Recall appointments were then made at 15 days and 30 days for additional follow-up and plaque control.
All the patients were recalled at 3 and 6 months post-surgery for follow-up. At 6 months recall visit, full mouth PI and GI were recorded. At each recall appointment, supragingival scaling was performed using ultrasonic scaler and oral hygiene instructions were reinforced. No periodontal probing or subgingival re-instrumentation was performed prior to six months.
DATA ANALYSIS
All the statistical analyses were made by using computer software package for statistical analysis SPSS version 11.5 (SPSS Inc., Chicago, Illinois). Wilcoxon Signed Rank Sum test was used for intragroup comparison in both test and control group. Mann Whitney U test was used for intergroup comparison between test and control group. For all the statistical analysis, P<0.05 was considered significant.
RESULTS
Fourteen of the 16 patients completed the 6-month follow-up period. Configuration and distribution of treated intrabony defects are depicted in Table 1. The postoperative healing was uneventful in all cases. Neither allergic reaction nor suppuration or abscess was observed in any of the patients. Membrane exposure occurred in one of the patient. The exposed part of the membrane disintegrates without any side effect.
Table 1.
Subjects and defects characteristics at baseline
The PI and GI values (Mean±SD) showed non-significant difference at baseline and 6 months between the test and control groups, as depicted in Table 2. The mean PI and GI score remained <1 throughout 6 month period. Thus, in general, the patients showed good oral hygiene throughout the study. No statistically significant differences were found between groups for any of the investigated parameters (PPD, CAL, REC, DD) at baseline [Table 2].
Table 2.
Test and control group at baseline and 6 months (Mean±SD) in mm

At six months, the mean PPD reduction was 4.33±0.50 mm for the test and 3.22±1.09 mm for the control group [Tables 2 and 3]. The Wilcoxon Signed Rank Sum test indicated that both the test (P=0.006) and control (P=0.007) groups showed a significantly greater mean PPD reduction at six months. Analysis by Mann Whitney U test demonstrated a statistically significantly (P=0.01) greater reduction in mean PPD favoring the test group, and an additional 1.11 mm PPD reduction was observed in test group [Table 3].
Table 3.
Comparison of change in Clinical and Radiographic parameter between test and control group at 6 months (Mean±SD) in mm
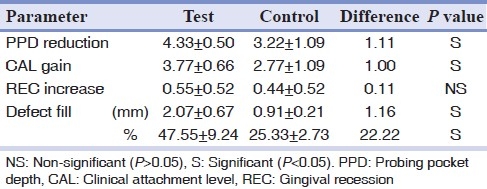
Statistically significant mean CAL gains of 3.77±0.66 mm was in the test, and 2.78±1.09 mm control group from baseline to 6 months [Tables 2 and 3]. There was statistically significantly greater CAL gains for the test group (3.77 mm) than the control group (2.77 mm) [Table 3]. The magnitude of the observed additional benefit was 1.00 mm in the test group.
At six months, the mean increase in REC was 0.55±0.52 mm and 0.44±0.52 mm in the test and control group, respectively [Table 3], although a statistically significant increase in REC was found in both the groups (P=0.025 test; P=0.046 control) [Table 3]. None of the sites treated in both test and control group resulted in an increase in REC of more than 1 mm.
The mean gain in radiographic defect fill was recorded as 2.07±0.67 mm (47.55%) in test and 0.91±0.21 mm (25.33%) in control group at 6 months [Tables 2 and 3]. Statistically significant (P<0.05) gain of radiographic defect fill was recorded in both the test as well as for the control group. At 6 months, statistically significant (P=0.001) greater reduction of radiographic DD was observed in test group in comparison with control group [Table 3]. There was also an additional 1.16 mm (22.22%) of radiographic bone fill in the test group [Table 3].
DISCUSSION
Conventional periodontal therapy has limited scope and results are not predictable. Bone grafts, their synthetic substitutes, and GTR techniques have been used in an attempt to gain this therapeutic endpoint. The present study was designed to compare the combined effect of BG+GTR (test group) with OFD (control group) in the treatment of intrabony periodontal defects.
A clinically and statistically significant improvement in PPD reduction, CAL gain, and radiographic defect fill was observed in both test and control group at 6 months postoperatively compared with baseline which was favoring to the test group.
In the control group, the mean reduction of PPD was 3.22±1.09 mm and CAL gain was 2.77±1.09 mm at 6 months. In a study by Kilic et al.,[19] OFD group showed the mean PPD reduction of 3.17 mm, which was in accordance to the results of the present study, and CAL gain of 2.1 mm, which was slightly lower than the present study. The difference in results of OFD group might be influenced by surgical technique, baseline defect characteristics, and operator skill.[21]
The reduction in PPD was slightly higher and the CAL gain was in agreement with the previous reported study by Kasaj et al.[27] that evaluated the clinical efficacy of NcHA paste in intrabony defects and reported PPD reduction of 3.9±1.2 mm and CAL gain of 3.6±1.6 mm. This slightly higher PPD reduction may be due to the effect of combination technique (BG+GTR) used in the present study. Kilic et al.[19] demonstrated that the combination of HA collagen bone graft with ePTFE membrane resulted in higher PPD reduction (5.85 mm) and greater CAL gain (3.80 mm) compared with the test group results of our study. The higher reduction of PPD might be explained by the higher REC in the above study (2.00 mm) compared with 0.55 mm in the present study.
It was suggested that intrabony defect configuration influences the results after GTR and larger amounts of CAL gain were reported in deep 3-wall defects than 2- or 1-wall defects following GTR treatment.[28] A systematic review concluded that in two-wall intrabony defect models of periodontal regeneration, the additional use of a grafting material gave superior histological results of bone repair to barrier membranes alone.[29] In the present study, configuration of defects treated were 2 and 2½-walled, which may have benefited from the combination technique.
Pocket depth reduction even though not necessarily a result attributed to regeneration, it is a major player in decision making in routine periodontal patient care scenarios. Initial pocket depth in the defects treated with BG+GTR in the present study were more than 7 mm and were reduced to value just slightly above 3 mm depth, accepted as manageable by current patient care standard.
Intergroup comparison of the results at 6 months showed that there was an additional 1.11 mm PPD reduction and 1.00 mm CAL gain in the test group. Trombelli[18] concluded that additional effect of the combination treatment (GTR + BG) is similar to GTR alone when compared with OFD with respect to attachment gain, but results in slightly more PPD reduction and greater gain in hard tissue probing at re-entry surgery.
In fact, collagen membranes are characterized by a lack of stiffness when they are dampened by biological fluids. The presence of a physical support under such a material allows the membrane to maintain its position when the flaps are sutured over the defect, exerting pressure onto the membrane itself. It should, however, be pointed out that in the present study, the collagen membrane was not fixed by means of bioresorbable sutures or pins. Thus, it cannot be excluded that the membrane was displaced during flap suturing or the healing process and may have acted only for stabilization of the graft particles.[21]
Periodontal therapies are usually associated with REC which is of esthetic concern for both patients and clinicians. Regenerative therapy potentially could help to overcome this unwanted side effect. Hence, it is important to assess the amount of REC. The REC in the present study showed no statistically significant difference between test and control sites at baseline and at 6 months. The increase in REC in present study was lower in both test and control groups compared with the previously reported similar study by Sculean et al.[21]
The ability of a probe to penetrate into a pocket is related to several factors including probing force and gingival tissue condition.[30] When evaluating results of periodontal regenerative therapy, results should be interpreted carefully. Camargo et al.[9] stated that the improvement in clinical parameter can result in gain in attachment; however, it should be remembered that placement of graft material into the defect may modify gingival tissue consistency and therefore interfere with the penetration of periodontal probe without necessarily having induced any gain in CAL.
Hence, bone fill data derived from surgical re-entry are important to substantiate routine postoperative measurements due to the above mentioned reason. However, it has certain inherent disadvantages, like inducing further resorption at the treated site and inability to ascertain the exact histological nature of the hard tissue. The second surgical procedure is also time consuming and may interrupt the regenerative process if healing is still ongoing.[30] Zybutz et al.[31] concluded that standardized radiographs reliably and permanently describe the hard tissue changes and thus can serve as substitute for probing to bone or re-entry measurements of bone changes.
In the present study, a change of the alveolar bone level was detected radiographically using consecutive pre- and postoperative radiographs. Projection geometry of consecutive radiographs should be standardized to minimize measurement errors. Prefabricated film holders[31] may provide projection standardization to a certain degree. In the present study, linear measurement from the crest to the base of the intrabony defect was measured by using the “J image” software, which can overcome errors in the linear measurement to a certain degree than manual measurements.
In the present study, the radiographic defect depth gain was 2.07±0.67 mm, which represents radiographic defect depth fill of 47.55±9.24% at 6 months in test group. Kilic et al.[19] reported 1.55 mm gain of radiographic defect depth in test group, which was slightly lower than the present study. Several reports indicate that the bone fill is enhanced by the addition of a graft material to GTR procedures. [29,32] Present study demonstrated additional 1.16 mm (22.22%) radiographic defect depth fill in the test group compared with the control group over six-month period, although complete regeneration was not achieved.
Wenzel et al.[33] reported that, no increased bone fill between 6 and 12 months may support the 6-month radiographic analysis of the present study. Question arises whether the radiographically assessed increase in the defect fill in the test group could represent the new bone formation or the presence of residual graft material or both, although NcHA was reported to be resorbable.
The present study demonstrated statistically significant but slight clinical improvement in CAL gain (1.00 mm) in the test group compared with OFD. Radiographic defect fill was more in favor of the test group. The most reliable outcome variable for assessing periodontal regeneration is histological analysis; however, due to ethical consideration and patient management limitation, no histological evidence was obtained to establish proof of periodontal regeneration in present study.
CONCLUSION
Adjunctive use of bioresorbable collagen membrane of fish origin in combination with NcHA bone graft resulted in clinically, radiographically and statistically significant compared with OFD alone, in terms of PPD reduction, CAL gain, and percentage of Bone fill.
Footnotes
Source of Support: The study was partially supported by funding provided by the Manipal University, Manipal, Karnataka, India
Conflict of Interest: None declared.
REFERENCES
- 1.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21:1803–10. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 2.Chris Arts JJ, Verdonschot N, Schreurs BW, Buma P. The use of a bioresorbable nano-crystalline hydroxyapatite paste in acetabular bone impaction grafting. Biomaterials. 2006;27:1110–18. doi: 10.1016/j.biomaterials.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Schnettler R, Dingeldein E. Inorganic bone substitutes. Tissue Engineering and Biodegradable Equivalents: Scientific and Clinical Applications. New York: Marcell Dekker; 2002. pp. 401–32. [Google Scholar]
- 4.Schnettler R, Stahl JP, Alt V, Pavlidis T, Dingeldein E, Wenisch S. Calcium phosphate-based bone substitutes. Eur J Trauma. 2004;4:219–29. [Google Scholar]
- 5.Huber FX, Belyaev O, Hillmeier J, Kock HJ, Huber C, Meeder PJ, et al. , First histological observations on the incorporation of a novel nanocrystalline hydroxyapatite paste (Ostims®) in human cancellous bone. BMC Musculoskelet Disord. 2006;7:50. doi: 10.1186/1471-2474-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strietzel FP, Reichart PA, Graf H. Lateral alveolar ridge augmentation using a synthetic nano-crystalline hydroxyapatite bone substitution material (Ostims®) Preliminary clinical and histological results. Clin Oral Implant Res. 2007;18:743–51. doi: 10.1111/j.1600-0501.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz F, Bieling K, Latz T, Nuesry E, Becker J. Healing of intrabony periimplantitis defects following application of a nanocrystalline hydroxyapatite (Ostims®) or a bovine-derived xenograft (Bio-Osst) in combination with a collagen membrane (Bio-Gide). A case series. J Clin Periodontol. 2006;33:491–99. doi: 10.1111/j.1600-051X.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal N, Steinberg J. The use of collagen membrane barriers in conjunction with combined demineralized bone collagen gel implants in human infrabony defects. J Periodontol. 1990;61:319–27. doi: 10.1902/jop.1990.61.6.319. [DOI] [PubMed] [Google Scholar]
- 9.Camargo PM, Lekovic V, Weinlaender M, Nedic M, Vasilic N, Wolinsky LE, et al. A controlled re-entry study on the effectiveness of bovine porous bone mineral used in combination with a collagen membrane of porcine origin in the treatment of intrabony defects in humans. J Clin Periodontol. 2000;27:889–96. doi: 10.1034/j.1600-051x.2000.027012889.x. [DOI] [PubMed] [Google Scholar]
- 10.Divya PV, Nandakumar K. Local drug delivery- Periocol® in periodontics. Trends Biomater Artif Organs. 2006;19:74–80. [Google Scholar]
- 11.Sella MN, Kohavi D, Krausz E, Steinberg D, Rosen G. Enzymatic degradation of collagen-guided tissue regeneration membranes by periodontal bacteria. Clin Oral Implants Res. 2003;14:263–8. doi: 10.1034/j.1600-0501.2003.140302.x. [DOI] [PubMed] [Google Scholar]
- 12.Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, Becker J. Biodegradation of differently cross-linked collagen membranes: An experimental study in rats. Clin Oral Implants Res. 2005;16:369–78. doi: 10.1111/j.1600-0501.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 13.Paolantonio M. Combined periodontal regenerative technique in human intrabony defects by collagen membranes and anorganic bovine bone. A controlled clinical study. J Periodontol. 2002;73:158–66. doi: 10.1902/jop.2002.73.2.158. [DOI] [PubMed] [Google Scholar]
- 14.Cortellini P, Pini PG, Tonetti MS. Periodontal regeneration of human intrabony defects with titanium reinforced membranes.A controlled clinical trial. J Periodontol. 1995;66:797–803. doi: 10.1902/jop.1995.66.9.797. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects: A systematic review. Ann Periodontol. 2003;8:227–65. doi: 10.1902/annals.2003.8.1.227. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz F, Sculean A, Bieling K, Ferrari D, Rothamel D, Becker J. Two-year clinical results following treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane. J Clin Periodontol. 2008;35:80–8. doi: 10.1111/j.1600-051X.2007.01168.x. [DOI] [PubMed] [Google Scholar]
- 17.Christgau M, Schamlz G, Wenzel A, Hiller KA. Periodontal regeneration of intrabony defects with resorbable and nonresorbable membranes: 30-month results. J Clin Periodontol. 1997;24:17–27. doi: 10.1111/j.1600-051x.1997.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 18.Trombelli L. Which reconstructive procedures are effective for treating the periodontal intraosseous defect? Periodontology 2000. 2005;37:88–105. doi: 10.1111/j.1600-0757.2004.03798.x. [DOI] [PubMed] [Google Scholar]
- 19.Kilic AR, Efeoglu E, Yilmaz S. Guided tissue regeneration in conjunction with hydroxyapatite-collagen grafts for intrabony defects.A clinical and radiological evaluation. J Clin Periodontol. 1997;24:372–83. doi: 10.1111/j.1600-051x.1997.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 20.Lundgarden D, Slotte C. Reconstruction of anatomically complicated periodontal defects using a bioresorbable GTR barrier supported by bone mineral. A 6-month follow-up study of 6 cases. J Clin Periodontol. 1999;26:56–62. doi: 10.1034/j.1600-051x.1999.260110.x. [DOI] [PubMed] [Google Scholar]
- 21.Sculean A, Berakdar M, Chiantella GC, Donos N, Arweiler NB, Brecx M. Healing of intrabony defects following treatment with a bovine-derived xenograft and collagen membrane.A controlled clinical study. J Clin Periodontol. 2003;30:73–80. doi: 10.1034/j.1600-051x.2003.10192.x. [DOI] [PubMed] [Google Scholar]
- 22.Tonetti MS, Cortellini P, Lang NP, Suvan JE, Adriaens P, Dubravec D, et al. Clinical outcomes following treatment of human intrabony defects with GTR/bone replacement material or access flap alone. A multicenter randomized controlled clinical trial. J Clin Periodontol. 2004;31:770–76. doi: 10.1111/j.1600-051X.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 23.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 24.Loe H, Silness J. Periodontal disease in pregnancy. I. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 25.Scabbia A, Trombelli L. A comparative study on the use of a HA/collagen/chondroitin sulphate biomaterial (Biostites) and a bovine-derived HA xenograft (Bio-Osss) in the treatment of deep intra-osseous defects. J Clin Periodontol. 2004;31:348–55. doi: 10.1111/j.1600-051X.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 26.Goldman HM, Cohen DW. The infrabony pocket: Classification and treatment. J Periodontol. 1958;10:272–91. [Google Scholar]
- 27.Kasaj A, Rohrig B, Zafiropoulos GG, Willershausen B. Clinical evaluation of nanocrystalline hydroxyapatite paste in the treatment of human periodontal bony defects-A randomized controlled clinical trial: 6-month results. J Periodontol. 2008;79:394–400. doi: 10.1902/jop.2008.070378. [DOI] [PubMed] [Google Scholar]
- 28.Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human intrabony defects. I. Clinical measures. J Periodontol. 1993;64:254–60. doi: 10.1902/jop.1993.64.4.254. [DOI] [PubMed] [Google Scholar]
- 29.Sculean A, Nikolidakis D, Schwarz F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials – biological foundation and preclinical evidence. A systematic review. J Clin Periodontol. 2008;35(Suppl 8):106–16. doi: 10.1111/j.1600-051X.2008.01263.x. [DOI] [PubMed] [Google Scholar]
- 30.Reddy MS, Jeffcoat MK. Methods of assessing periodontal Regeneration. Periodontol 2000. 1999;19:87–103. doi: 10.1111/j.1600-0757.1999.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 31.Zybutz M, Rapoport D, Laurell L, Persson GR. Comparisons of clinical and radiographic measurements of interproximal vertical defects before and 1 year after surgical treatments. J Clin Periodontol. 2000;27:179–86. doi: 10.1034/j.1600-051x.2000.027003179.x. [DOI] [PubMed] [Google Scholar]
- 32.Needleman I, Tucker R, Giedrys-Leeper E, Worthington H. A systematic review of guided tissue regeneration for periodontal infrabony defects. J Periodontol Res. 2002;37:380–8. doi: 10.1034/j.1600-0765.2002.01369.x. [DOI] [PubMed] [Google Scholar]
- 33.Wenzel A, Warrer K, Karring T. Digital subtraction radiography in assessing bone changes in periodontal defects following guided tissue regeneration. J Clin Periodontol. 1992;19:208–13. doi: 10.1111/j.1600-051x.1992.tb00641.x. [DOI] [PubMed] [Google Scholar]


